Photo Racemization and Polymerization of (R)-1,1′-Bi(2-naphthol)
Abstract
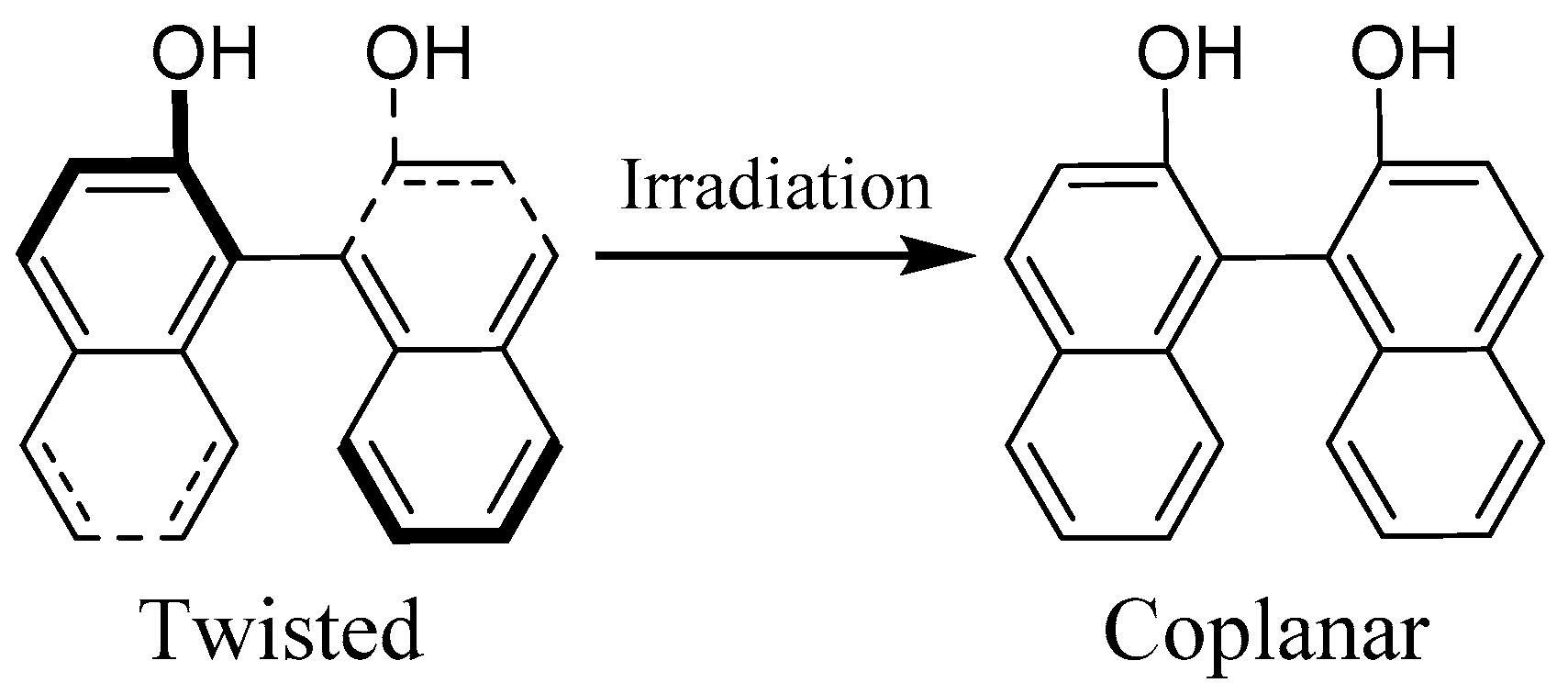
:1. Introduction
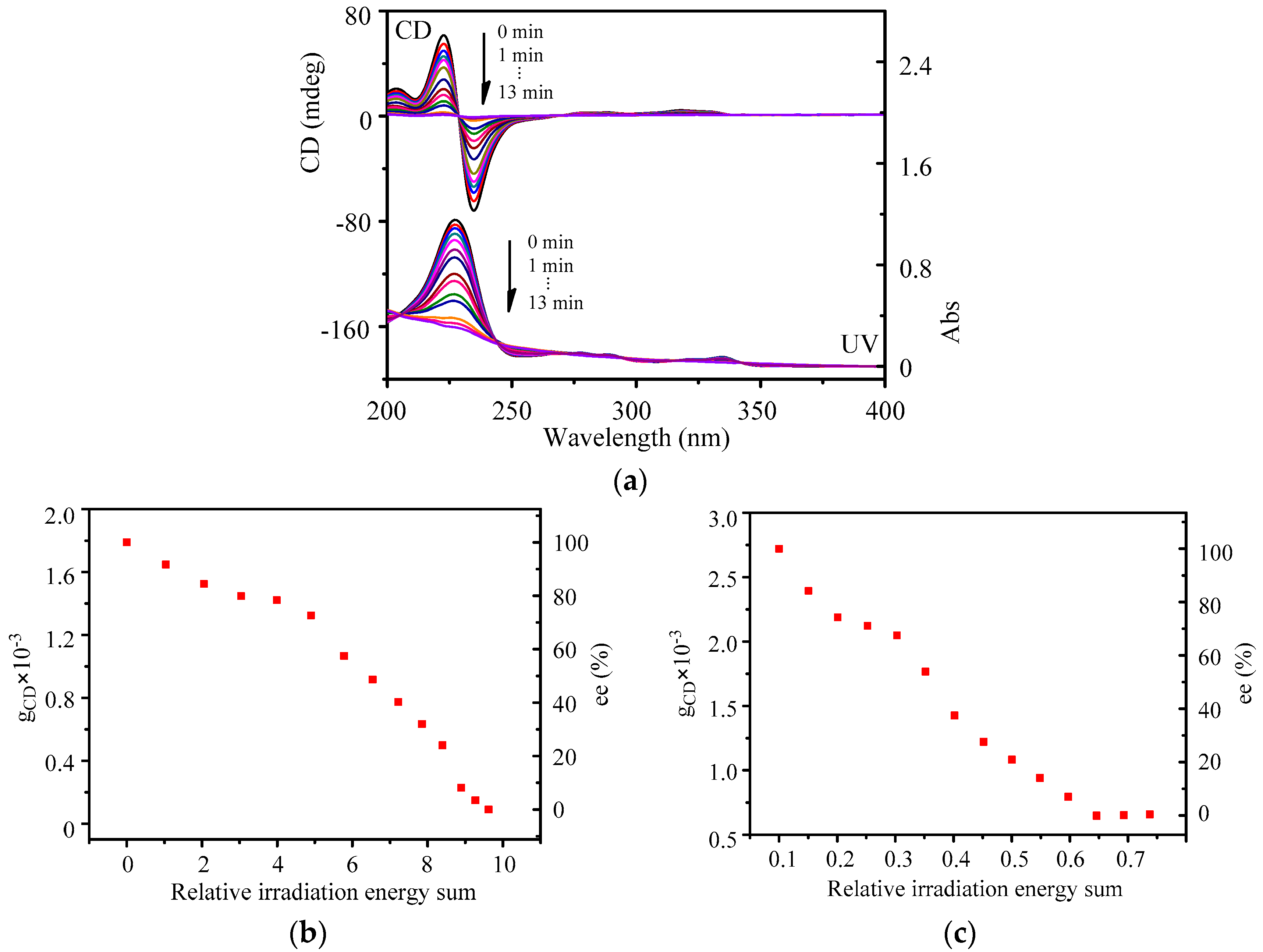
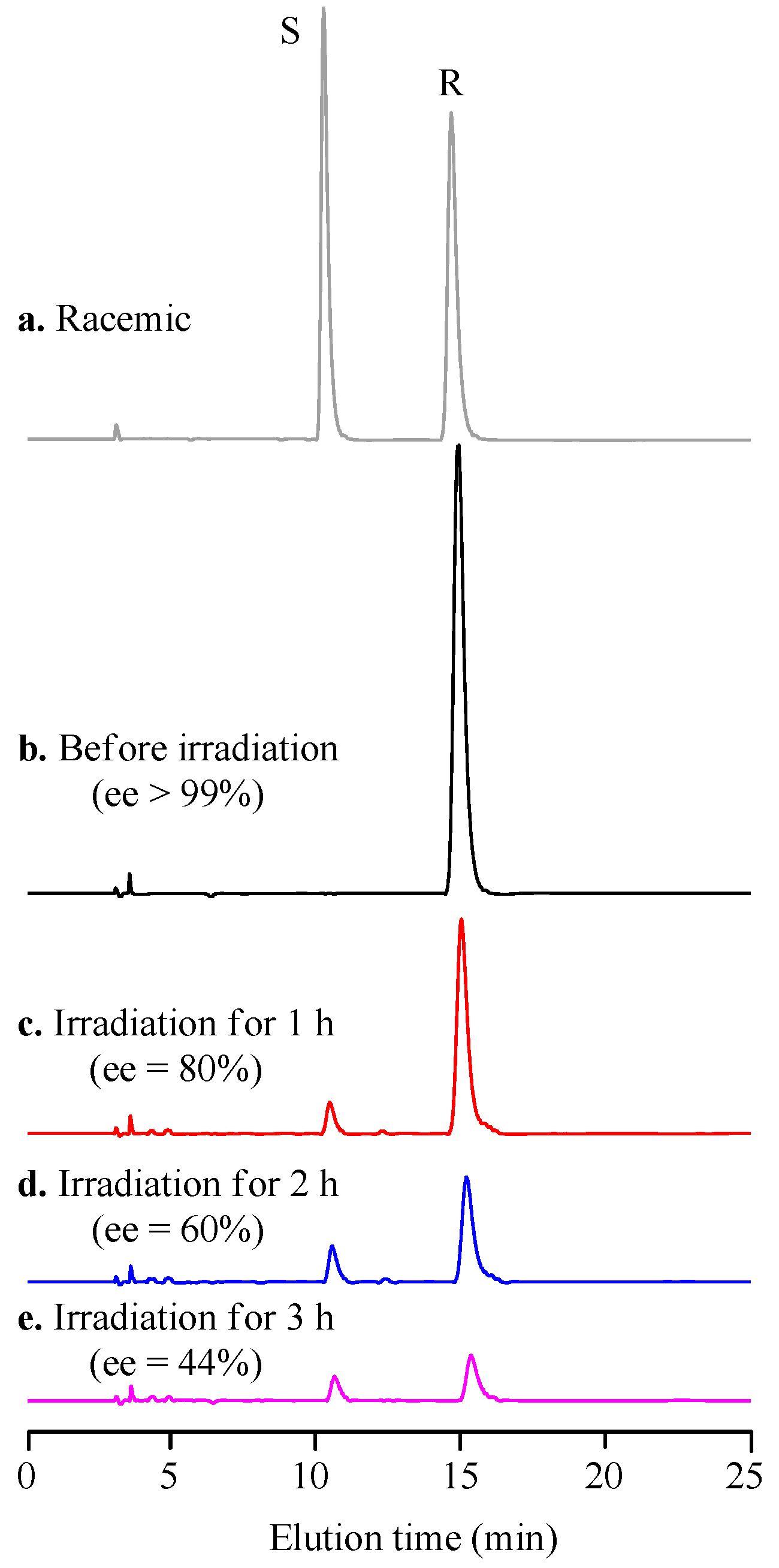
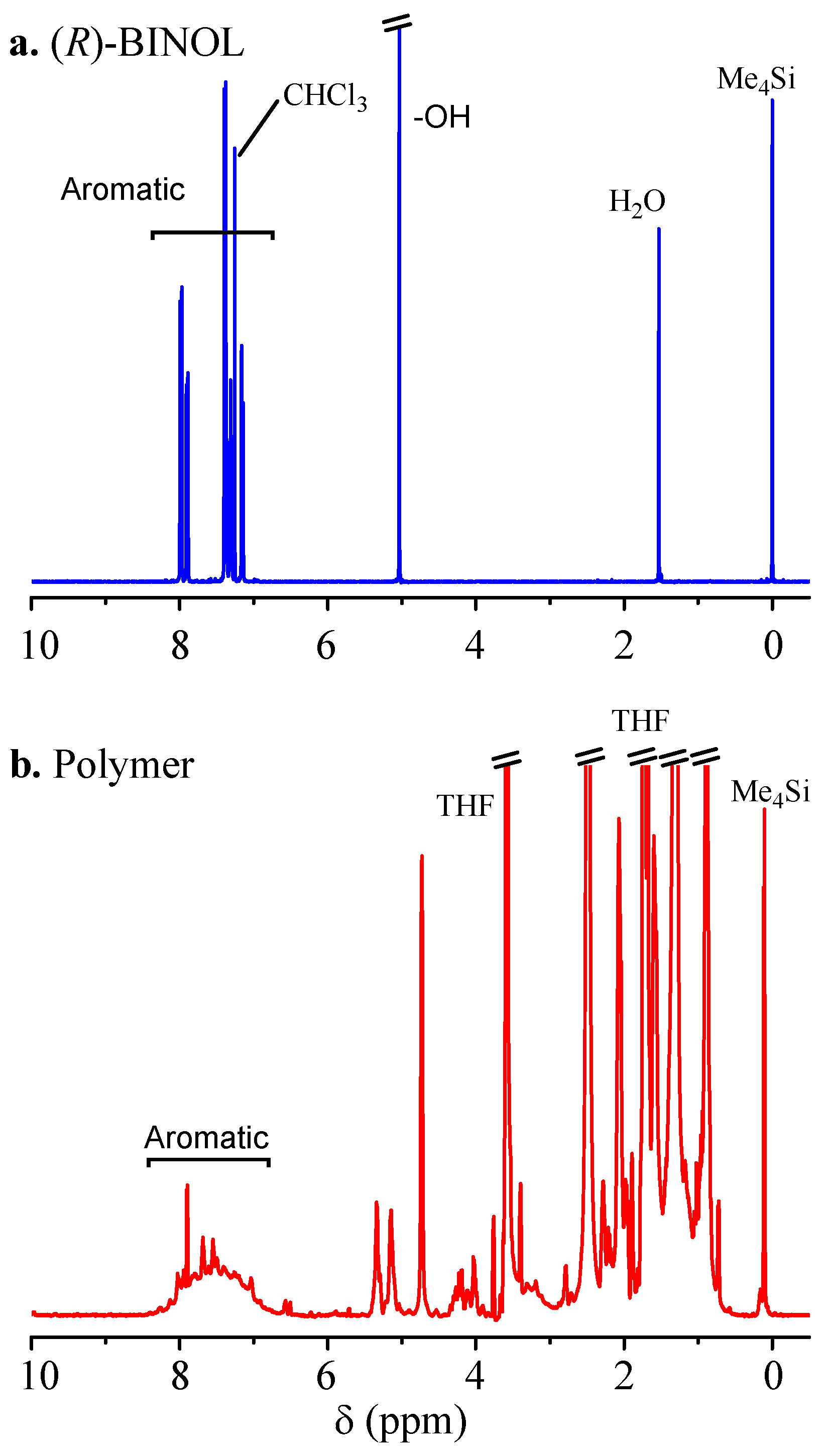
2. Results and Discussion
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, USA, 2010. [Google Scholar]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Balzani, V.; Ceroni, P.; Juris, A. Photochemistry and Photophysics: Concepts, Research, Applications; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Asandei, A.D. Photomediated Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Keukeleire, D.D.; He, S.-L. Photochemical Strategies for the Construction of Polycyclic Molecules. Chem. Rev. 1993, 93, 359–380. [Google Scholar] [CrossRef]
- Dilling, W.L. Intramolecular Photochemical Cycloaddition of Nonconjugated Olefins. Chem. Rev. 1966, 66, 373–393. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Poplata, S.; Troster, A.; Zou, Y.Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2+2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef] [PubMed]
- Remy, R.; Bochet, C.G. Arene-Alkene Cycloaddition. Chem. Rev. 2016, 116, 9816–9849. [Google Scholar] [CrossRef] [PubMed]
- Le Bell, J.A. Sur les Relations Qui Existent Entre les Formules Atomiques des Corps Organiques et le Pouvoir Rotatoire de Leurs Dissolutions. Bull. Soc. Chim. Fr. 1874, 22, 337–347. [Google Scholar]
