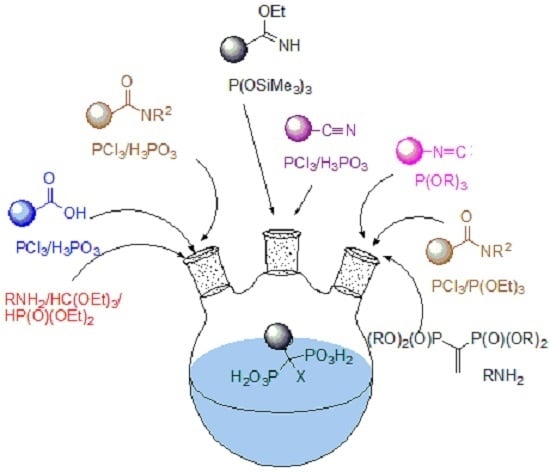
Synthetic Procedures Leading towards Aminobisphosphonates
Abstract
:1. Introduction
2. Overview of Synthetic Procedures
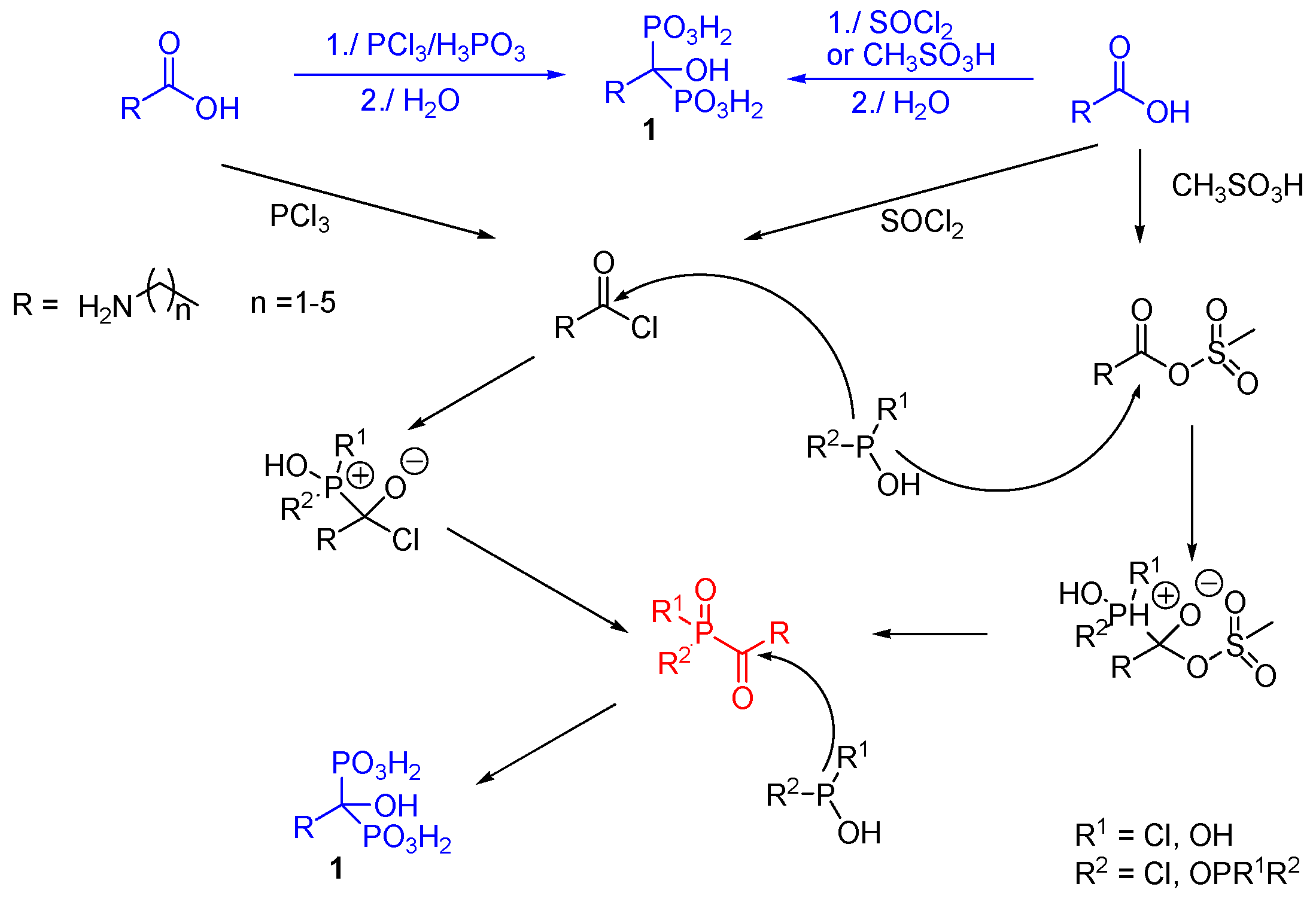
2.1. Synthesis from Carboxylic Acids
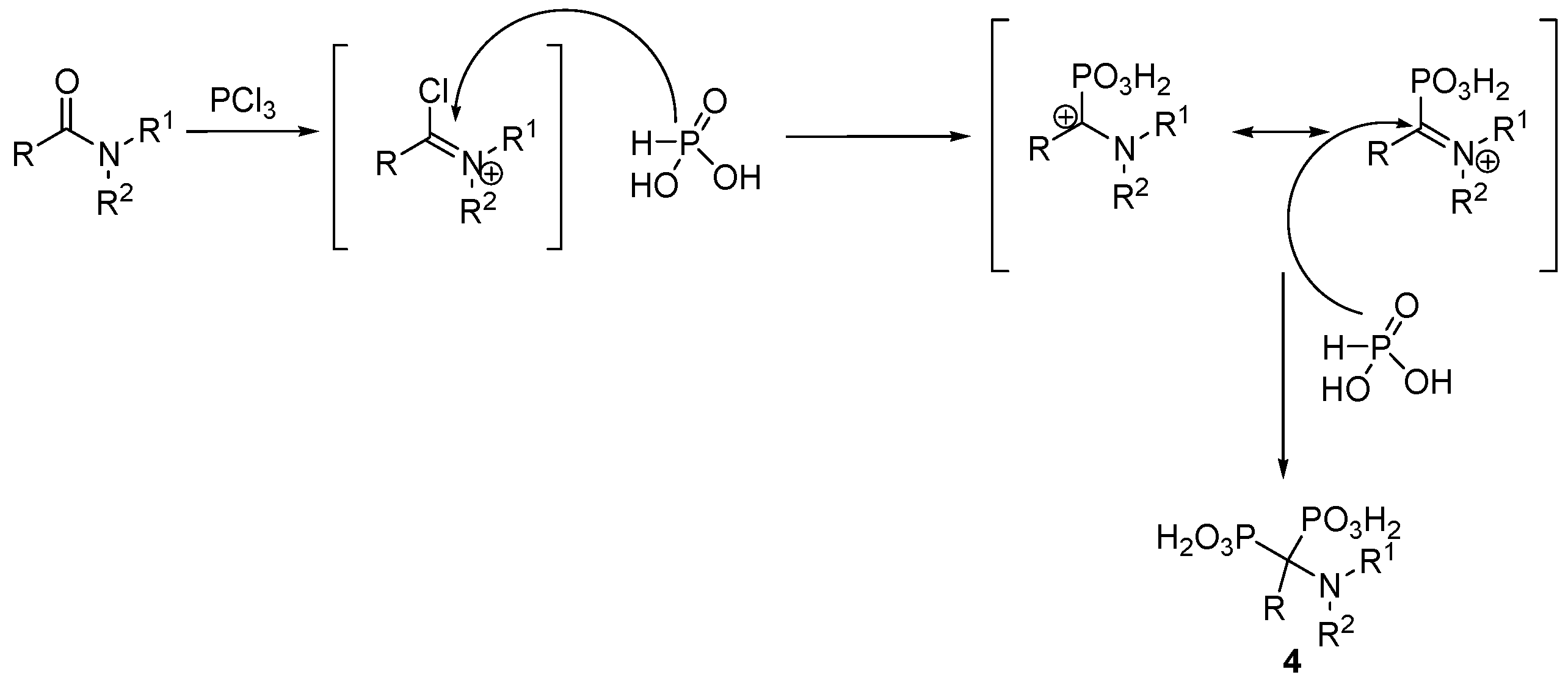
2.2. Synthesis from Amides
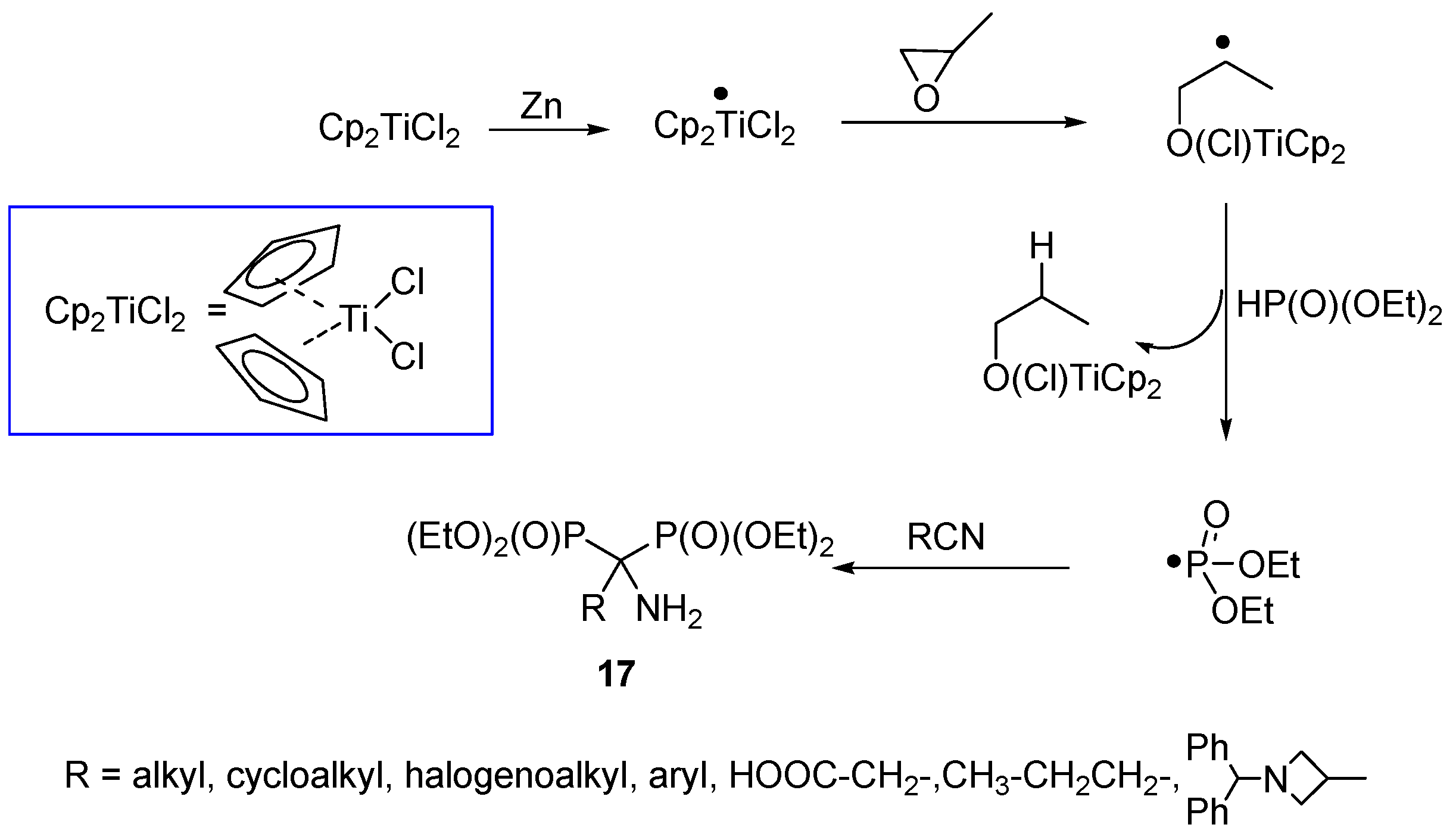
2.3. Synthesis from Nitriles
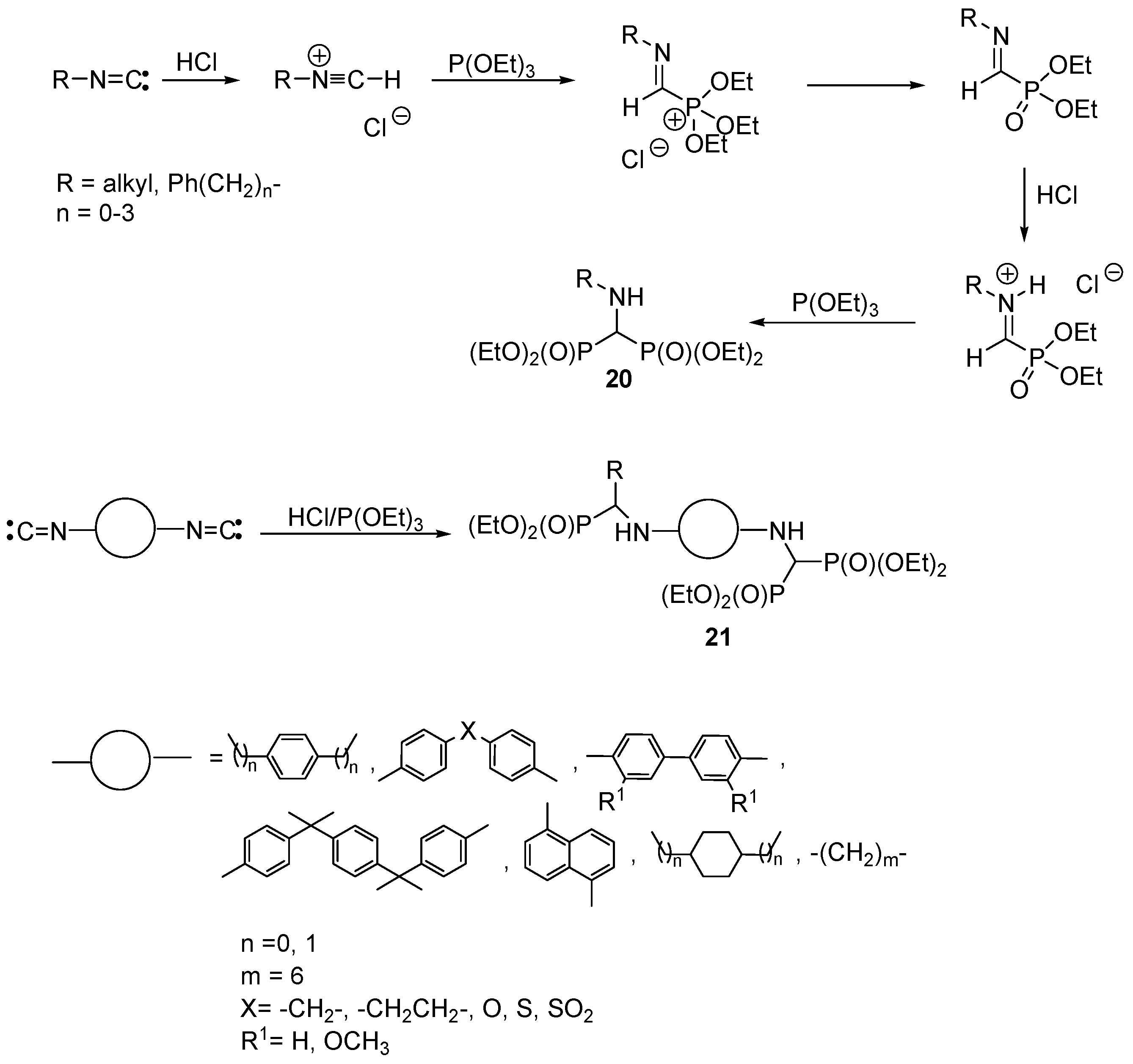
2.4. Synthesis from Isonitriles
2.5. Synthesis via Ketophosphonates
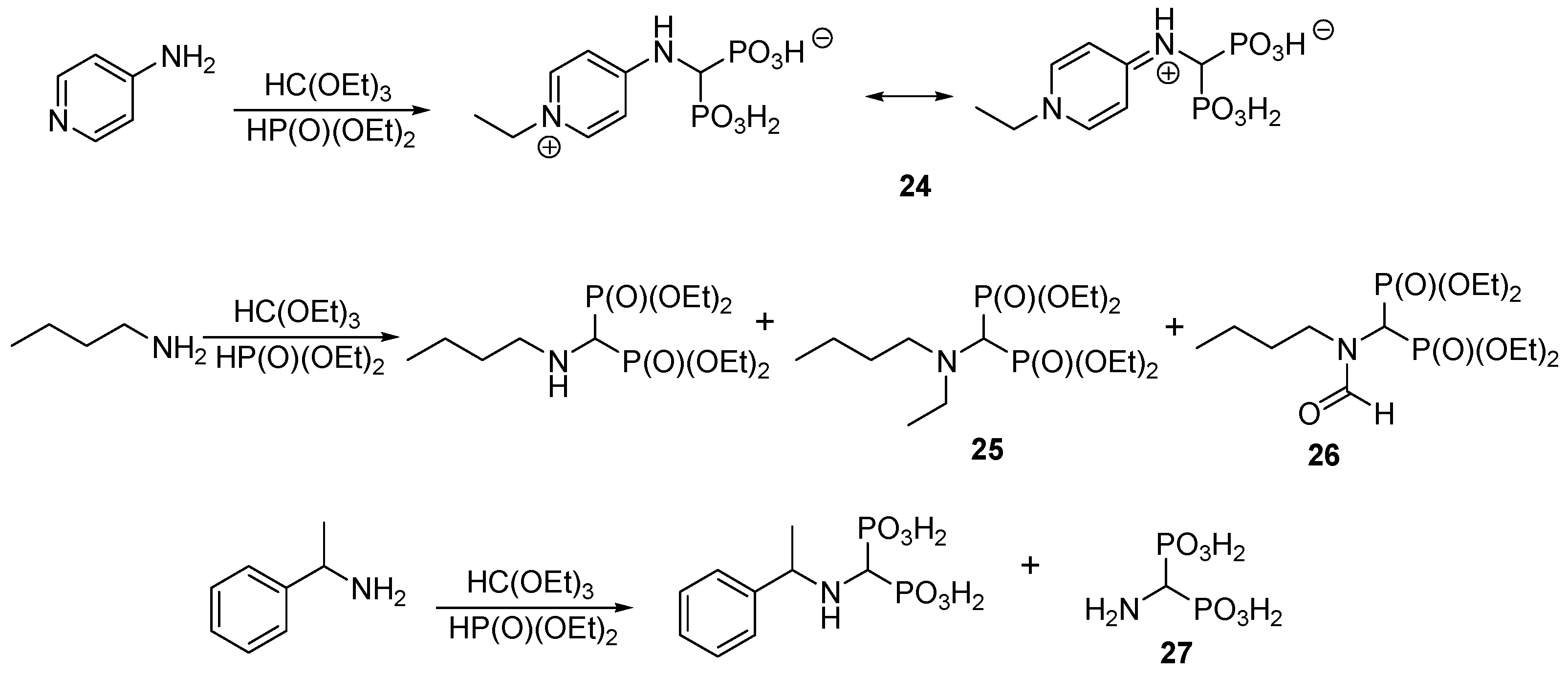
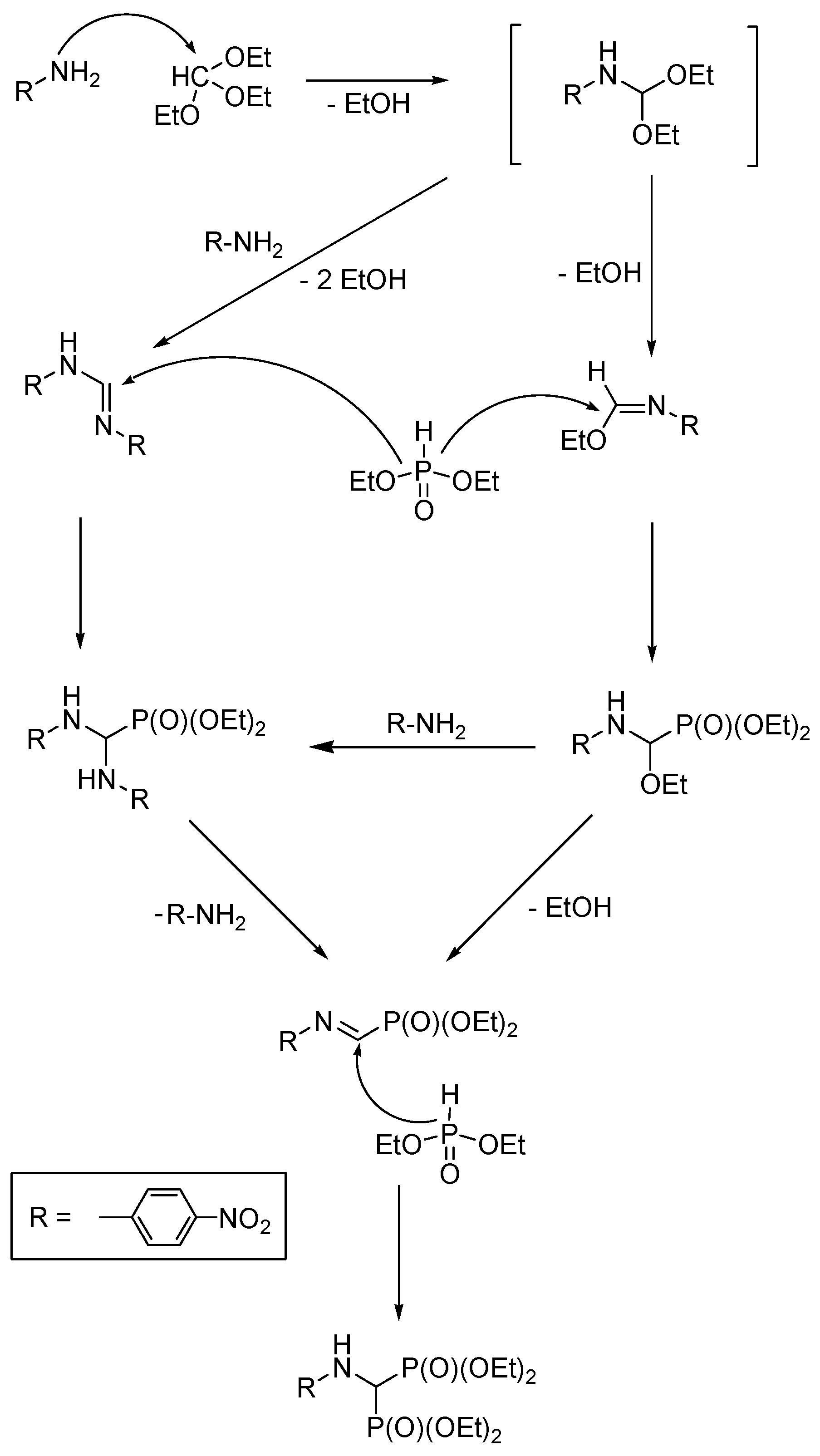
2.6. Three-Component Condensation of Amines with Triethyl Orthoformate and Diethylphosphite
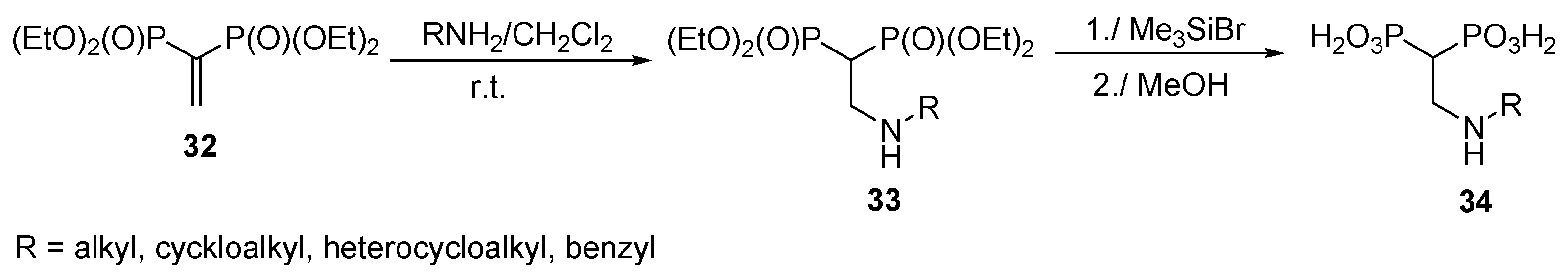
2.7. Addition of Amines to Vinylidenebisphosphonates
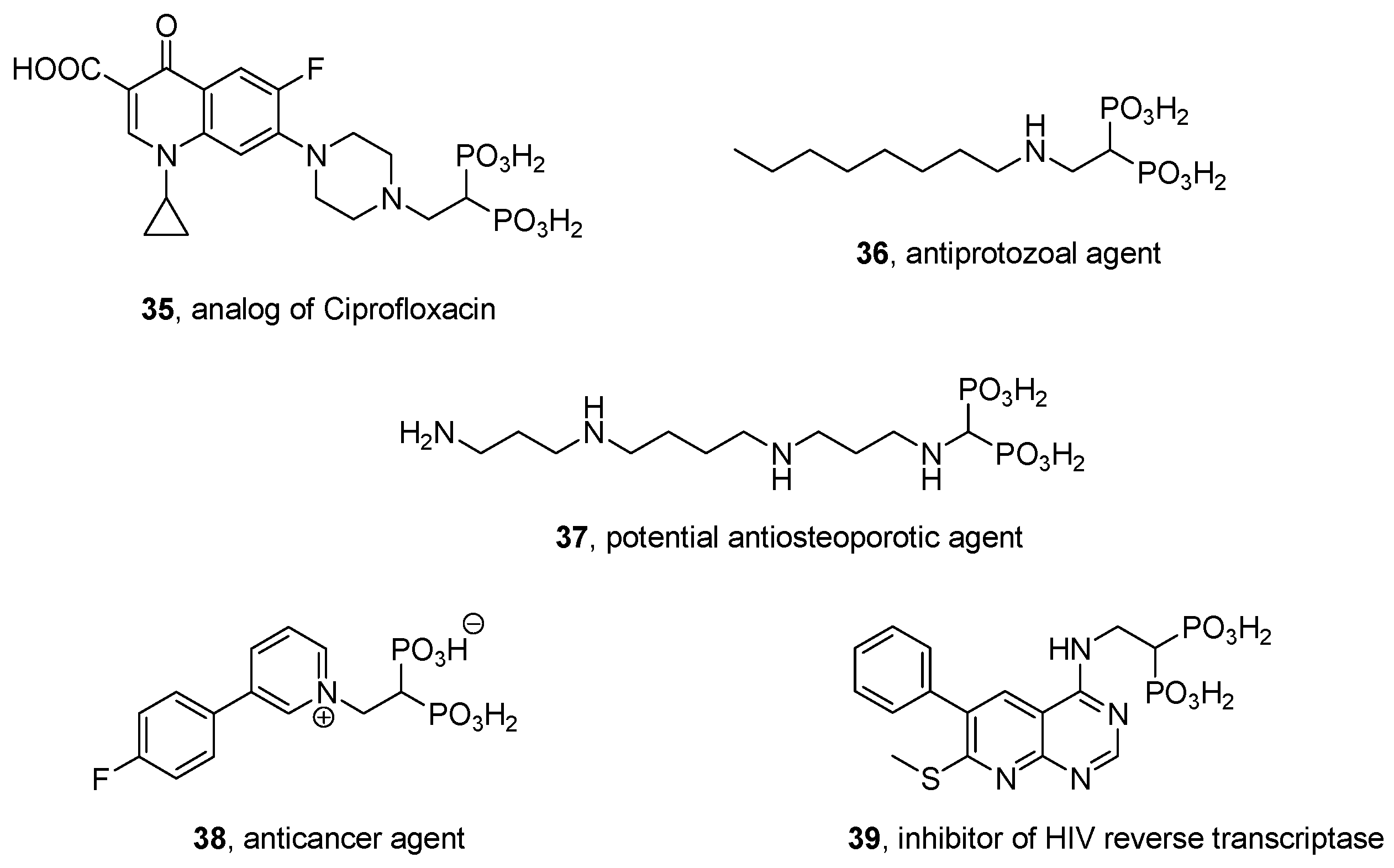
2.8. Miscellaneous Procedures
3. Functionalization of Aminobisphosphonates
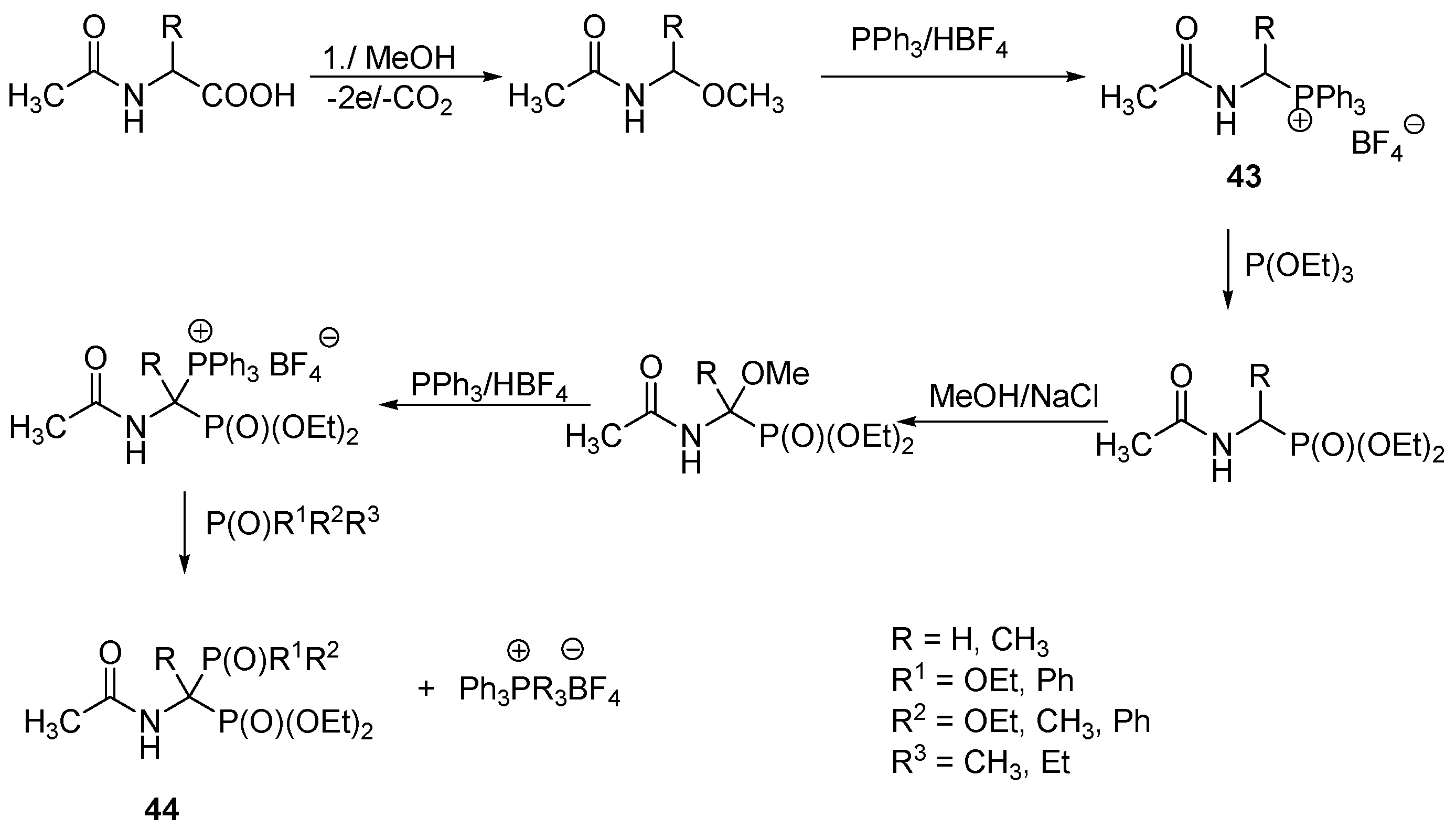
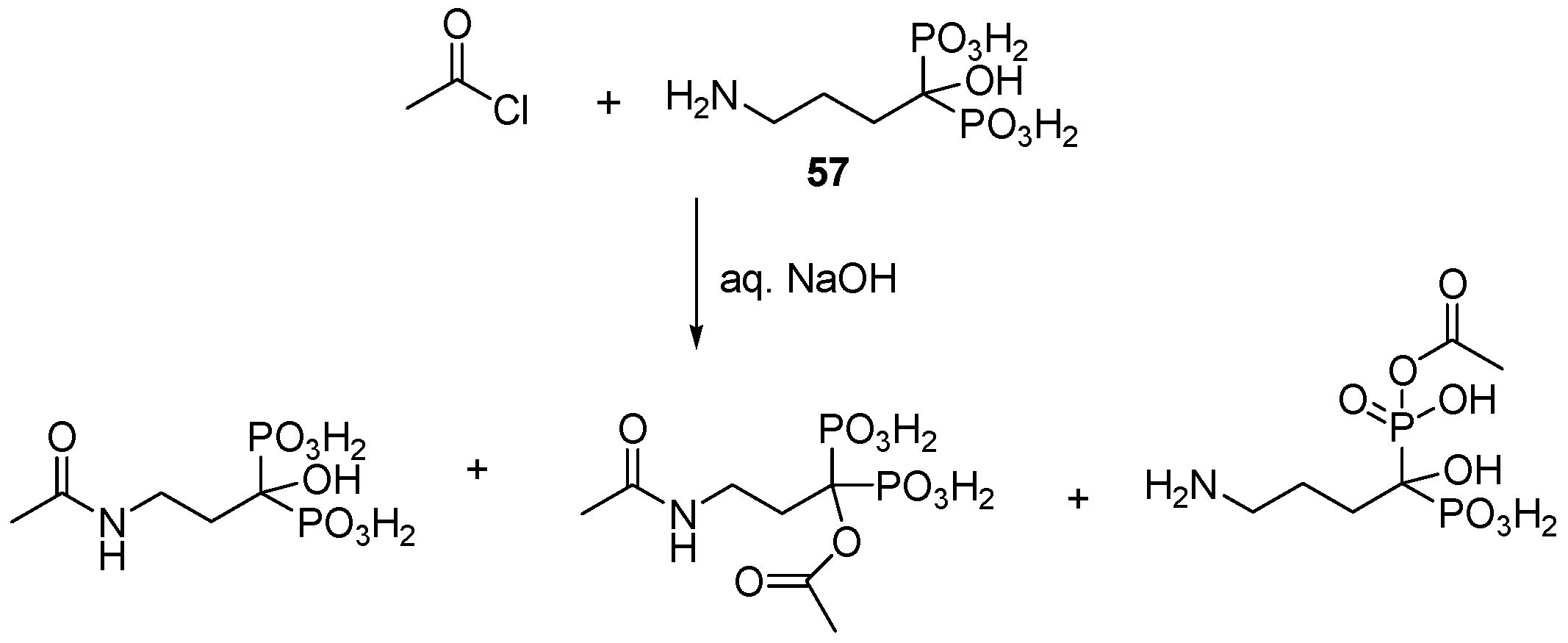
3.1. Direct Acylation of Aminobisphosphonic Acids
3.2. Direct Acylation of Tetraethyl Aminobisphosphonates
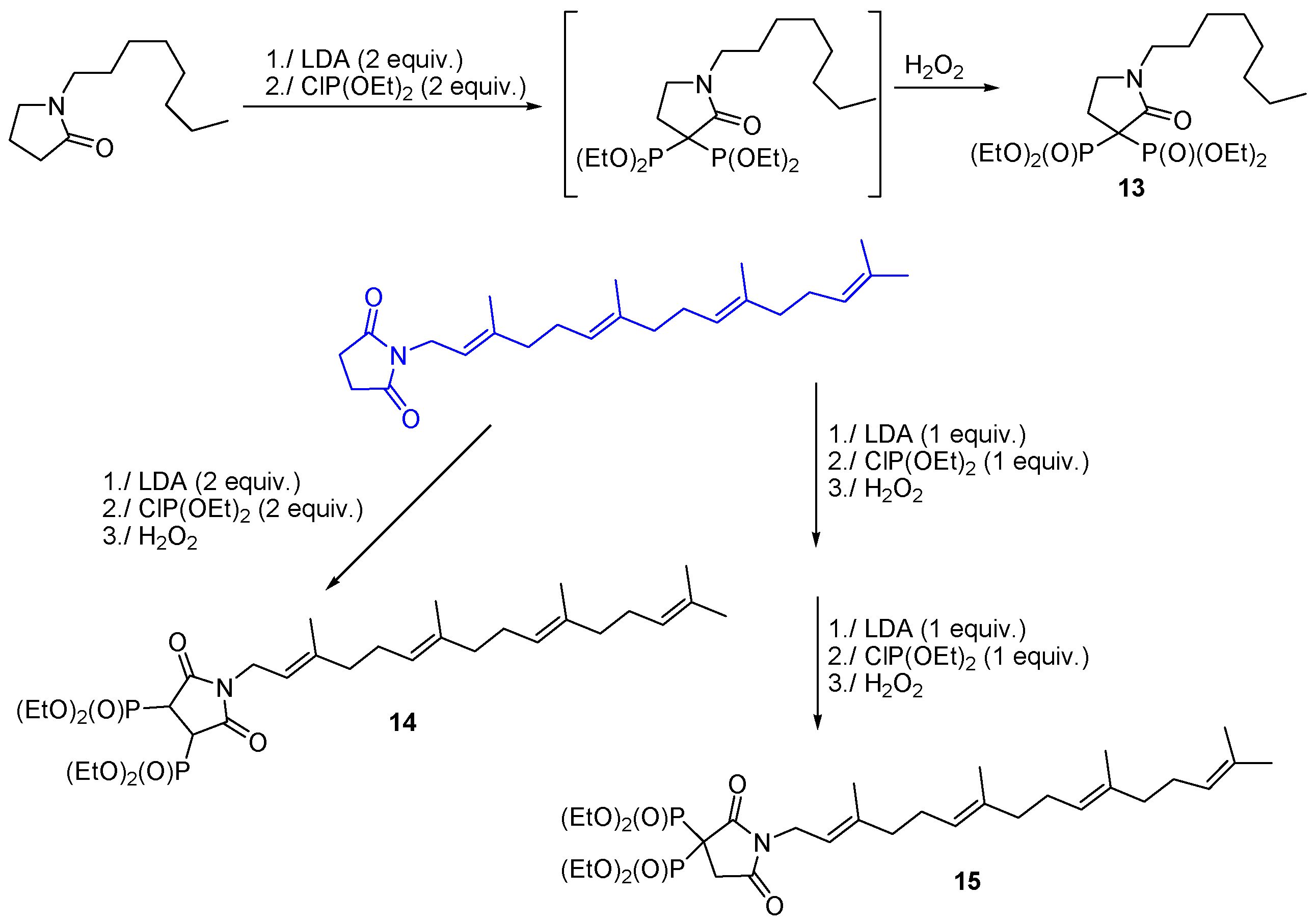
3.3. Synthesis of Building Blocks for Polymer Chemistry
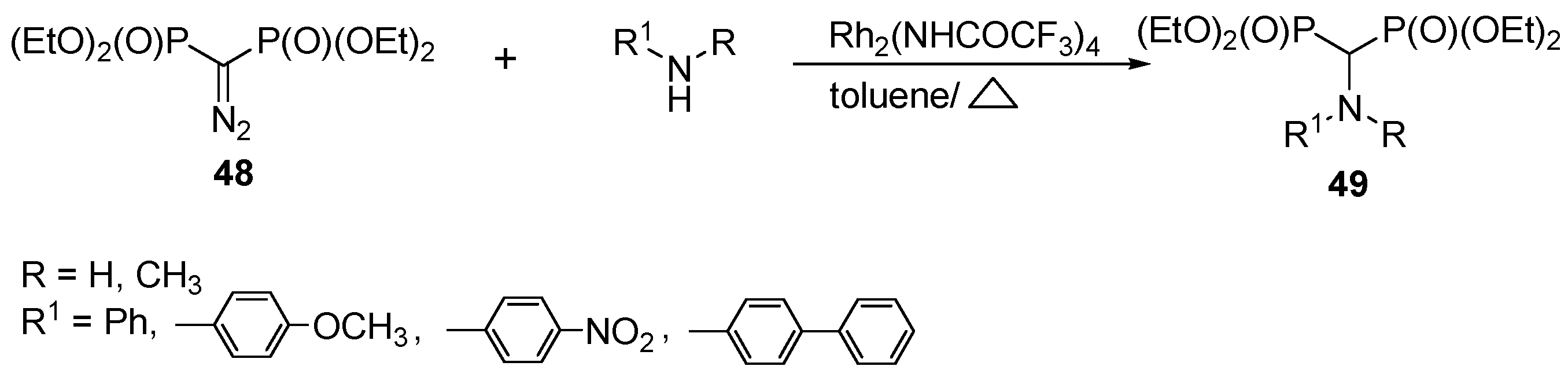
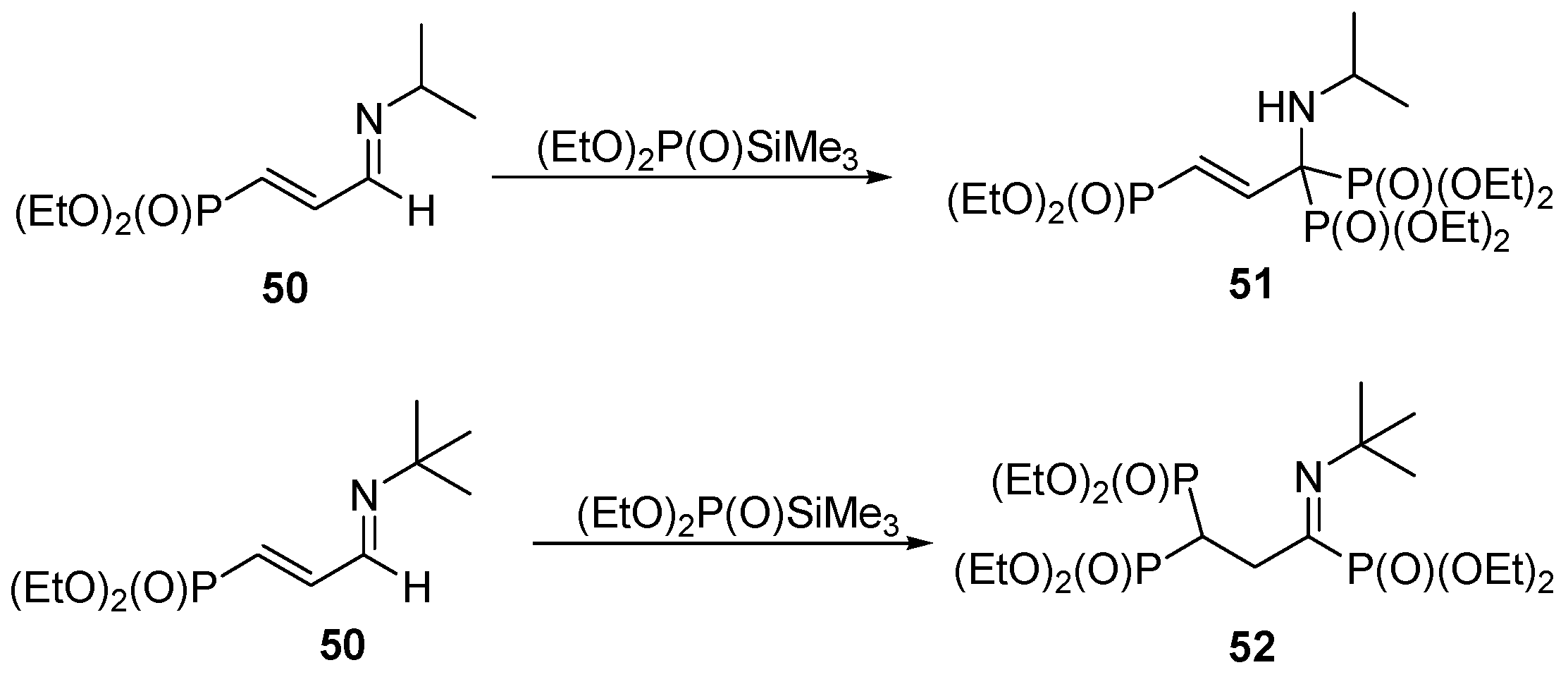
3.4. Miscellaneous
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Turhanen, P.A. Synthesis of triple bond containing 1-hydroxy-1,1-bisphosphonic acid derivatives to be used as precursors in “click” chemistry: Two examples. J. Org. Chem. 2014, 79, 6330–6335. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Ebetino, F.H.; Hogan, A.M.; Sun, S.; Tsuompra, M.K.; Duan, X.; Triffitt, J.T.; Kwaasi, A.A.; Dunford, J.E.; Barnett, B.L.; Oppermann, U.; et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Kennel, K.A. Bisphosphonates for the prevention and treatment of osteoporosis. Br. Med. J. 2015, 351, h3783. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.G.; Zhang, Y.; Yuan, W. Efficacy of bisphosphonates on bone mineral density and fracture Rate in patients with osteogenesis imperfecta: A systematic review and meta-analysis. Am. J. Ther. 2016, 3, e894–e904. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, E.; Kafarski, P. Physiologic Activity of Bisphosphonates—Recent Advances. Open Pharm. Sci. J. 2016, 3, 56–78. [Google Scholar] [CrossRef]
- Demkowicz, S.; Rachoń, J.; Daśko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Studnik, H.; Liebsch, S.; Forlani, G.; Wieczorek, D.; Kafarski, P.; Lipok, J. Amino polyphosphonates—Chemical features and practical uses, environmental durability and biodegradation. New Biotechnol. 2015, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Turhanen, P.A.; Vepsäläinen, J.J.; Peräniemi, S. Advanced material and approach for metal ions removal from aqueous solutions. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Gałęzowska, J.; Gumienna-Kontecka, E. Phosphonates, their complexes and bio-applications: A spectrum of surprising diversity. Coord. Chem. Rev. 2012, 256, 105–124. [Google Scholar] [CrossRef]
- Abdou, W.M.; Shaddy, A.A. The development of bisphosphonates for therapeutic uses, and bisphosphonate structure-activity consideration. Arch. Org. Chem. 2008, 2009, 143–182. [Google Scholar]
- Romaneneko, V.D.; Kukhar, V.A. 1-Amino-1,1-bisphosphonates. Fundamental syntheses and new developments. Arch. Org. Chem. 2012, 2012, 127–166. [Google Scholar]
- Petroianu, G.A. Pharmacist Theodor Salzer (1833–1900) and the discovery of bisphosphonates. Pharmazie 2011, 66, 804–807. [Google Scholar] [PubMed]
- Kieczykowski, G.R.; Jobson, R.B.; Melillo, D.G.; Reinhold, D.F.; Grenda, V.J.; Shinkai, I. Preparation of (4-amino-1-hydroxybutylidene)bisphosphonic acid sodium salt, MK-217 (alendronate sodium). An improved procedure for the preparation of 1-hydroxy-1,1-bisphosphonic acids. J. Org. Chem. 1995, 60, 8310–8312. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Antunes, I.F.; Curto, M.J.M.; Neves, M.; Teixeira, A.P.S. Novel 1-hydroxy-1,1-bisphosphonates derived from indazole: Synthesis and characterization. Arch. Org. Chem. 2009, 2009, 69–84. [Google Scholar]
- Yanvarev, D.V.; Koovina, A.N.; Usanov, N.N.; Kochetkov, S.N. Non-hydrolysable analogues of inorganic pyrophosphate as inhibitors of Hepatitis C virus RNA-dependent RNA polymerase. Russ. J. Bioorg. Chem. 2012, 38, 224–229. [Google Scholar] [CrossRef]
- Srinivasa Rao, D.V.N.; Dandala, R.; Narayan, G.K.A.S.S.; Lenin, R.; Sivakumaran, N.; Naidu, A. Novel procedure for the synthesis of 1-hydroxy-1,1-bisphosphonic acids using phenols as medium. Synth. Commun. 2007, 37, 4359–4365. [Google Scholar] [CrossRef]
- Grün, A.; Nagy, D.I.; Németh, O.; Mucsi, Z.; Garadnay, S.; Greiner, I.; Keglevich, G. The Synthesis of 3-Phenylpropidronate Applying Phosphorus Trichloride and Phosphorous Acid in Methanesulfonic Acid. Curr. Org. Chem. 2016, 20, 1745–1752. [Google Scholar] [CrossRef]
