The aldehydes, ketones, carboxylic acids and potassium allyltrifluoroborate were purchased from Sigma Aldrich Chemical Co. (São Paulo, SP, Brazil) or Alfa Aesar (São Paulo, SP, Brazil) and used as received. The reactions were monitored by thin-layer chromatography on 0.25 mm silica gel 60 plates (F254) (E. Merck, Darmstadt, Germany) and GC HP5890 Series II system (HP, Palo alto, CA, USA), equipped with a HP-1 25 m × 0.32 microns column).
The products were purified, when necessary, by column chromatographic using silica gel 60 (230–400 mesh). 1H- and 13C-NMR data were obtained on a Varian Unity plus 300 or Varian UNMRS 400 spectrometer (Varian, Palo Alto, CA, USA) in CDCl3 using the solvent residual peak as the internal reference.
The experiments were performed with the Mini-Bead Beater from BioSpec (Bartlesville, OK, USA) at 70 Hz using 2.0 mL polypropylene screwcap microvials and glass beads of 1 mm de diameter and density of 2.5 g·cm−3 and with the vibratory mill MM200 model from Retsch (Haan, Germany) at 25 Hz using 5 mL stainless steel grinding jars and 10 mm stainless steel balls or 2 mL disposable Eppendorf jars and 1 mm glass beads. When using the disposable Eppendorf jars it is necessary to use a PTFE adapter for 5 samples, allowing tone to use 10 samples at once.
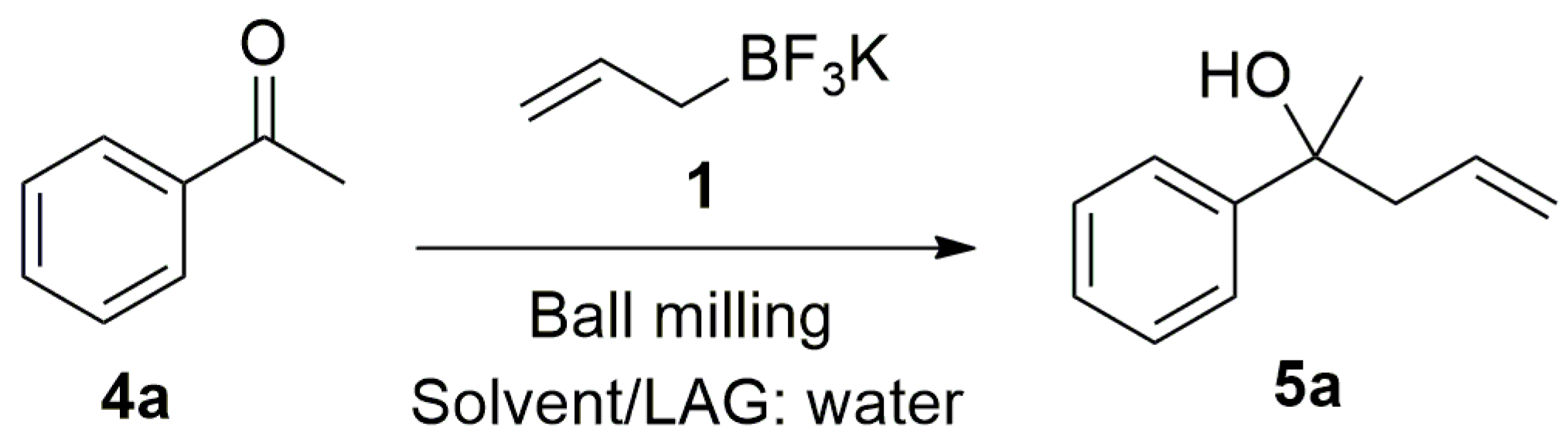
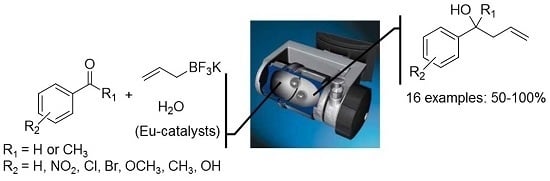
4.4. General Procedure for the Allylation of Acetophenone with Potassium Allyltrifluoroborate (1) Using EuFum or MandEu Catalysts in Solution
To a solution of the acetophenone (0.1 mmol) and the catalyst (10 mol %) in 1.1 mL of CH
2Cl
2:H
2O (1:0.1) was added potassium allyltrifluoroborate (0.11 mmol). The biphasic mixture was stirred for the time indicated in
Table 6. The reaction mixture was extracted with CH
2Cl
2 (3 × 5 mL) and dried over anhydrous magnesium sulfate. The solvent was removed in vacuum to yield compound
5a.