- Van’t Hoff, J.H. Sur Les Formules de Structure dans L’espace; Pamphlet: Utrecht, The Netherlands, 1874. [Google Scholar]
- Inoue, Y. Asymmetric Photochemical Reactions in Solution. Chem. Rev. 1992, 92, 741–770. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R.A. Absolute Asymmetric Synthesis: The Origin, Control, and Amplification of Chirality. Angew. Chem. Int. Ed. Engl. 1999, 38, 3418–3438. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sato, M.; Ishiguro, S.; Saito, T.; Morishita, Y.; Sato, I.; Nishino, H.; Inoue, Y.; Soai, K. Enantioselective Synthesis of Near Enantiopure Compound by Asymmetric Autocatalysis Triggered by Asymmetric Photolysis with Circularly Polarized Light. J. Am. Chem. Soc. 2005, 127, 3274–3275. [Google Scholar] [CrossRef] [PubMed]
- Balavoine, G.; Moradpour, A.; Kagan, H. Preparation of Chiral Compounds with High Optical Purity by Irradiation with Circularly Polarized Light, a Model Reaction for the Prebiotic Generation of Optical Activity. J. Am. Chem. Soc. 1974, 96, 5152–5158. [Google Scholar] [CrossRef]
- Rau, H. Asymmetric Photochemistry in Solution. Chem. Rev. 1983, 83, 535–547. [Google Scholar] [CrossRef]
- Kagan, H.; Moradpour, A.; Nicoud, J.F.; Balavoine, G.; Tsoucaris, G. Photochemistry with Circularly Polarized Light. Synthesis of Optically Active Hexahelicene. J. Am. Chem. Soc. 1971, 93, 2353–2354. [Google Scholar] [CrossRef]