- Lecouvey, M.; Leroux, I. Synthesis of 1-hydroxy-1,1-bisphosphonates. Heteroat. Chem. 2000, 11, 556–561. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Kovács, R.; Garadnay, S.; Greiner, I. Green chemical synthesis of bisphosphonic/dronic derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 664–667. [Google Scholar] [CrossRef]
- Troev, K.; Todorov, P.; Naydenova, E.; Mitova, V.; Vassiliev, N. A study of the reaction of phosphorus trichloride with paraformaldehyde in the presence of carboxylic acids. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1147–1155. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Synthesis of hydroxymethylenebisphosphonic acid derivatives in different solvents. Molecules 2016, 21, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kachbi Khelfallah, S.; Monteil, M.; Deschamp, J.; Gager, O.; Migianu-Griffoni, E.; Lecouvey, M. Synthesis of novel polymerisable molecules bearing bisphosphonate. Org. Biomol. Chem. 2015, 13, 11382–11392. [Google Scholar] [CrossRef] [PubMed]
- Grün, A.; Kovács, R.; Nagy, D.L.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient synthesis of benzidronate applying of phosphorus trichloride and phosphorous acid. Lett. Drug Des. Discov. 2015, 12, 78–84. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Aradi, K.; Garadnay, S.; Greiner, I. Optimized synthesis of N-heterocyclic dronic acids; closing a black-box era. Tetrahedron Lett. 2011, 21, 2744–2746. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Garadnay, S.; Greiner, I. Rational synthesis of dronic acid derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2116–2124. [Google Scholar] [CrossRef]
- Ratrout, S.S.; Al Sarabi, A.L.; Sweidan, K.A. A one-pot and efficient synthesis of zolendronic acid starting from tert-butyl imodazol-1-yl acetate. Pharm. Chem. J. 2015, 48, 837–841. [Google Scholar] [CrossRef]
- Mizrahi, D.A.; Waner, T.; Segall, Y. α-Amino acid derived bisphosphonates: Synthesis and anti-resorptive activity. Phosphorus Sulfur Silicon Relat. Elem. 2001, 173, 1–25. [Google Scholar] [CrossRef]
- Lenin, R.; Raju, M.R.; Srinisava Rao, D.V.N.; Ray, U.K. Microvawe-assistant efficient synthesis of bisphosphonate libraries: A useful procedure for the preparation of bisphosphonates containing nitrogen and sulfur. Med. Chem. Res. 2013, 22, 1624–1629. [Google Scholar] [CrossRef]
- Roth, A.G.; Drescher, A.; Yang, Y.; Redmer, S.; Uhling, S.; Arenz, C. Potent and selective inhibition of acid sphingomyelinase by bisphosphonates. Angew. Chem. Int. Ed. 2009, 48, 7560–7563. [Google Scholar] [CrossRef] [PubMed]
- Szajnman, S.H.; Ravaschino, E.L.; Docampo, R.; Rodrigues, J.B. Synthesis and biological evaluation of 1-amino-1,1-bisphosphonates derived from fatty acids against Trypanosoma cruzi targeting farnesyl pyrophosphate synthase. Bioorg. Med. Chem. Lett. 2005, 15, 4685–4690. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, P.; Huang, Y. Convenient synthesis of analogs of aminomethylene gem-diphosphonic acid from amines without catalyst. Synth. Commun. 2004, 34, 1393–1398. [Google Scholar] [CrossRef]
- Fukuda, M.; Okamoto, Y.; Sakurai, H. Synthesis of dialkylaminomethylenediphosphonic acids. Bull. Chem. Soc. Jpn. 1975, 48, 1030–1031. [Google Scholar] [CrossRef]
- Yu, C.M.; Wang, B.; Chen, Z.W. A Novel synthesis of alkylamino substituted methylenediphosphonates using bis(trichloromethyl) carbonate and RCONR1R2. Chin. J. Chem. 2008, 26, 1899–1901. [Google Scholar] [CrossRef]
- Plöger, W.; Schindler, N.; Wollmann, K.; Worms, K.H. Herstellung von 1-Aminoalkan-1,1-diphosphonsäuren. Z. Anorg. Allg. Chem. 1972, 389, 119–128. [Google Scholar] [CrossRef]
- Olive, G.; le Moigne, F.; Mercier, A.; Rockenbauer, A.; Tordo, P. Synthesis of tetraalkyl (pyrrolidine-2,2-diyl)bisphosphonates and 2,2-bis(diethoxyphosphoryl)-3,4-dihydro-2H-pyrrole 1-oxide; ESR study of derived nitroxides. J. Org. Chem. 1998, 63, 9095–9099. [Google Scholar] [CrossRef]
- Olive, G.; Jacques, A. Tetraethyl(pyrrolidine-2,2-diyl)bisphosphonate. Molecules 2001, 6, M275. [Google Scholar] [CrossRef]
- Olive, G.; Jacques, A. Optimization, continuation and lack of the one-step diphosphorylation reaction assay of modification of the tetraethyl(pyrrolidine-2,2-diyl)bisphosphonate. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 33–46. [Google Scholar] [CrossRef]
- Olive, G.; Rockenbauer, A.; Rozanska, X.; Jacques, A.; Peeters, D.; German, A. Synthesis of new tetraethyl(N-alkyl-1-aminoethan-1,1-diyl)bisphosphonates and ESR analysis of chemical exchange of derived nitroxides of acyclic aminobisphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2008, 182, 2359–2369. [Google Scholar] [CrossRef]
- Wang, A.-E.; Chang, Z.; Sun, W.-T.; Huang, P.-Q. General and chemoselective bisphosphonylation of secondary and tertiary amides. Org. Lett. 2015, 17, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Prishchenko, A.A.; Livantsov, N.V.; Novikova, O.P.; Livantsova, L.I.; Petrosyan, V.S. Synthesis of the new types of N-substituted aminomethylenebisorganophosphorus acids and their derivatives. Heteroatom Chem. 2009, 20, 319–324. [Google Scholar] [CrossRef]