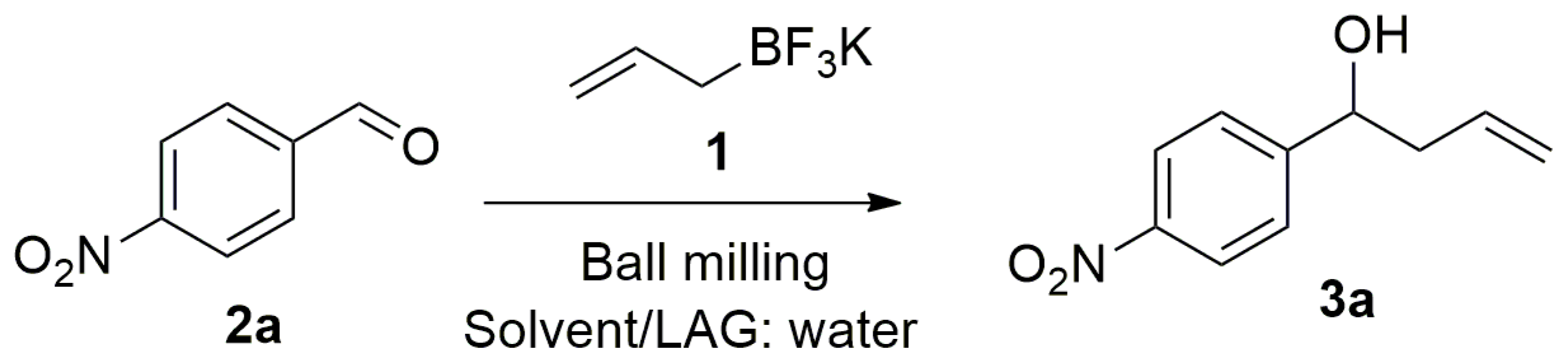
1-(4-Nitrophenyl)but-3-en-1-ol (
3a):
1H-NMR (400 MHz, CDCl
3) δ 8.20 (d,
J = 8.0 Hz, 2H), 7.53 (d,
J = 8.0 Hz, 2H), 5.95–5.62 (m, 1H), 5.27–5.10 (m, 2H), 4.87 (dd,
J = 8.0, 4,7 Hz, 1H), 2.70–2.38 (m, 2H), 2.28 (br. s, 1H).
13C-NMR (75.5 MHz, CDCl
3) δ 151.0, 147.2, 133.2, 126.5, 123.6, 119.6, 72.1, 43.8 [
23].
1-(3-Nitrophenyl)but-3-en-1-ol (
3b):
1H-NMR (300 MHz, CDCl
3) δ8.25 (s, 1H), 8.18–8.09 (m, 1H), 7.71 (d,
J = 7.6 Hz, 1H), 7.53 (t,
J = 7.9 Hz, 1H), 7.26 (s, 1H), 5.80 (m, 1H), 5.26–5.13 (m, 2H), 4.87 (dd,
J = 7.9, 4.7 Hz, 1H), 2.66–2.41 (m, 2H), 2.25 (br. s, 1H).
13C-NMR (75.5 MHz, CDCl
3) δ 148.4, 145.9, 133.2, 131.9, 129.3, 122.5, 120.8, 119.7, 72.0, 43.9 [
25].
1-(2-Nitrophenyl)but-3-en-1-ol (
3c):
1H-NMR (300 MHz, CDCl
3) δ 7.93 (dd,
J = 7.9, 1.5 Hz, 1H), 7.83 (d,
J = 7.6 Hz, 1H), 7.65 (t,
J = 7.6 Hz, 1H), 7.48–7.36 (m, 1H), 6.00–5.78 (m, 1H), 5.37–5.27 (m, 1H), 5.27–5.11 (m, 2H), 2.71 (ddd,
J = 14.4, 6.75, 2.9 Hz, 1H), 2.51–2.32 (m, 2H).
13C-NMR (75 MHz, CDCl
3) δ 147.7, 139.2, 133.9, 133.4, 128.1, 128.0, 124.3, 119.0, 68.3, 42.8 [
48].
1-(4-Chlorophenyl)but-3-en-1-ol (
3d):
1H-NMR (300 MHz, CDCl
3) δ 7.35–7.25 (m, 4H), 5.87–5.65 (m, 1H), 5.20–5.15 (m, 2H), 5.13 (d,
J = 0.6 Hz, 1H), 4.71 (dd,
J = 7.5, 5.3 Hz, 1H), 2.54–2.37 (m, 2H), 2.19 (br. s, 1H).