- Wang, Y.; Sakamoto, T.; Nakano, T. Molecular Chirality Induction to an Achiral π-Conjugated Polymer by Circularly Polarized Light. Chem. Commun. 2012, 48, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, A.; Wang, Y.; Nakano, T. Predicting the Switchable Screw Sense in Fluorene-Based Polymers. Angew. Chem. Int. Ed. 2015, 54, 2688–2692. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kanibolotsky, A.L.; Skabara, P.J.; Nakano, T. Chirality Induction Using Circularly Polarized Light into a Branched Oligofluorene Derivative in the Presence of an Achiral Aid Molecule. Chem. Commun. 2016, 52, 1919–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, A.; Hoffmann, R. Electronic Structure and Torsional Potentials in Ground and Excited States of Biphenyl Fulvalene and Related Compounds. J. Am. Chem. Soc. 1968, 90, 5379–5385. [Google Scholar] [CrossRef]
- Fujiki, M.; Yoshida, K.; Suzuki, N.; Zhang, J.; Zhang, W.; Zhu, X. Mirror Symmetry Breaking and Restoration within μm-Sized Polymer Particles in Optofluidic Media by Pumping Circularly Polarised Light. RSC Adv. 2013, 3, 5213–5219. [Google Scholar] [CrossRef]
- Yeom, J.; Yeom, B.; Chan, H.; Smith, K.W.; Dominguez-Medina, S.; Bahng, J.H.; Zhao, G.; Chang, W.-S.; Chang, S.-J.; Chuvilin, A. Chiral Templating of Self-assembling Nanostructures by Circularly Polarized Light. Nat. Mater. 2015, 14, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pu, L. 1,1′-Binaphthyl Dimers, Oligomers, and Polymers: Molecular Recognition, Asymmetric Catalysis, and New Materials. Chem. Rev. 1998, 98, 2405–2494. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Furusho, Y.; Murakawa, K.; Endo, T.; Matsuoka, H.; Hirasa, T.; Matsuo, J.; Sisido, M. Optically Active Poly(aryl carbonates) Consisting of Axially Chiral Units. Chiral Binaphthyl Group Induced Helical Polymer. J. Am. Chem. Soc. 1998, 120, 4530–4531. [Google Scholar] [CrossRef]