- Prishchenko, A.A.; Livantsov, N.V.; Novikova, O.P.; Livantsova, L.I.; Ershov, I.R.; Petrosyan, V.S. Synthesis and reactivity of the new trimethylsilyl esters of aminomethylenebisorganophosphorus acids. Heteroatom Chem. 2013, 24, 355–360. [Google Scholar] [CrossRef]
- Prishchenko, A.A.; Livantsov, N.V.; Novikova, O.P.; Livantsova, L.I.; Petrosyan, V.S. Synthesis of aminomethylenediphosphonates and their derivatives containing PCNH2 fragments. J. Gen. Chem. 2014, 83, 608–610. [Google Scholar] [CrossRef]
- Chmielewska, E.; Miszczyk, P.; Kozłowska, J.; Prokopowicz, M.; Młynarz, P.; Kafarski, P. Reaction of benzolactams with triethyl phosphite prompted by phosphoryl chloride affords benzoannulated monophosphonates instead of expected bisphosphonates. J. Organomet. Chem. 2015, 785, 84–91. [Google Scholar] [CrossRef]
- Prishchenko, A.A.; Livantsov, N.V.; Novikova, O.P.; Livantsova, L.I.; Ershov, I.R.; Petrosyan, V.S. Synthesis of new types of aminomethylenediphosphorus-containing acids and their derivatives. Russ. J. Gen. Chem. 2015, 85, 370–379. [Google Scholar] [CrossRef]
- Du, Y.; Jung, K.Y.; Wimer, D.F. A one-flask synthesis of α,α-bisphosphonates via enolate chemistry. Tetrahedron Lett. 2002, 43, 8665–8668. [Google Scholar] [CrossRef]
- Srinivasa Rao, D.V.N.; Dandala, R.; Lenin, R.; Sivakumaran, N.; Shivashankar, S.; Naidu, A. A facile one pot synthesis of bisphosphonic acids and their sodium salts from nitriles. Arch. Org. Chem. 2007, 2007, 34–38. [Google Scholar]
- Kaabak, L.V.; Kuz’mina, N.E.; Khudneko, A.V.; Tomilov, A.P. Improved synthesis of 1-aminoethylidenediphosphonic acid. Russ. J. Gen. Chem. 2006, 76, 1673–1674. [Google Scholar] [CrossRef]
- Bandurina, T.A.; Konyukhov, V.N.; Panomareva, O.A.; Barybin, O.S.; Pushkareva, Z.N. Synthesis and antitumor activity of aminophosphonic acids. Pharm. Chem. J. 1978, 12, 1428–1431. [Google Scholar] [CrossRef]
- Midier, C.; Lantsoght, M.; Volle, J.-N.; Pirat, J.L.; Virieux, D.; Stevens, C.V. Hydrophosphonylation of alkenes or nitriles by double radical transfer mediated by titanocene/propylene oxide. Tetrahedron Lett. 2011, 52, 6693–6696. [Google Scholar] [CrossRef]
- Váradi, A.; Palmer, T.C.; Notis Dardashti, R.; Majumdar, S. Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules 2016, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Han, L.-B. Palladium-catalyzed insertion of isocyanides into P(O)−H bonds: Selective formation of phosphinoyl imines and bisphosphinoylaminomethanes. J. Am. Chem. Soc. 2006, 128, 7422–7423. [Google Scholar] [CrossRef] [PubMed]
- Goldemen, W.; Kluczyński, A.; Soroka, M. The preparation of N-substituted aminomethylidenebisphosphonates and their tetraalkyl esters via reaction of isonitriles with trialkyl phosphites and hydrogen chloride. Part 1. Tetrahedron Lett. 2012, 53, 5290–5292. [Google Scholar]
- Goldeman, W.; Nasulewicz-Goldeman, A. Synthesis and antiproliferative activity of aromatic and aliphatic bis(aminomethylidene(bisphosphonic)) acids. Bioorg. Med. Chem. Lett. 2014, 24, 3475–3479. [Google Scholar] [CrossRef] [PubMed]
- Kurzak, B.; Goldeman, W.; Szpak, M.; Matczak-Jon, E.; Kamecka, E. Synthesis of N-methyl alkylaminomethane-1,1-diphosphonic acids and evaluation of their complex-formation abilities towards copper(II). Polyhedron 2015, 85, 675–684. [Google Scholar] [CrossRef]
- Goldeman, W.; Nasulewicz-Goldeman, A. Synthesis and biological evaluation of aminomethylidenebisphosphonic derivatives of β-arylethylamines. Tetrahedron 2015, 71, 3282–3289. [Google Scholar] [CrossRef]
- Bhushan, K.R.; Tanaka, E.; Frangioni, J.V. Synthesis of conjugatable bisphosphonates for molecular imaging of large animals. Angew. Chem. Int. Ed. 2007, 46, 7969–7971. [Google Scholar] [CrossRef] [PubMed]
- Ziora, Z.; Maly, A.; Lejczak, B.; Kafarski, P.; Holband, J.; Wójcik, G. Reactions of N-phthalylamino acid chlorides with trialkyl phosphites. Heteroatom Chem. 2000, 11, 232–239. [Google Scholar] [CrossRef]
- Yanvarev, D.V.; Korovina, A.N.; Usanov, N.N.; Khomich, O.A.; Vepsäläinen, J.; Puljula, E.; Kukhanova, M.K.; Kochetkov, S.N. Data on synthesis of methylene bisphosphonates and screening of their inhibitory activity towards HIV reverse transcriptase. Data Brief 2016, 8, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Guenin, E.; Degache, E.; Liquier, J.; Lecouvey, M. Synthesis of 1-hydroxymethylene-1,1-bis(phosphonic acids) from acid anhydrides: Preparation of a new cyclic 1-acyloxymethylene-1,1-bis(phosphonic acid). Eur. J. Org. Chem. 2004, 2983–2987. [Google Scholar] [CrossRef]
- Kachbi-Khelfallah, S.; Monteil, M.; Cortes-Clerget, M.; Migianu-Griffoni, E.; Pirat, J.-L.; Gager, O.; Deschamp, J.; Lecouvey, M. Towards potential nanoparticle contrast agents: Synthesis of new functionalized PEG bisphosphonates. Beilstein J. Org. Chem. 2016, 12, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.C.; Rangel, C.M.; Teixeira, A.P.S. Synthesis of new azole phosphonate precursors for fuel cells proton exchange membranes. Heteroatom Chem. 2015, 26, 238–248. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Lucas, C.; Curto, M.J.M.; Neves, M.; Duarte, M.T.; André, V.; Teixeira, A.P.S. New 1-Hydroxy-1,1-bisphosphonates derived from 1H-pyrazolo[3,4-b]pyridine: Synthesis and characterization. J. Braz. Chem. Soc. 2013, 24, 1295–1306. [Google Scholar] [CrossRef]