13C-NMR (75 MHz, CDCl
3) δ 141.8, 133.5, 133.1, 128.1, 126.7, 118.4, 72.1, 43.4 [
29].
1-(4-Bromophenyl)but-3-en-1-ol (
3e):
1H-NMR (300 MHz, CDCl
3) δ 7.47 (d,
J = 8.2 Hz, 2H), 7.24 (d,
J = 8.2 Hz, 2H), 5.90–5.64 (m, 1H), 5.27–5.05 (m, 2H), 4.80–4.57 (m, 1H), 2.65–2.31 (m, 2H), 2.13 (br. s., 1H).
13C-NMR (75 MHz, CDCl
3) δ 142.8, 133.9, 131.4, 127.5, 121.2, 118.9, 72.6, 43.8 [
29].
1-(2-Methoxyphenyl)but-3-en-1-ol (
3f):
1H-NMR (300 MHz, CDCl
3) δ 7.35 (dd,
J = 7.6, 1.7Hz, 1H), 7.30–7.20 (m, 1H), 7.01–6.92 (m, 1H), 6.87 (d,
J = 8.2, 1H), 5.97–5.76 (m,1H), 5.21–5.06 (m, 2H), 4.97 (dd,
J = 7.9 Hz, 5.0 Hz, 1H), 3.85 (s, 3H), 2.68–2.43 (m, 3H).
13C-NMR (75 MHz, CDCl
3) δ 156.2, 135.1, 131.7, 128.2, 126.7, 120.6, 117.4, 110.3, 6959, 55.1, 41.7 [
49].
1-(3-Methoxyphenyl)but-3-en-1-ol (
3g):
1H-NMR (300 MHz, CDCl
3) δ 7.32–7.21 (m, 1H), 6.96–6.89 (m, 2H), 6.81 (dd,
J = 8.0, 2.3 Hz, 1H), 5.81 (ddt,
J = 17.2, 10.2, 7.1 Hz, 1H), 5.22–5.09 (m, 2H), 4.71 (dd,
J = 7.4, 5.5 Hz, 1H), 3.81 (s, 3H), 2.49 (ddd,
J = 12.5, 7.6, 4.5 Hz, 2H), 2.06 (s, 1H).
13C-NMR (75 MHz, CDCl
3) δ 159.7, 145.6, 134.4, 129.4, 118.5, 118.1, 112.9, 111.2, 73.2, 55.2, 43.8 [
25].
1-(4-Methoxyphenyl)but-3-en-1-ol (
3h):
1H-NMR (300 MHz, CDCl
3) δ 7.30–7.23 (m, 2H), 6.90–6.85 (m, 2H), 5.78 (m, 1H), 5.18–5.06 (m, 2H), 4.66 (t,
J = 6.5 Hz, 2H), 3.79 (s, 3H), 2.48 (t,
J = 6.8 Hz, 1H).
13C-NMR (75 MHz, CDCl
3) δ 158.9, 136.0, 134.6, 127.0, 118.1, 113.7, 72.9, 55.2, 43.7 [
16].
1-(2-Methylphenyl)but-3-en-1-ol (
3i):
1H-NMR (400 MHz, CDCl
3) δ 7.50–7.44 (m, 1H), 7.27–7.20 (m, 1H), 7.20–7.10 (m, 2H), 5.92–5.80 (m, 1H), 5.23–5.10 (m, 2H), 4.97 (m, 1H), 2.55–2.39 (m, 2H), 2.34 (s, 3H), 1.89 (s, 1H).
13C-NMR (100 MHz, CDCl
3) δ 141.9, 134.7, 134.3, 130.3, 127.2, 126.2, 125.1, 118.2, 69.7, 42.6, 19.0 [
50].
1-(1-Naphthyl)but-3-en-1-ol (
3j):
1H-NMR (300 MHz, CDCl
3) δ 8.13 (d,
J = 8.2 Hz, 1H), 7.86 (d,
J = 7.6 Hz, 1H), 7.72 (dd,
J = 15.8, 7.6 Hz, 2H), 7.57–7.35 (m, 3H), 5.97 (m, 1H), 5.51 (m, 1H), 5.19–4.93 (m, 2H), 2.83–2.48 (m, 2H), 2.08 (d,
J = 1.7 Hz, 1H).
13C-NMR (75 MHz, CDCl
3) δ 139.8, 134.6, 132.9, 129.5, 127.8, 126.5, 124.8, 124.5, 124.4, 122.4, 122.1, 115.7, 69.1, 42.3 [
50].