- Habaue, S.; Seko, T.; Okamoto, Y. Asymmetric Oxidative Coupling Polymerization of Optically Active Tetrahydroxybinaphthalene Derivative. Macromolecules 2002, 35, 2437–2439. [Google Scholar] [CrossRef]
- Cavazza, M.; Zandomeneghi, M.; Ouchi, A.; Koga, Y. Photochromism in 1,1′-Bi-2-naphthols. J. Am. Chem. Soc. 1996, 118, 9990–9991. [Google Scholar] [CrossRef]
- Flegel, M.; Lukeman, M.; Wan, P. Photochemistry of 1,1′-Bi-2-naphthol (BINOL)-ESIPT Is Responsible for Photoracemization and Photocyclization. Can. J. Chem. 2008, 86, 161–169. [Google Scholar] [CrossRef]
- Solntsev, K.M.; Bartolo, E.A.; Pan, G.; Muller, G.; Bommireddy, S.; Huppert, D.; Tolbert, L.M. Excited-State Proton Transfer in Chiral Environments: Photoracemization of BINOLs. Isr. J. Chem. 2009, 49, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, C.; Lavalette, D.; Cabaret, D.; Geraghty, N.; Welvart, Z. Bridged Derivatives of Binaphthol- Kinetic Factors Governing the Enantiomeric Stability of Atropisomers in Their Triplet-State. Nouv. J. Chim. 1982, 6, 461–465. [Google Scholar]
- Irie, M.; Yoshida, K.; Hayashi, K. Laser Photolysis Study of the Photoracemization of 1,1′-Binaphthyl. J. Phys. Chem. 1977, 81, 969–972. [Google Scholar] [CrossRef]