- Kaboudin, B.; Ezzati, A.; Faghihi, M.R.; Barati, A.; Kazemi, F.; Abdollahi, H.; Yokomatsu, T. Hydroxy‑bisphosphinic acids: Synthesis and complexation properties with transition metals and lanthanide ions in aqueous solution. J. Iran. Chem. Soc. 2016, 13, 747–752. [Google Scholar] [CrossRef]
- Crossey, K.; Migaud, M.E. Solventless synthesis of acyl phosphonamidates, precursors to masked bisphosphonates. Chem. Commun. 2015, 51, 11088–11091. [Google Scholar] [CrossRef] [PubMed]
- Egorov, M.; Aoun, S.; Padrines, M.; Redini, F.; Heymann, D.; Lebreton, J.; Mathé-Allainmat, M.A. One-pot synthesis of 1-hydroxy-1,1-bis(phosphonic acid)s starting from the corresponding carboxylic acids. Eur. J. Org. Chem. 2011, 7148–7154. [Google Scholar] [CrossRef]
- Suzuki, F.; Fujikawa, Y.; Yamamoto, S.; Mizutani, H.; Funabashi, C.; Ohya, T.; Ikai, T.; Oguchi, T. Pharmaceutical Compositions Containing Geminal Diphosphonates. US 5583122 A, 1 February 1979. [Google Scholar]
- Maier, L. Organishe phosphorverbindungen 75: Herstellung und Eigenschaften von Aminomethylendiphosphinaten und -diphosphonaten, RR1NCH[P(O)R2(OR3)]2 und Derivaten. Phosphorus Sulfur Silicon Relat. Elem. 1981, 11, 311–332. [Google Scholar] [CrossRef]
- Kaboudin, B.; Kalipour, S. A microwave-assisted solvent- and catalyst-free synthesis of aminomethylene bisphosphonates. Tetrahedron Lett. 2009, 50, 4243–4245. [Google Scholar] [CrossRef]
- Minaeva, L.; Patrikeeva, L.S.; Kabachnik, M.M.; Beletskaya, I.P.; Orlinson, B.S.; Novakov, I.A. Synthesis of novel aminomethylenebisphosphonates and bisphosphonic acids, containing adamantyl fragment. Heteroatom Chem. 2011, 22, 55–58. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, A.; Dziełak, A.; Hägele, G.; Keglevich, G. Microwave-assisted synthesis of (aminomethylene)bisphosphine oxides and (aminomethylene)bisphosphonates by a three-component condensation. Beilstein J. Org. Chem. 2016, 12, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.B.; Sundar, C.S.; Krishna, B.S.; Reddy, K.M.K.; Reddy, C.S. Synthesis and in-vitro antimicrobial activity of aminomethylene bisphosphonates. Iran. J. Org. Chem. 2014, 6, 1227–1234. [Google Scholar]
- Krutikov, V.I.; Erkin, A.V.; Pautov, P.A.; Zolotukhina, M.M. Heteryl- and arylaminomethylenebisphosphonates: Synthesis and biologic activity. Russ. J. Gen. Chem. 2003, 73, 187–191. [Google Scholar] [CrossRef]
- Siva Prasad, S.; Jayaprakash, S.H.; Syamasundar, Ch.; Sreelakshmi, P.; Bhuvaneswar, C.; Vijaya Bhaskar, B.; Rajendra, W.; Nayak, S.K.; Suresh Reddy, C. Tween 20-/H2O promoted green synthesis, computational and antibacterial activity of amino acid substituted methylene bisphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2040–2050. [Google Scholar] [CrossRef]
- Mimura, M.; Hayashida, M.; Nomiyama, K.; Ikegami, S.; Iida, Y.; Tamura, M.; Hiyama, Y.; Ohishi, Y. Synthesis and evaluation of (piperidinomethylene)bis(phosphonic acid) derivatives as anti-osteoporosis agents. Chem. Pharm. Bull. 1993, 41, 1973–1986. [Google Scholar] [CrossRef]
- De Schutter, J.W.; Shaw, J.; Lin, Y.-S.; Tsantrizos, Y.S. Design of potent bisphosphonate inhibitors of the human farnesyl pyrophosphate synthase via targeted interactions with the active site “capping” phenyls. Bioorg. Med. Chem. 2012, 20, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.Y.; Langille, A.M.; Mancuso, J.; Tsantrizos, Y.S. Discovery of thienopyrimidine-based inhibitors of the human farnesyl pyrophosphate synthase—Parallel synthesis of analogs via a trimethylsilyl ylidene intermediate. Bioorg. Med. Chem. 2013, 21, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.Y.; Park, J.; de Schutter, J.W.; Sebag, M.; Berghuis, A.M.; Tsantrizos, Y.S. Thienopyrimidine bisphosphonate (ThPBP) inhibitors of the human farnesyl pyrophosphate synthase: Optimization and characterization of the mode of inhibition. J. Med. Chem. 2013, 56, 7939–7950. [Google Scholar] [CrossRef] [PubMed]
- Rubino, M.T.; Agamennone, M.; Campestre, C.; Campiglia, P.; Cremasco, V.; Faccio, R.; Laghezza, A.; Loiodice, F.; Maggi, D.; Panza, E.; et al. Biphenyl sulfonylamino methyl bisphosphonic acids as inhibitors of matrix metalloproteinases and bone resorption. ChemMedChem 2011, 6, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Tauro, M.; Lagezza, A.; Loiodice, F.; Agamennone, M.; Campestre, C.; Tortorella, P. Arylamino methylene bisphosphonate derivatives as bone seeking matrix metalloproteinase inhibitors. Bioorg. Med. Chem. 2013, 21, 6456–6465. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, E.; Mazur, Z.; Kempińska, K.; Wietrzyk, J.; Piątek, A.; Kuryszko, J.J.; Kiełbowicz, Z.; Kafarski, P. N-Arylaminomethylenebisphosphonates bearing fluorine atoms: Synthesis and antiosteoporotic activity. Phosphorus Sulfur Silicon Relat. Elem. 2016, 190. [Google Scholar] [CrossRef]
