Surface Modification of a Nanoporous Carbon Photoanode upon Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Performance of the Nanoporous Carbon Anodes
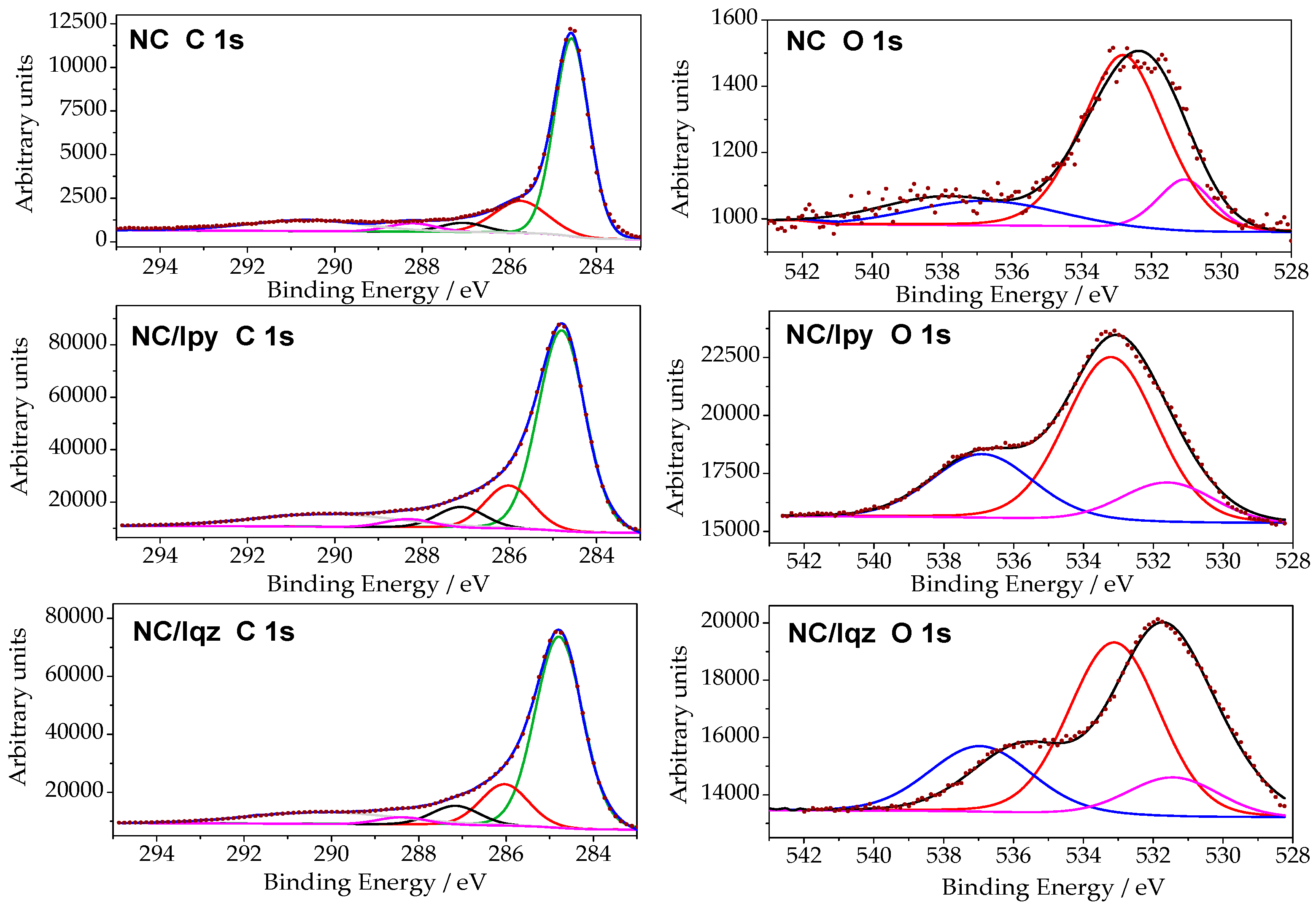
2.2. Surface Modification of the Nanoporous Carbon under Solar Irradiation
3. Materials and Methods
3.1. Materials
3.2. Photocatalytic Measurements
3.3. Irradiation Conditions for Surface Modification
3.4. Textural Characterization
3.5. Chemical Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nocera, D.G. The artificial leaf. Acc. Chem. Res. 2012, 45, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Gewirth, A.A.; Thorum, M.S. Electroreduction of Dioxygen for Fuel-Cell Applications: Materials and Challenges. Inorg. Chem. 2010, 49, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Wang, D.; Hisatomi, T.; Takata, T.; Pan, C.; Katayama, M.; Kubota, J.; Domen, K. Core/shell photocatalyst with spatially separated co-catalysts for efficient reduction and oxidation of water. Angew. Chem. 2013, 52, 11252–11256. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Lu, D.; Domen, K. Direct water splitting into hydrogen and oxygen under visible light by using modified TaON photocatalysts with d0 electronic configuration. Chem. Eur. J. 2013, 19, 4986–4991. [Google Scholar] [CrossRef] [PubMed]
- Marschalla, R.; Wang, L.Z. Non-metal doping of transition metal oxides for visible-light photocatalysis. Catal. Today 2014, 225, 111–135. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Kamat, P.V. Emergence of New Materials for Light–Energy Conversion: Perovskites. J. Phys. Chem. Lett. 2014, 5, 4167–4168. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Etacheria, V.; Valentinc, C.D.; Schneiderd, J.; Bahnemannd, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Chena, J.; Qiub, F.; Xua, W.; Caoc, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.R.; Fu, X.; Xu, Y.J. TiO2—Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2—Graphene Truly Different from Other TiO2—Carbon Composite Materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Xu, Y.J. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, N.; Xu, Y.J. Structural diversity of graphene materials and their multifarious roles in heterogeneous photocatalysis. Nano. Today 2016, 11, 351–372. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.F.; Teng, C.Y.; Chen, S.J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.F.; Chen, S.J.; Yeh, C.S.; Teng, H. Tuning the electronic structure of graphite oxide through ammonia treatment for photocatalytic generation of H2 and O2 from water splitting. J. Phys. Chem. C 2013, 117, 6516–6524. [Google Scholar] [CrossRef]
- Velasco, L.F.; Lima, J.C.; Ania, C.O. Visible-light photochemical activity of nanoporous carbons under monochromatic light. Angew. Chem. Int. Ed. 2014, 53, 4146–4148. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.F.; Fonseca, I.M.; Parra, J.B.; Lima, J.C.; Ania, C.O. Photochemical behaviour of activated carbons under UV irradiation. Carbon 2012, 50, 249–258. [Google Scholar] [CrossRef]