- Zimmerman, H.E.; Crumrine, D.S. Duality of Mechanism in Photoracemization of Optically Active Biphenyl. Mechanistic and Exploratory Organic Photochemistry. LXV. J. Am. Chem. Soc. 1972, 94, 498–506. [Google Scholar] [CrossRef]
- Hattori, T.; Shimazumi, Y.; Goto, H.; Yamabe, O.; Morohashi, N.; Kawai, W.; Miyano, S. Synthesis, Resolution, and Absolute Stereochemistry of (−)-Blestriarene C. J. Org. Chem. 2003, 68, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Shimazumi, Y.; Yamabe, O.; Koshiishi, E.; Miyano, S. First Determination of the Absolute Stereochemistry of a Naturally Occurring 1,1′-Biphenanthrene, (−)-Blestriarene C, and Its Unexpected Photoracemization. Chem. Commun. 2002, 19, 2234–2235. [Google Scholar] [CrossRef]
- Zandomeneghi, M. Photochemical Activation of Racemic Mixtures in Biological Matrices. J. Am. Chem. Soc. 1991, 113, 7774–7775. [Google Scholar] [CrossRef]
- Kuhn, W.; Knopf, E. The Preparation of Optically Active Compounds by the Aid of Light. Z. Phys. Chem. 1930, 7, 292–310. [Google Scholar]
- Hamai, S.; Hirayama, F. Actinometric Determination of Absolute Fluorescence Quantum Yields. J. Phys. Chem. 1983, 87, 83–89. [Google Scholar] [CrossRef]
- Hassan, K.; Yamashita, K.-I.; Hirabayashi, K.; Shimizu, T.; Nakabayashi, K.; Imai, Y.; Matsumoto, T.; Yamano, A.; Sugiura, K.-I. π-Expanded Axially Chiral Biaryls and Their Emissions: Molecular Design, Syntheses, Optical Resolution, Absolute Configuration, and Circularly Polarized Luminescence of 1,1′-Bipyrene-2,2′-diols. Chem. Lett. 2015, 44, 1607–1609. [Google Scholar] [CrossRef]