1-(2-Naphthyl)but3-en-1-ol (
3k):
1H-NMR (300 MHz, CDCl
3): δ 7.88–7.81 (m, 4H), 7.55–7.42 (m, 3H), 5.84 (m, 1H), 5.26–5.11 (m, 2H), 4.92 (m, 1H), 2.68–2.54 (m, 2H), 1.83 (br. s, 1H).
13C-NMR (75 MHz, CDCl
3): δ 141.2, 134.3, 128.2; 127.9, 127.6, 126.1, 125.8, 118.5, 73.4, 43.7 [
50].
1-(3-Hydroxyphenyl)but-3-en-1-ol (
3l):
1H-NMR (300 MHz, CDCl
3) δ 7.49–7.29 (m, 1H), 7.28–7.08 (m, 2H), 6.96–6.84 (m, 2H), 6.75 (dt,
J = 8.4, 1.7 Hz, 1H), 5.80 (s, 1H), 5.23–5.09 (m, 2H), 5.01 (br. s, 1H), 4.71 (dd,
J = 7.6, 5.3 Hz, 1H), 2.60–2.41 (m, 2H).
13C-NMR (75 MHz, CDCl
3) δ: 155.7, 145.8, 134.3, 129.7, 118.6, 118.2, 114.5, 112.7, 73.0, 43.7 [
51].
1-(4-Hydroxyphenyl)but-3-en-1-ol (
3m):
1H-NMR (300 MHz, CDCl
3) δ 7.25–7.19 (m, 2 H), 6.82-6.76 (m, 2 H), 5.89–5.62 (m, 1 H), 5.31 (br. s., 1H), 5.16–5.02 (m, 2 H), 4.68 (t,
J = 6.5Hz, 1H), 2.62–2.39 (m, 2 H), 2.10 (br. s, 1 H).
13C-NMR (75 MHz, CDCl
3) δ 155.1, 135.9, 134.5, 127.3, 118.4, 115.2, 73.0, 43.6 [
52,
53].
1-Phenylbut-3-en-1-ol (
3n):
1H-NMR (300 MHz, CDCl
3) δ 7.25–7.41 (m, 5H), 5.75–5.90 (m, 1H), 5.10–5.25 (m, 2H), 4.76 (t, 1H,
J = 6.6 Hz), 2.45–2.60 (m, 2H).
13C-NMR (75 MHz, CDCl
3) δ 43.7, 73.2, 118.3, 125.7, 127.5, 128.3, 134.4, 143.8 [
48].
1-(4-(Trifluoromethyl)phenyl)but-3-em-1-ol (
3o):
1H-NMR (400 MHz, CDCl
3) δ: 7.61 (d,
J = 8.2 Hz, 2H), 7,47 (d,
J = 8.2Hz, 2H), 5.85–5.72 (m, 1H), 5.22–5.14 (m, 2H), 4.80 (dd,
J = 8.0, 4.8 Hz, 1H), 2.50 (m, 2H), 2.06 (br. s, 1H).
13C-NMR (100 MHz, CDCl
3) δ: 147.7, 133.7, 129.7 (q,
J = 32.7 Hz), 126.1, 125.3 (q,
J = 3.8 Hz), 124.1(q,
J = 270 Hz), 119.2, 72.5, 43.9 [
54].
Dec-1-en-4-ol (
3p):
1H-NMR (400 MHz, CDCl
3) δ 5.96–5.75 (m, 1H), 5.22–5.03 (m, 2H), 3.65 (br. s, 1H), 2.51–2.00 (m, 2H), 1.52–1.18 (m, 10H), 0.89 (br. s, 3H).
13C-NMR (100 MHz, CDCl
3) δ 134.9, 118.0, 70.7, 41.9, 36.8, 31.0, 29.3, 25.6, 22.6, 14.1 [
16].
1-Cyclohexylbut-3-en-1-ol (
3q):
1H-NMR (400 MHz, CDCl
3) δ 5.84 (td,
J = 16.4, 7.8 Hz, 1H), 5.24–4.88 (m, 2H), 3.40 (br. s, 1H), 2.32 (br. s, 1H), 2.18–2.00 (m, 1H), 1.96–1.57 (m, 6H), 1.52–0.96 (m, 6H).
13C-NMR (101 MHz, CDCl
3) δ 135.4, 117.9, 74.7, 43.1, 38.8, 29.1, 28.1, 26.5, 26.3, 26.1 [
48].