- Velasco, L.F.; Maurino, V.; Laurenti, E.; Ania, C.O. Light-induced generation of radicals on semiconductor-free carbon photocatalysts. Appl. Catal. A 2013, 453, 310–315. [Google Scholar] [CrossRef]
- Velasco, L.F.; Gomis-Berenguer, A.; Lima, J.C.; Ania, C.O. Tuning the Surface Chemistry of Nanoporous Carbons for Enhanced Nanoconfined Photochemical Activity. ChemCatChem 2015, 7, 3012–3019. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Iniesta, J.; Moro, A.; Maurino, V.; Lima, J.C.; Ania, C.O. Boosting visible light conversion in the confined pore space of nanoporous carbons. Carbon 2016, 96, 98–104. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Matos, J.; Seredych, M.; Islam, M.S.Z.; Alfano, R. Photoactivity of S-doped nanoporous activated carbons: A new perspective for harvesting solar energy on carbon-based semiconductors. Appl. Catal. A 2012, 445–446, 159–165. [Google Scholar] [CrossRef]
- Ania, C.O.; Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T.J. Visible light driven photoelectrochemical water splitting on metal free nanoporous carbon promoted by chromophoric functional groups. Carbon 2014, 79, 432–441. [Google Scholar] [CrossRef]
- Radovic, L.R.; Bockrath, B. On the chemical nature of graphene edges: Origin of stability and potential for magnetism in carbon materials. J. Am. Chem. Soc. 2005, 127, 5917–5927. [Google Scholar] [CrossRef] [PubMed]
- Modestov, A.D.; Gun, J.; Lev, O. Graphite photoelectrochemistry study of glassy carbon, carbon-fiber and carbon-black electrodes in aqueous electrolytes by photocurrent response. Surf. Sci. 1998, 417, 311–322. [Google Scholar] [CrossRef]
- Seredych, M.; Messali, L.; Bandosz, T.J. Analysis of factors affecting visible and UV enhanced oxidation of dibenzothiophenes on sulfur-doped activated carbons. Carbon 2013, 62, 356–364. [Google Scholar]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Surface modifications of activated carbon by gamma irradiation. Carbon 2014, 67, 236–249. [Google Scholar] [CrossRef]
- Voudrias, E.A.; Larson, R.A.; Snoeyink, V.L. Importance of surface free radicals in the reactivity of granular activated carbon under water treatment conditions. Carbon 1987, 25, 503–515. [Google Scholar] [CrossRef]
- Henning, G.R. Electron microscopy of reactivity changes near lattice defects in graphite. Chem. Phys. Carbons 1966, 2, 1–49. [Google Scholar]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Activated carbon as photocatalyst of reactions in aqueous phase. Appl. Catal. B Environ. 2013, 142–143, 694–704. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Kuhn, H.K.; Braslavsky, S.E.; Schmidt, R. Chemical actinometry (IUPAC technical report). Pure Appl. Chem. 2004, 76, 2105–2146. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the carbon photoanodes are available from the authors.