- Chmielewska, E.; Kempińska, K.; Wietrzyk, J.; Piątek, A.; Kuryszko, J.J.; Kiełbowicz, Z.; Kafarski, P. Novel Bisphosphonates and Their Use. WO2015159153 A1, 22 October 2015. [Google Scholar]
- Kubiček, V.; Rudovský, J.; Kotek, J.; Hermann, P.; Elst, L.V.; Muller, R.N.; Kolar, Z.I.; Wolterbeek, H.Th.; Peters, J.A.; Lukeš, I. A Bisphosphonate monoamide analogue of DOTA: A potential agent for bone targeting. J. Am. Chem. Soc. 2005, 127, 16477–16485. [Google Scholar] [CrossRef] [PubMed]
- Årstad, E.; Hoff, P.; Skattebøl, L.; Skretting, A.; Breistøl, K. Studies on the synthesis and biological properties of non-carrier-added [125I and 131I]-labeled arylalkylidenebisphosphonates: Potent bone-seekers for diagnosis and therapy of malignant osseous lesions. J. Med. Chem. 2003, 46, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.B.; Sanders, J.M.; Kendrick, H.; de Luca-Fradley, K.; Lewis, J.C.; Grimley, J.S.; van Brussel, E.M.; Olsen, J.R.; Meints, G.A.; Burzyńska, A.; et al. Activity of Bisphosphonates against Trypanosoma brucei rhodesiense. J. Med. Chem. 2002, 45, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.M.; Gomez, A.O.; Mao, J.; Meints, G.A.; van Brussel, E.M.; Burzyńska, A.; Kafarski, P.; Gonzalez-Pacanowska, D.; Oldfield, E. 3-D QSAR investigations of the inhibition of Leishmania major farnesyl pyrophosphate synthase by bisphosphonates. J. Med. Chem. 2003, 46, 5171–5183. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chan, J.M.; Lea, C.R.; Meints, G.A.; Lewis, J.C.; Tovian, Z.S.; Flessner, R.M.; Loftus, T.C.; Bruchhaus, I.; Kendrick, H.; et al. Effects of bisphosphonates on the growth of Entamoeba histolytica and Plasmodium species in vitro and in vivo. J. Med. Chem. 2004, 47, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Sahota, D.; Odeh, S.; Chan, J.M.; Araujo, F.G.; Moreno, S.N.; Oldfield, E. Bisphosphonate inhibitors of Toxoplasma gondi growth: In vitro, QSAR, and in vivo investigations. J. Med. Chem. 2005, 48, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.S.E.; Houghton, T.J.; Kang, T.; Dietrich, E.; Delorme, D.; Ferreira, S.S.; Caron, L.; Viens, F.; Arhin, S.S.; Sarmiento, I.; et al. Bisphosphonated fluoroquinolone esters as osteotropic prodrugs for the prevention of osteomyelitis. Bioorg. Med. Chem. 2008, 16, 9217–9229. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Petrolino, D.; Fusetti, M.; Romanini, L.; Nocek, B.; Joachimiak, A.; Berlicki, L.; Kafarski, P. δ1-Pyrroline-5-carboxylate reductase as a new target for therapeutics: Inhibition of the enzyme from Streptococcus pyogenes and effects in vivo. Amino Acids 2012, 42, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Brel, V.K. Synthesis of gem-bisphosphonates with (3-aryl-4,5-dihydroxisoxazol-5-yl)methylamino moiety. Mendeleev Commun. 2015, 25, 234–235. [Google Scholar] [CrossRef]
- Kosikowska, P.; Bochno, M.; Macegoniuk, K.; Forlani, G.; Kafarski, P.; Berlicki, Ł. Bisphosphonic acids as effective inhibitors of Mycobacterium tuberculosis glutamine synthetase. J. Enzyme Inhib. Med. Chem. 2016, 31, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Lacbay, C.M.; Mancuso, J.; Lin, Y.-S.; Bennett, N.; Götte, M.; Tsantrizos, Y.S. Modular assembly of purine-like bisphosphonates as inhibitors of HIV-1 reverse transcriptase. J. Med. Chem. 2014, 57, 7435–7449. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, A.A.; Kamel, A.A.; Abdou, W.M. Synthesis, quantitative structure-activity relationship, and anti-inflammatory profiles of substituted 5- and 6-N-heterocycle bisphosphonate esters. Chem. Commun. 2013, 43, 236–252. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B.; Forlani, G. Herbicidally active aminomethylenebisphosphonic acids. Heteroat. Chem. 2000, 11, 449–453. [Google Scholar] [CrossRef]
- Forlani, G.; Berlicki, Ł.; Duò, M.; Dziędzioła, G.; Giberti, S.; Bertazzini, M.; Kafarski, P. Synthesis and Evaluation of Effective Inhibitors of Plant δ1-Pyrroline-5-carboxylate Reductase. J. Agric. Food Chem. 2013, 61, 6792–6798. [Google Scholar] [CrossRef] [PubMed]
- Giberti, S.; Bertazzini, M.; Liboni, M.; Berlicki, Ł.; Kafarski, P.; Forlani, G. Phytotoxicity of aminobisphosphonates targeting both δ1-pyrroline-5-carboxylate reductase and glutamine synthetase. Pest Manag. Sci. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, A.; Spychała, J.; Ptak, T.; Chmielewska, E.; Maciejewska, G.; Kafarski, P.; Młynarz, P. Electrochemical and spectroscopic investigations of selected N-heteroalkylaminomethylene-bisphosphonic acids with Pb(II) ions. Coord. Chem. Rev. 2016, in press. [Google Scholar] [CrossRef]
- Dąbrowska, E.; Burzyńska, A.; Mucha, A.; Matczak-Jon, E.; Sawka-Dobrowolska, W.; Berlicki, Ł.; Kafarski, P. Insight into the mechanism of three component condensation leading to aminomethylenebisphosphonates. J. Organomet. Chem. 2009, 6943, 3806–3813. [Google Scholar] [CrossRef]