2-Phenylpent-4-en-2-ol (
5a):
1H-NMR (300 MHz, CDCl
3) δ 7.48–7.21 (m, 5H), 5.68–5.62 (m, 1H), 5.19–5.12 (m, 2H), 2.74–2.62 (dd,
J = 12,0, 6.0 Hz, 1H), 2.54–2,46 (m, 1.0 Hz, 1H), 1,89 (br. s, 1H), 1.55 (s, 3H).
13C-NMR (75 MHz, CDCl
3) δ 147.6, 133.6, 128.1, 126.6, 124.7, 119.45, 73.6, 48.4, 29.9 [
55].
2-(4-Nitrophenyl)pent-4-en-2-ol (
5b):
1H-NMR (400 MHz, CDCl
3) δ 8.20 (d,
J = 8.3 Hz, 2H), 7.62 (d,
J = 8.3 Hz, 2H), 5.59 (dt,
J = 16.9, 7.8 Hz, 1H), 5.23–5.09 (m, 2H), 2.61 (ddd,
J = 22.2, 13.8, 7.9 Hz, 1H), 2.16 (s, 1H), 1.58 (s, br., 1H), 1.26 (s, 3H).
13C-NMR (101 MHz, CDCl
3) δ 154.9, 132.4, 125.9, 123.4, 120.6, 73.5, 48.6, 29.9 [
56].
4-(2-Hydroxypent-4-en-2-yl)-phenol (
5c):
1H-NMR (400 MHz, CDCl
3) δ 9.11 (s, 1H), 7.23–6.79 (m, 4H), 5.82 (td,
J = 17.2, 7.5 Hz, 1H), 5.31–5.13 (m, 2H), 2.85 (dd,
J = 14.0, 6.9 Hz, 1H), 2.74 (s, 1H), 2.57 (dd,
J = 13.9, 7.8 Hz, 1H), 1.65 (s, 3H).
13C-NMR (101 MHz, CDCl
3) δ 156.0, 132.9, 129.1, 125.9, 120.6, 119.45, 117.7, 46.5, 28.3 [
49].
2-(2-Methoxyphenyl)pent-4-en-2-ol (
5d):
1H-NMR (400 MHz, CDCl
3) δ 7.31 (d,
J = 7.7 Hz, 1H), 7.28–7.21 (m, 3H), 6.99–6.89 (m, 2H), 5.64 (td,
J = 17.0, 7.1 Hz, 1H), 5.11–4.92 (m, 2H), 3.97–3.80 (m, 3H), 2.81 (dd,
J = 13.7, 6.9 Hz, 1H), 2.60 (dd,
J = 15.2, 6.1 Hz, 1H), 1.58 (s, 3H).
13C-NMR (101 MHz, CDCl
3) δ 156.6, 134.7, 134.52, 128.2, 126.8, 120.8, 117.7, 111.24, 74.2, 55.3, 46.6, 26.9 [
56].
2-(4-Methoxyphenyl)pent-4-en-2-ol (
5e):
1H-NMR (400 MHz, CDCl
3) δ 7.36 (d,
J = 8.3 Hz, 2H), 6.87 (d,
J = 8.3 Hz, 2H), 5.63 (dt,
J = 17.4, 7.4 Hz, 1H), 5.19–5.05 (m, 1H), 3.80 (s, 3H), 2.64 (dt,
J = 35.9, 18.0 Hz, 1H), 2.48 (dd,
J = 13.7, 8.2 Hz, 1H), 1.89 (s, 1H), 1.53 (s, 3H).
13C-NMR (100 MHz, CDCl
3) δ 158.3, 139.8, 133.8, 125.9, 119.3, 113.4, 73.3, 55.2, 48.5, 29.9 [
57].
1-Allylcyclohexanol (
5f):
1H-NMR (400 MHz, CDCl
3) δ 5.97–5.82 (m, 1H), 5.15 (m, 2H), 2.22 (d,
J = 7.5 Hz, 2H), 1.72–1.37 (m, 10H), 1.35–1.21 (m, 1H).
13C-NMR (101 MHz, CDCl
3) δ 133.7, 118.6, 70.9, 46.7, 37.4, 25.7, 22.2 [
58].
3-Methylhex-5-en-3-ol (
5g): (400 MHz, CDCl
3) d 5.92–5.80 (m, 1H), 5.20–5.05 (m, 2H), 2.20 (d,
J = 7.4 Hz, 2H), 1.51 (q,
J = 7.4 Hz, 2H), 1.16 (s, 3H), 0.92 (t,
J = 7.4 Hz, 3H);
13C-NMR (100 MHz, CDCl
3) d 134.1, 118.5, 72.3, 45.8, 34.2, 26.1, 8.1 [
59].