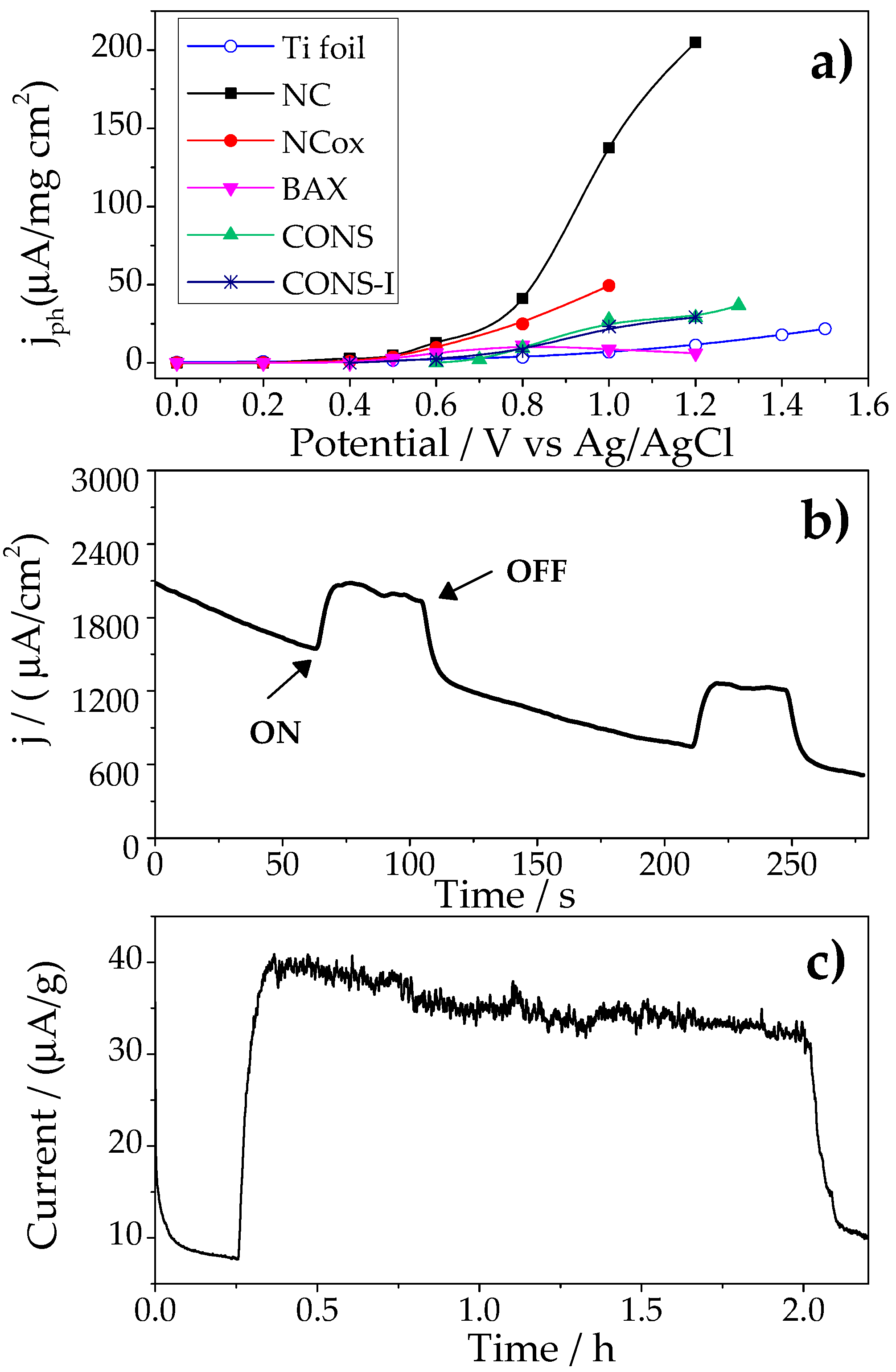

| Samples | SBET (m2/g) | Vtotal (cm3/g) A | Vmicropores (cm3/g) B | Vmesopores (cm3/g) B |
|---|---|---|---|---|
| NC | 1027 | 0.524 | 0.320 | 0.090 |
| NCox | 989 | 0.500 | 0.310 | 0.070 |
| BAX | 2010 | 1.463 | 0.472 | 0.991 |
| CONS | 38 | 0.022 | 0.001 | 0.021 |
| CONS-I | 727 | 0.363 | 0.260 | 0.103 |
| Samples | CO2 Evolved (mmol/g) | CO Evolved (mmol/g) | Oxygen (wt. %) A |
|---|---|---|---|
| NC photoanode (as prepared) | 0.320 | 1.139 | 1.9 |
| NC photoanode irradiated (2 h) | 0.502 | 1.251 | -- |
| NC photoanode irradiated (2 h) and polarized (1 V vs. Ag/AgCl) | 0.555 | 1.603 | -- |
| NC (pristine carbon) | 0.164 | 0.557 | 1.9 |
| NC/Ipy ( irradiation 360 < λ < 600 nm) | 0.292 | 0.630 | 6.9 |
| NC/Iqz (irradiation 200 < λ < 600 nm) | 0.282 | 0.652 | 5.8 |
| NC | NC/Iqz | NC/Ipy | |
|---|---|---|---|
| –C–C in graphitic carbon (284.3–284.9 eV) | 64.2 | 64.6 | 64.2 |
| –C–(O, S, H) in phenolic, alcoholic (285.2–286.0 eV) | 15.8 | 14.0 | 13.7 |
| –C=O in carbonyl or quinone (286.56–287.1 eV) | 3.9 | 6.4 | 6.7 |
| –O–C=O in carboxyl or ester (287.6–288.22 eV) | 3.8 | 2.4 | 2.5 |
| –carbonate, occluded CO, ð-electrons in aromatic ring (289.3–289.5 eV) | 12.3 | 12.6 | 12.9 |
| Total C 1s (at. %) | 96.4 | 92.3 | 92.2 |
| –C=O in carbonyl, quinone (530.6–531.9eV) | 13.8 | 24.1 | 23.9 |
| –C–O in phenol/epoxy, ether, thioethers (532.3–533.9 eV) | 70.3 | 64.9 | 49.5 |