- Lejczak, B.; Boduszek, B.; Kafarski, P.; Forlani, G.; Wojtasek, H.; Wieczorek, P. Mode of action of herbicidal derivatives of aminomethylenebisphosphonic acid. I. Physiologic activity and inhibition of anthocyanin biosynthesis. J. Plant Growth Regul. 1996, 15, 109–113. [Google Scholar] [CrossRef]
- Bochno, M.; Berlicki, Ł. A three-component synthesis of aminomethylene bis-H-phosphinates. Tetrahedron Lett. 2014, 55, 219–223. [Google Scholar] [CrossRef]
- Rodriguez, J.B. Tetraethyl vinylidenebisphosphonate: A versatile synthon for the preparation of bisphosphonates. Synthesis 2014, 46, 1129–1142. [Google Scholar] [CrossRef]
- Rosso, V.S.; Szajman, S.H.; Malayil, L.; Galizzi, M.; Moreno, S.N.J.; Docampo, R.; Rodriguez, J.B. Synthesis and biological evaluation of new 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase. Bioorg. Med. Chem. 2011, 19, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Szajnman, S.H.; Liñares, G.E.G.; Li, Z.-H.; Jiang, C.; Galizzi, M.; Bontempi, E.; Ferella, M.; Moreno, S.N.J.; Docampo, R.; Rodriguez, J.B. Synthesis and biological evaluation of 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase. Bioorg. Med. Chem. 2008, 15, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Herczegh, P.; Buxton, T.B.; McPherson, J.C., III; Kovács-Kulyassa, A.; Brewer, P.D.; Sztaricskai, F.; Stroebel, G.C.; Plowman, K.M.; Farcasiu, D.; Hartmann, J.F. Osteoadsorptive bisphosphonate derivatives of fluoroquinolone antibacterials. J. Med. Chem. 2002, 45, 2338–2341. [Google Scholar] [CrossRef] [PubMed]
- Houghton, T.J.; Tanaka, K.S.E.; Kang, T.; Dietrich, E.; Lafontaine, Y.; Delorme, D.; Ferreira, S.S.; Viens, F.; Arhin, F.F.; Sarmiento, I.; et al. Linking bisphosphonates to the free amino groups in fluoroquinolones: Preparation of osteotropic prodrugs for the prevention of osteomyelitis. J. Med. Chem. 2008, 51, 6955–6969. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewski, M.; Hejmanowska, J.; Albrecht, Ł. A convenient approach to a novel group of quaternary amino acids containing a geminal bisphosphonate moiety. Synthesis 2014, 46, 3233–3239. [Google Scholar] [CrossRef]
- Simoni, D.; Gebbia, N.; Invidiata, M.P.; Eleopra, M.; Marchetti, P.; Rondanin, R.; Baruchello, R.; Provera, S.; Marchioro, C.; Tolomeo, M.; et al. Design, synthesis, and biological evaluation of novel aminobisphosphonates possessing an in vivo antitumor activity through a γδ-T lymphocytes-mediated activation mechanism. J. Med. Chem. 2008, 51, 6800–6807. [Google Scholar] [CrossRef] [PubMed]
- Sankala, E.; Weisell, J.M.; Huhtala, T.; Närvänen, A.T.O.; Vepsäläinen, J.J. Synthesis of novel bisphosphonate polyamine conjugates and their affinity to hydroxyapatite. Arch. Org. Chem. 2012, 2012, 233–241. [Google Scholar]
- Chen, K.M.C.; Hudock, M.P.; Zhang, Y.; Guo, R.T.; Cao, R.; No, J.H.; Liang, P.H.; Ko, T.-P.; Chang, T.H.; Chang, S.; et al. Inhibition of geranylgeranyl diphosphate synthase by bisphosphonates: A crystallographic and computational investigation. J. Med. Chem. 2008, 51, 5594–5607. [Google Scholar]
- Zhang, Y.; Leon, A.; Song, Y.; Studer, D.; Haase, C.; Kościelski, Ł.A.; Oldfield, E. Activity of nitrogen-containing and non-nitrogen-containing bisphosphonates on tumor cell lines. J. Med. Chem. 2006, 49, 5804–5814. [Google Scholar] [CrossRef] [PubMed]
- Makarov, M.V.; Rybalkina, E.Y.; Röschenthaler, G.-V. New 3,5-bis(arylidene)-4-piperidones with bisphosphonate moiety: Synthesis and antitumor activity. Russ. Chem. Bull. Int. Ed. 2014, 63, 1181–1186. [Google Scholar] [CrossRef]
- Skarpos, H.; Osipov, S.N.; Vororb’eva, D.V.; Odinets, I.L.; Lork, E.; Röschenthaler, G.-V. Synthesis of functionalized bisphosphonates via click chemistry. Org. Biomol. Chem. 2007, 5, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bi, W.; Li, X.; Chen, X.; Qu, N.; Zhao, Y. A Practical method to synthesize 1,2,3-triazole-amino-bisphosphonate derivatives. Phosphorus Sulfur Relat. Elem. 2015, 190, 1735–1742. [Google Scholar] [CrossRef]
- Gouault-Bironneau, S.; Deprèle, S.; Sutor, A.; Montchamp, J.-L. Radical reaction of sodium hypophosphite with terminal alkynes: Synthesis of 1,1,-bis-H-phosphinates. Org. Lett. 2005, 7, 5909–5912. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik, A.; Mazurkiewicz, R.; Grymel, M.; Zielińska, K.; Adamek, J.; Chmielewska, E.; Bochno, M.; Kubica, S. A new method for the synthesis of α-aminoalkylidenebisphosphonates and their asymmetric phosphonyl-phosphinyl and phosphonyl-phosphinoyl analogues. Beilstein J. Org. Chem. 2015, 11, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.S.; Dietrich, E.; Ciblat, S.; Métayer, C.; Arhin, F.F.; Sarmiento, I.; Moeck, G.; Parr, T.R., Jr.; Far, A.R. Synthesis and in vitro evaluation of bisphosphonate glycopeptide prodrugs for the treatment of osteomyelitis. Bioorg. Med. Chem. Lett. 2010, 20, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Bala, J.L.F.; KAshemirov, B.A.; McKenna, C.E. Synthesis of novel bisphosphonic acid alkene monomer. Synth. Commun. 2010, 40, 3577–3584. [Google Scholar] [CrossRef]