- He, Y.; Lv, Y.; Hu, J.; Qi, L.; Hou, X. Simple, Sensitive and On-Line Fluorescence Monitoring of Photodegradation of Phenol and 2-Naphthol. Luminescence 2007, 22, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Taupier, G.; Boeglin, A.; Crégut, O.; Mager, L.; Barsella, A.; Gąsior, K.; Rehspringer, J.-L.; Dorkenoo, K.D. Beating Photo-Degradation in Sum-Frequency Imaging of Chiral Organic Media. Opt. Mater. 2015, 45, 22–27. [Google Scholar] [CrossRef]
- Meca, L.; Reha, D.; Havlas, Z. Racemization Barriers of 1,1′-Binaphthyl and 1,1′-Binaphthalene-2,2′-diol: A DFT Study. J. Org. Chem. 2003, 68, 5677–5680. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Fukuda, Y.; Sato, S.I.; Nakano, T. Photoinduced Racemization of an Optically Active Helical Polymer Formed by the Asymmetric Polymerization of 2,7-Bis (4-tert-butylphenyl) fluoren-9-yl Acrylate. Angew. Chem. Int. Ed. 2009, 121, 9472–9475. [Google Scholar] [CrossRef]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Y.; Nakano, T. Photo Racemization and Polymerization of (R)-1,1′-Bi(2-naphthol). Molecules 2016, 21, 1541. https://doi.org/10.3390/molecules21111541
Zhang Z, Wang Y, Nakano T. Photo Racemization and Polymerization of (R)-1,1′-Bi(2-naphthol). Molecules. 2016; 21(11):1541. https://doi.org/10.3390/molecules21111541
Chicago/Turabian StyleZhang, Zhaoming, Yue Wang, and Tamaki Nakano. 2016. "Photo Racemization and Polymerization of (R)-1,1′-Bi(2-naphthol)" Molecules 21, no. 11: 1541. https://doi.org/10.3390/molecules21111541