| –O– in carboxyl, water or chemisorbed oxygen species (534.7–535.0 eV) | 15.9 | 11.0 | 26.6 |
| Total O 1s (at. %) | 3.6 | 7.7 | 7.8 |
| Samples | SBET (m2/g) | Vtotal (cm3/g) A | W0, N2 (cm3/g) B | W0, CO2 (cm3/g) B |
|---|---|---|---|---|
| NC (pristine carbon) | 1027 | 0.524 | 0.386 | 0.197 |
| NC/Iqz ( irradiation 200 < λ < 600 nm) | 1018 | 0.537 | 0.488 | 0.168 |
| NC/Ipy (irradiation 360 < λ < 600 nm) | 1033 | 0.579 | 0.580 | 0.170 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomis-Berenguer, A.; Velo-Gala, I.; Rodríguez-Castellón, E.; Ania, C.O. Surface Modification of a Nanoporous Carbon Photoanode upon Irradiation. Molecules 2016, 21, 1611. https://doi.org/10.3390/molecules21111611
Gomis-Berenguer A, Velo-Gala I, Rodríguez-Castellón E, Ania CO. Surface Modification of a Nanoporous Carbon Photoanode upon Irradiation. Molecules. 2016; 21(11):1611. https://doi.org/10.3390/molecules21111611
Chicago/Turabian StyleGomis-Berenguer, Alicia, Inmaculada Velo-Gala, Enrique Rodríguez-Castellón, and Conchi O. Ania. 2016. "Surface Modification of a Nanoporous Carbon Photoanode upon Irradiation" Molecules 21, no. 11: 1611. https://doi.org/10.3390/molecules21111611
APA StyleGomis-Berenguer, A., Velo-Gala, I., Rodríguez-Castellón, E., & Ania, C. O. (2016). Surface Modification of a Nanoporous Carbon Photoanode upon Irradiation. Molecules, 21(11), 1611. https://doi.org/10.3390/molecules21111611