- Lecerclé, D.; Gabillet, S.; Gomis, J.-M.; Taran, F. A facile synthesis of aminomethylene bisphosphonates through rhodium carbenoid mediated N-H insertion reaction. Application to the preparation of powerful uranyl ligands. Tetrahedron Lett. 2008, 49, 2083–2087. [Google Scholar] [CrossRef]
- Masschelein, K.G.R.; Stevens, C.V. Double nucleophilic 1,2-addition of silylated dialkyl phosphites to 4-phosphono-1-aza-1,3-dienes: Synthesis of γ-phosphono-α–aminobisphosphonates. J. Org. Chem. 2007, 72, 9248–9252. [Google Scholar] [CrossRef] [PubMed]
- Rassukana, Y.V.; Davydova, K.O.; Onys’ko, P.P.; Sinitsa, A.D. Synthesis and rearrangements of N-trichloroacetylfluoroacetimidolyl chloride and its phosphorylation products. J. Fluor. Chem. 2002, 117, 107–113. [Google Scholar] [CrossRef]
- Rassukana, Y.V.; Onys’ko, P.P.; Davydova, K.O.; Sinitsa, A.D. A new reaction of phosphorylated N-sulfonylimines with hydrophosphoryl agents involving C-N transfer of phosphoryl groups. Tetrahedron Lett. 2004, 45, 3899–3902. [Google Scholar] [CrossRef]
- Kolotilo, N.V.; Sinitsa, A.D.; Rassukanaya, Y.V.; Onys’ko, P.P. N-Sulfonyl- and N-phosphorylbenzimidoylphosphonates. Russ. J. Gen. Chem. 2006, 76, 1210–1218. [Google Scholar] [CrossRef]
- Romanenko, V.D.; Kukhar, V.P. Methylidynetrisphosphonates: Promising C1 building block for the design of phosphate mimetics. Beilstein J. Org. Chem. 2013, 9, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Lejczak, B. A facile conversion of aminoalkanephosphonic acids into their diethyl esters. The use of unblocked aminophosphonic acids in phosphono peptide synthesis. Synthesis 1988, 4, 307–310. [Google Scholar] [CrossRef]
- Turhanen, P.A.; Weisell, J.; Vepsäläinen, J.J. Preparation of mixed trialkyl alkylcarbonate derivatives of etidronic acid via an unusual route. Beilstein J. Org. Chem. 2012, 8, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Turhanen, P.A.; Vepsäläinen, J.J. Synthesis of novel (1-alkanoyloxy-4-alkanoylaminobutylidene)-1,1-bisphosphonic acid derivatives. Beilstein J. Org. Chem. 2006, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, C.; Bo, L.; Wu, X.; Xie, Y. Synthesis of new potential chelating agents: Catechol-bisphosphonate conjugates for metal intoxication therapy. Heteroatom Chem. 2004, 15, 251–257. [Google Scholar] [CrossRef]
- Agyin, J.K.; Santhamma, B.; Roy, S.S. Design, synthesis, and biological evaluation of bone-targeted proteasome inhibitors for multiple myeloma. Bioorg. Med. Chem. 2013, 23, 5455–6458. [Google Scholar] [CrossRef] [PubMed]
- Ezra, A.; Hoffman, A.; Breuer, E.; Alferiev, A.S.; Mönkkönen, J.; El Hanany-Rozen, N.; Weiss, G.; Stepensky, D.; Gati, I.; Cohen, H.; et al. A Peptide prodrug approach for improving bisphosphonate oral absorption. J. Med. Chem. 2000, 43, 3641–3652. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Błażewska, K.; Kadina, A.P.; Kashemirov, B.A.; Duan, X.; Triffitt, J.T.; Dunford, J.E.; Russell, R.G.R.; Ebetino, F.H.; Roelofs, A.J.; et al. Fluorescent bisphosphonate and carboxyphosphonate probes: A versatile imaging toolkit for applications in bone biology and biomedicine. Bioconj. Chem. 2016, 27, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jo, J.; Kawai, Y.; Aoki, I.; Tanaka, C.; Yamamoto, M.; Tabata, Y. Preparation of polymer-based multimodal imaging agent to visualize the process of bone regeneration. J. Control. Release 2012, 157, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, O.P.; Sun, W.; Hilborn, J.; Ossipov, D.A. In situ cross-linkable high molecular weight hyaluronan-bisphosphonate conjugate for localized delivery and cell-specific targeting: A hydrogel linked prodrug approach. J. Am. Chem. Soc. 2009, 131, 8781–8783. [Google Scholar] [CrossRef] [PubMed]
- Vitha, T.; Kubiček, V.; Hermann, P.; Elst, L.V.; Muller, R.N.; Kollar, Z.I.; Wolterbeek, H.T.; Breeman, W.A.P.; Lukeš, I.; Peters, J.A. Lanthanide(III) Complexes of Bis(phosphonate) Monoamide Analogues of DOTA: Bone-Seeking Agents for Imaging and Therapy. J. Med. Chem. 2008, 51, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Xu, Y.; Jiao, Y.; Zhou, C. Synthesis and characterization of an alendronate-chitosan conjugate. Appl. Mech. Mater. 2012, 140, 53–57. [Google Scholar] [CrossRef]
- Morioka, M.; Kamizono, A.; Takikawa, H.; Mori, A.; Ueno, H.; Kadowaki, S.; Nakano, Y.; Kato, K.; Umezawa, K. Design, synthesis, and biological evaluation of novel estradiol-bisphosphonate conjugates as bone-specific estrogens. Bioorg. Med. Chem. 2010, 18, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Dadiboyena, S. Recent advances in the synthesis of raloxifene: A selective estrogen receptor modulator. Eur. J. Med. Chem. 2012, 51, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Oliveira, B.M.; Correira, J.D.G.; Gano, L.; Maria, L.; Santos, I.C.; Santos, I. A new bisphosphonate-containing 99mTc(I) tricarbonyl complex potentially useful as bone-seeking agent: Synthesis and biological evaluation. J. Biol. Inorg. Chem. 2007, 12, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Dietrich, E.; Lafontaine, Y.; Houghton, T.J.; Belanger, O.; Dubois, A.; Arhin, F.F.; Sarmiento, I.; Fadhil, I.; Laquerre, K.; et al. Bisphosphonated benzoxazinorifamycin prodrugs for the prevention and treatment of osteomyelitis. ChemMedChem 2008, 3, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Bekker, K.S.; Chukanov, N.V.; Grigor’ev, I.A. Synthesis of a bisphosphonate derivative of folic acid. Chem. Nat. Prod. 2013, 49, 495. [Google Scholar] [CrossRef]
- Bekker, K.S.; Chukanov, N.V.; Popov, S.A.; Grigor’ev, I.A. New Amino-Bisphosphonate Building Blocks in the Synthesis of Bisphosphonic Derivatives Based on Lead Compounds. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1209–1212. [Google Scholar] [CrossRef]
- Bekker, K.S.; Chukanov, N.V.; Popov, S.A.; Grigor’ev, I.A. Conjugates of bisphosphonates with derivatives of ursolic, betulonic, and betulinic acids. Chem. Nat. Prod. 2013, 49, 581–582. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Gao, J.; Xu, K.; Gu, H.; Zhang, B.; Zhang, X.; Xu, B. A biocompatible method of decorporation: Bisphosphonate-modified magnetite nanoparticles to remove uranyl ions from blood. J. Am. Chem. Soc. 2006, 131, 13358–13359. [Google Scholar] [CrossRef] [PubMed]
- Bekker, K.S.; Chukanov, N.V.; Grigor’ev, I.A. Synthesis of a bis-phosphonic acid derivative of trolox, a new potential antioxidant. Chem. Nat. Prod. 2013, 49, 785–786. [Google Scholar] [CrossRef]
- Altin, A.; Akgun, B.; Bilgici, Z.S.; Turker, S.B.; Avci, D. Synthesis, photopolymerization, and adhesive properties of hydrolytically stable phosphonic acid-containing (meth)acrylamides. J. Polym. Sci. A Polym. Chem. 2014, 52, 512–522. [Google Scholar] [CrossRef]
- Akgun, B.; Avci, D. Synthesis and evaluations of bisphosphonate-containing monomers for dental materials. J. Polym. Sci. A Polym. Chem. 2012, 50, 4854–4863. [Google Scholar] [CrossRef]
- Yang, X.; Akhtar, X.; Rubino, S.; Leifer, K.; Hilborn, J.; Ossipov, D. Direct “click” synthesis of hybrid bisphosphonate—Hyaluronic acid hydrogel in aqueous solution for biomineralization. Chem. Mater. 2012, 24, 1690–1697. [Google Scholar] [CrossRef]
- Gluz, E.; Grinberg, I.; Corem-Salkmon, E.; Mizrahi, D.; Margel, S. Engineering of new crosslinked near-infrared fluorescent polyethylene glycol bisphosphonate nanoparticles for bone targeting. J. Polym. Sci. A Polym. Chem. 2013, 51, 4282–4291. [Google Scholar] [CrossRef]
- Mukaya, H.E.; Mbianda, X.Y. Macromolecular co-conjugate of ferrocene and bisphosphonate: Synthesis, characterization and kinetic drug release study. J. Inorg. Organomet. Polym. 2015, 25, 411–418. [Google Scholar] [CrossRef]
- Beck, J.; Gharbi, S.; Herteg-Fernea, A.; Vercheval, L.; Berbone, C.; Lassaux, P.; Zervosen, A.; Marchant-Bryanert, J. Aminophosphonic acids and aminobis(phosphonic acids) as potential inhibitors of penicillin-binding proteins. Eur. J. Org. Chem. 2009, 2009, 85–97. [Google Scholar] [CrossRef]
- Chuiko, A.L.; Filonenko, L.P.; Borisevich, A.M.; Lozinskii, M.O. pH-Dependent isomerism of (iso)thioureidomethylenebisphosphonates. Russ. J. Gen. Chem. 2009, 79, 72–77. [Google Scholar] [CrossRef]
- Chuiko, A.L.; Filonenko, L.P.; Lozinskii, M.O. Reaction of thioureidomethylenebisphosphonic acids with α-haloketones: II. Effect of pH on the reaction pathway. Russ. J. Gen. Chem. 2009, 79, 57–66. [Google Scholar] [CrossRef]
- Chuiko, A.L.; Filonenko, L.P.; Lozinskii, M.O. Synthesis of ((thiazol-2-ylamino)-methylene)bisphosphonic acids. Chem. Heterocycl. Compd. 2011, 47, 1137–1140. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hasa, T.; Ahola, J.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Phosphonated nanocelluloses from sequential oxidative-reductive treatment—Physicochemical characteristics and thermal properties. Carbohydr. Polym. 2015, 133, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Bonzi, G.; Salmaso, S.; Scomparin, A.; Edar-Boock, A.; Satchi-Fainaro, R.; Caliceti, P. Novel pullulan bioconjugate for selective breast cancer bone metastases treatment. Bioconj. Chem. 2015, 26, 489–501. [Google Scholar] [CrossRef] [PubMed]

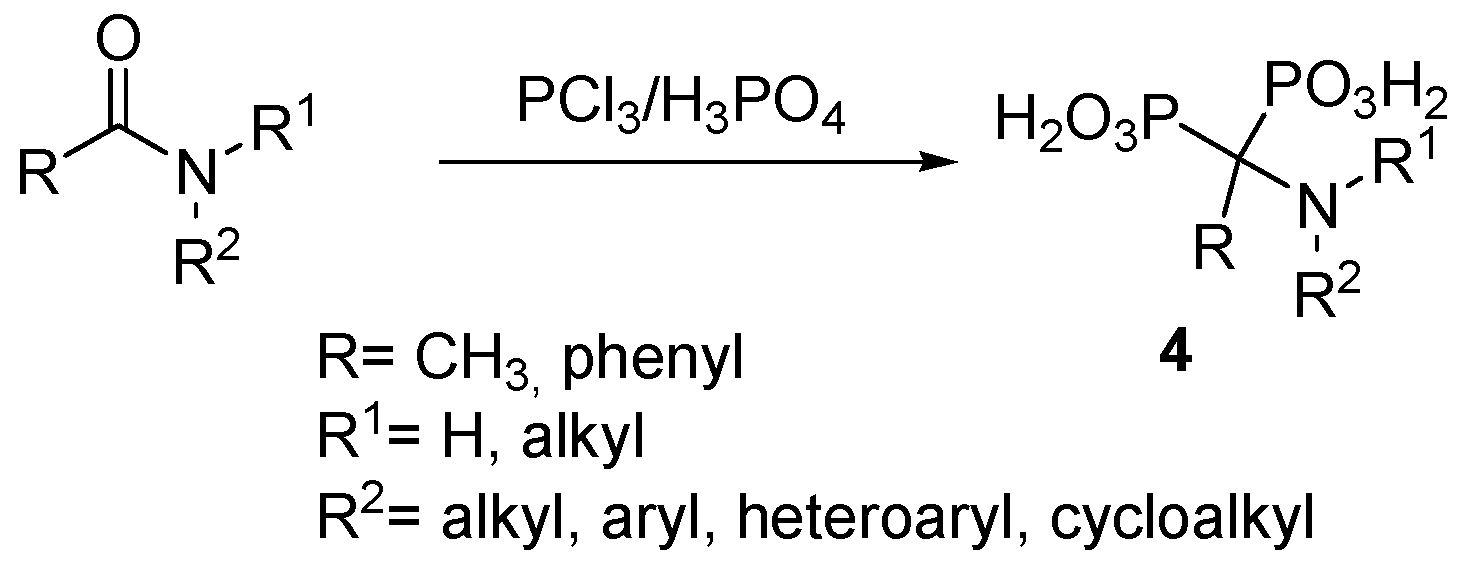



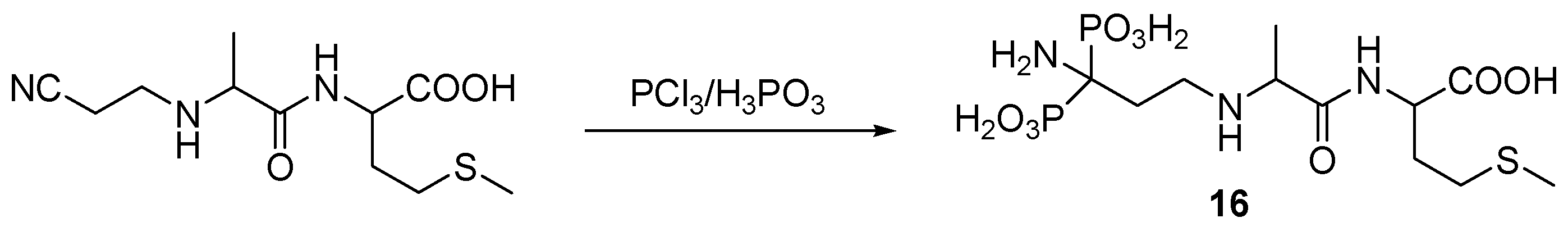
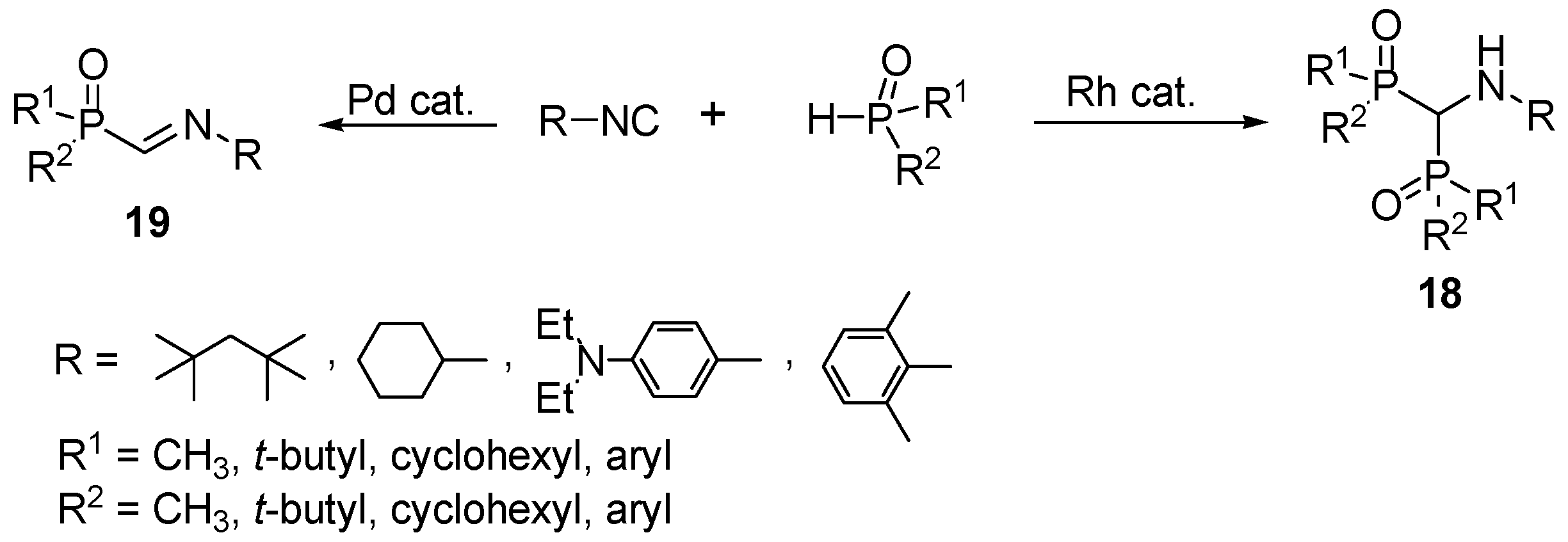

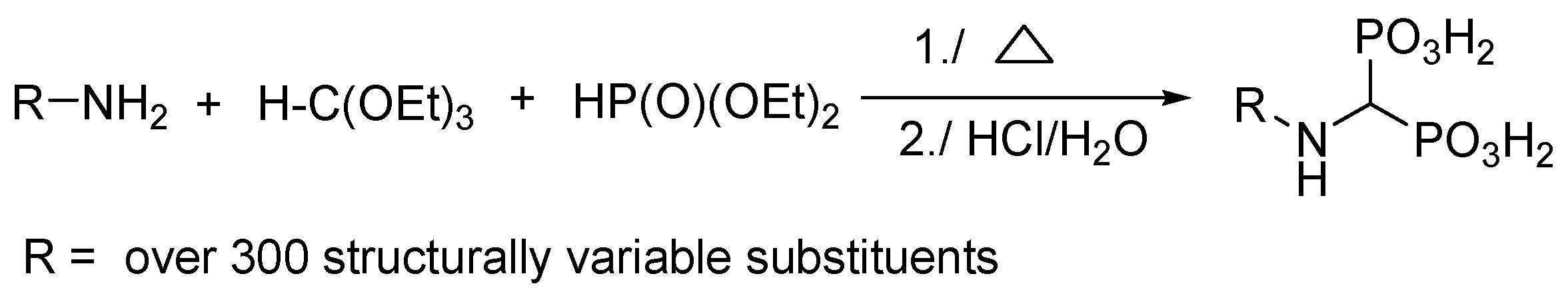

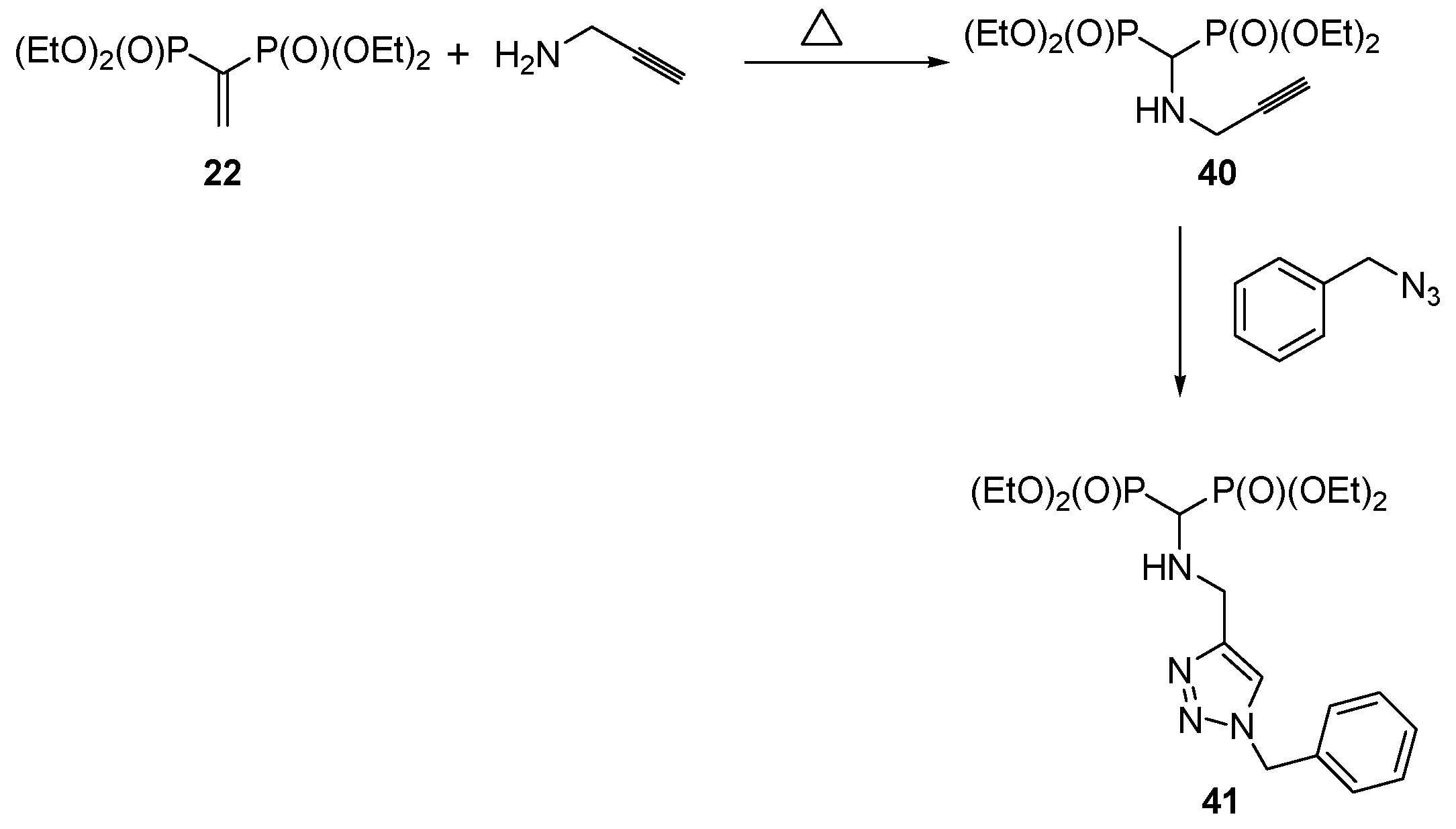
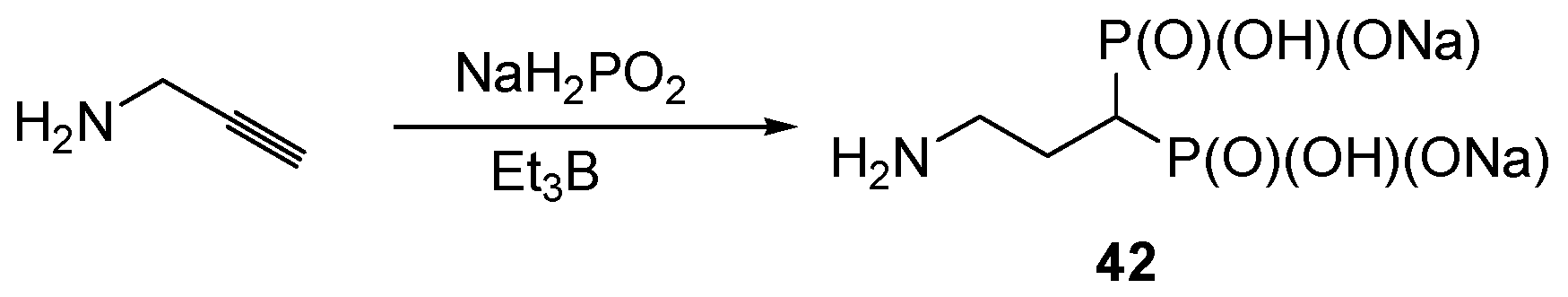



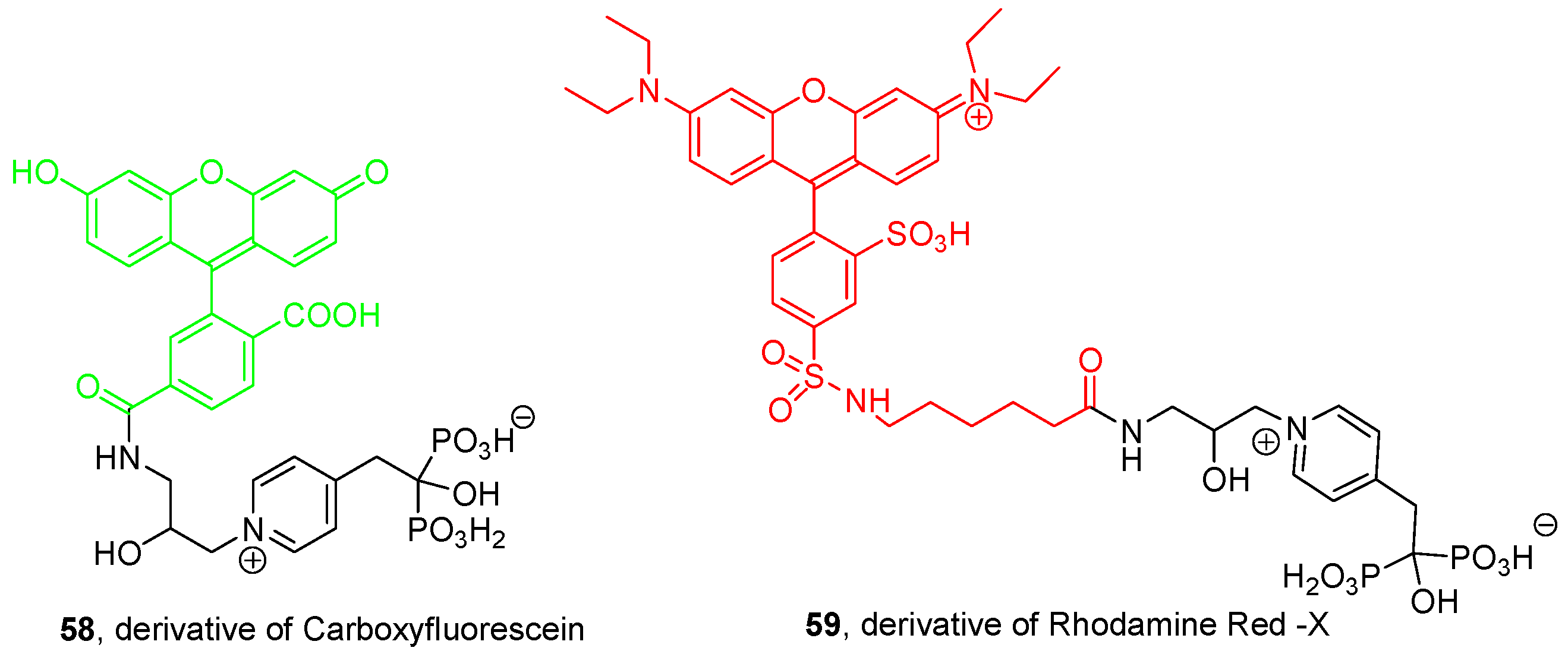
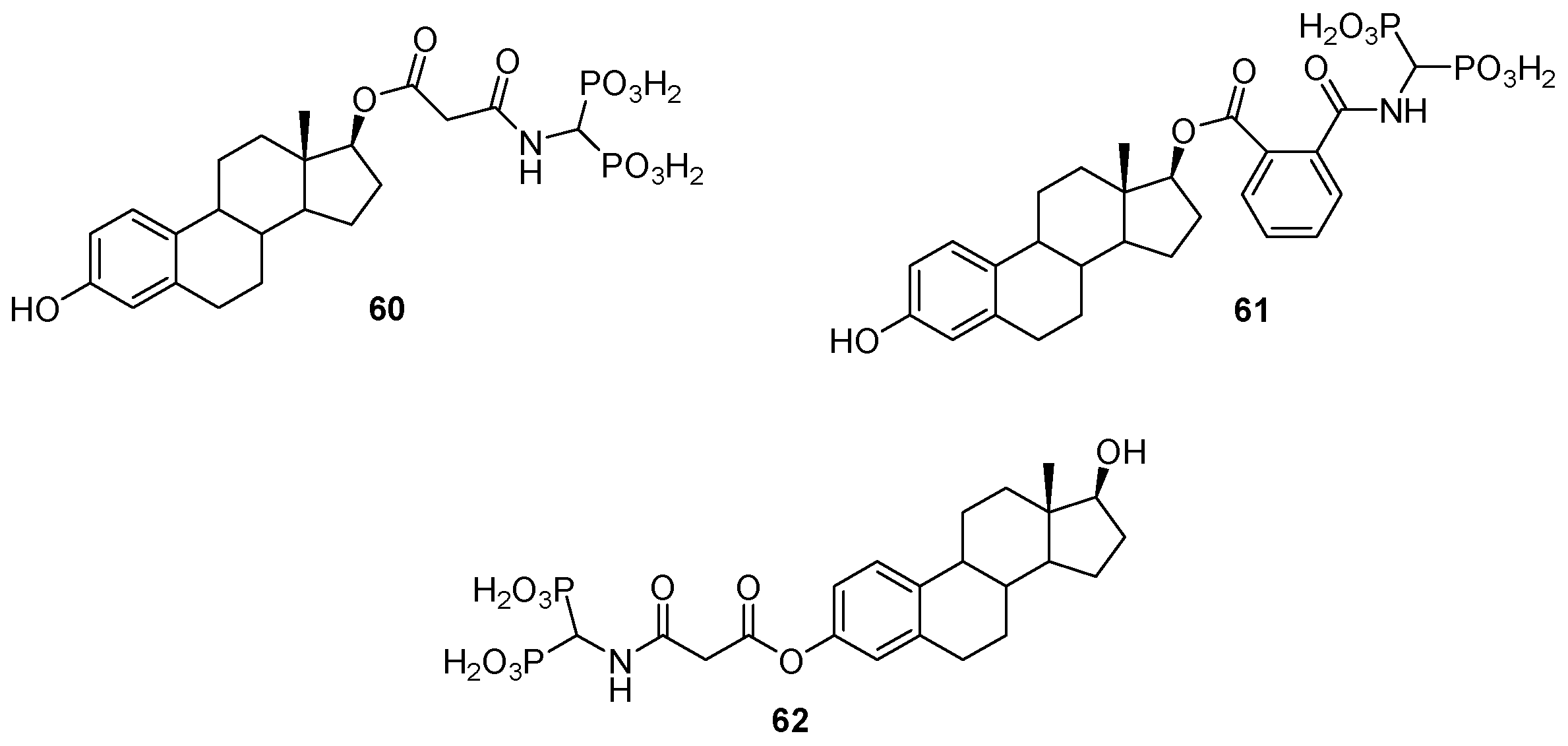
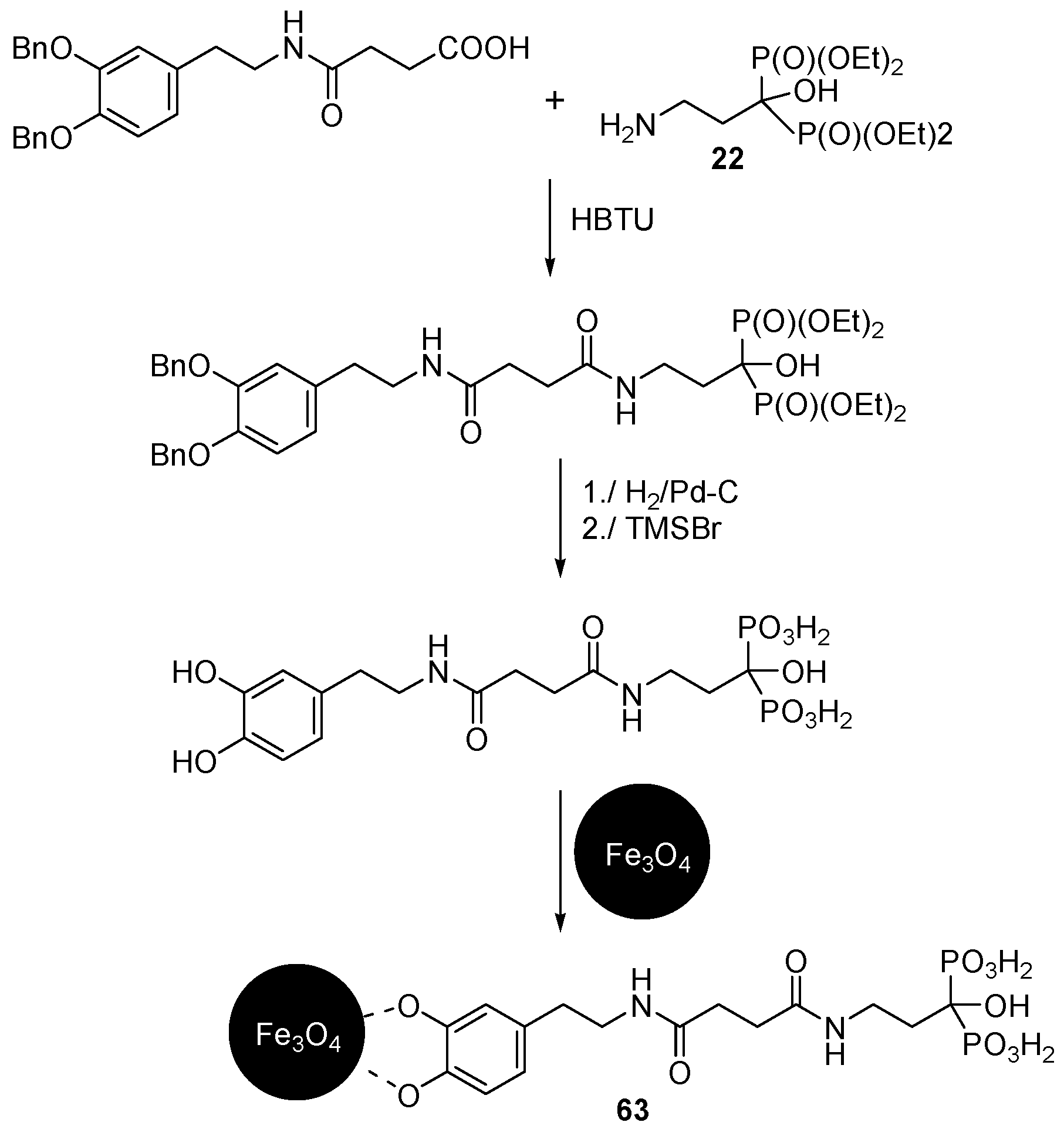



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewska, E.; Kafarski, P. Synthetic Procedures Leading towards Aminobisphosphonates. Molecules 2016, 21, 1474. https://doi.org/10.3390/molecules21111474
Chmielewska E, Kafarski P. Synthetic Procedures Leading towards Aminobisphosphonates. Molecules. 2016; 21(11):1474. https://doi.org/10.3390/molecules21111474
Chicago/Turabian StyleChmielewska, Ewa, and Paweł Kafarski. 2016. "Synthetic Procedures Leading towards Aminobisphosphonates" Molecules 21, no. 11: 1474. https://doi.org/10.3390/molecules21111474