Chlorella sorokiniana Extract Improves Short-Term Memory in Rats
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of Chlorella Sorokiniana Extract
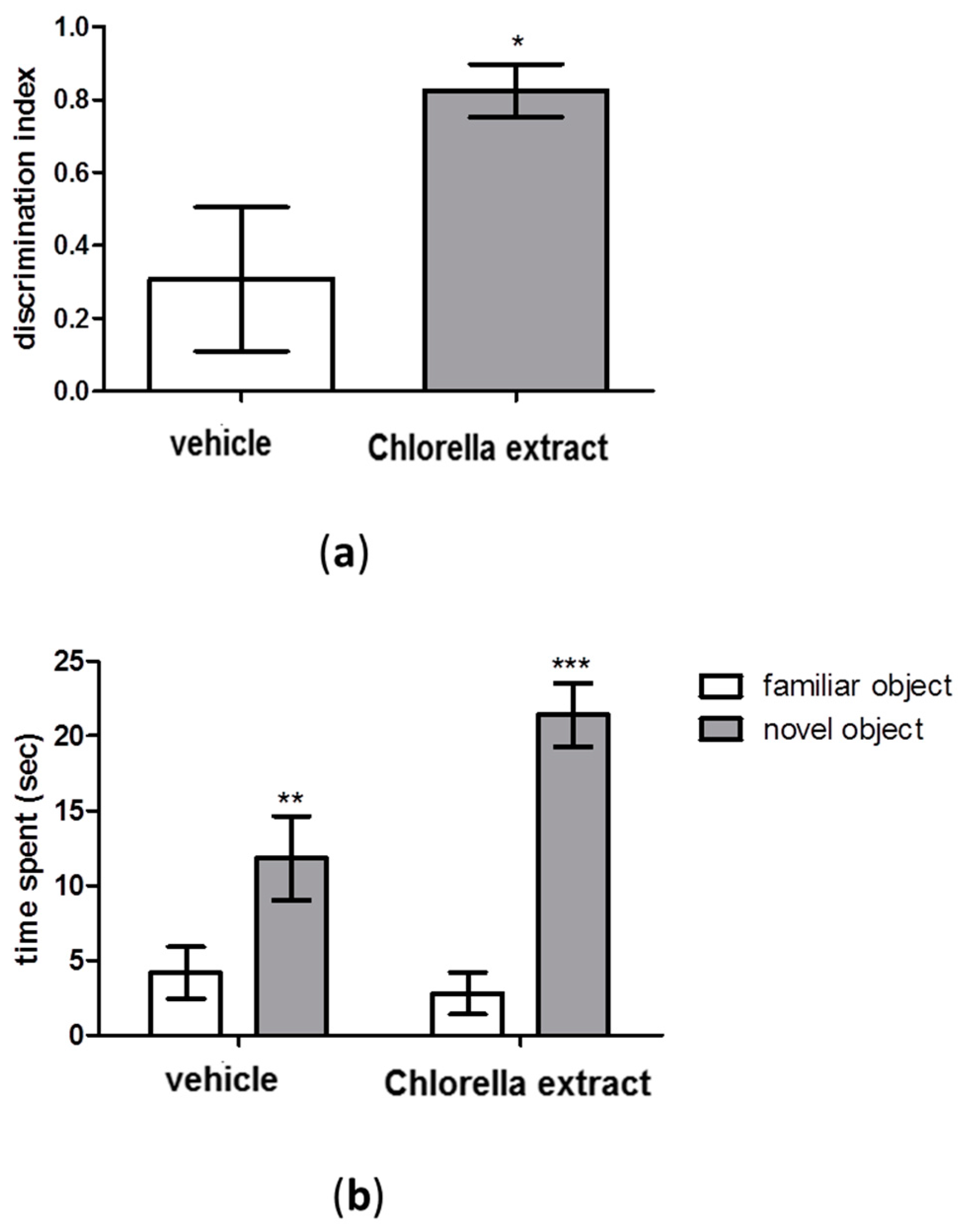
2.2. Effects of Acute Oral Administration of Chlorella Extract on Cognition and Short-Term Memory
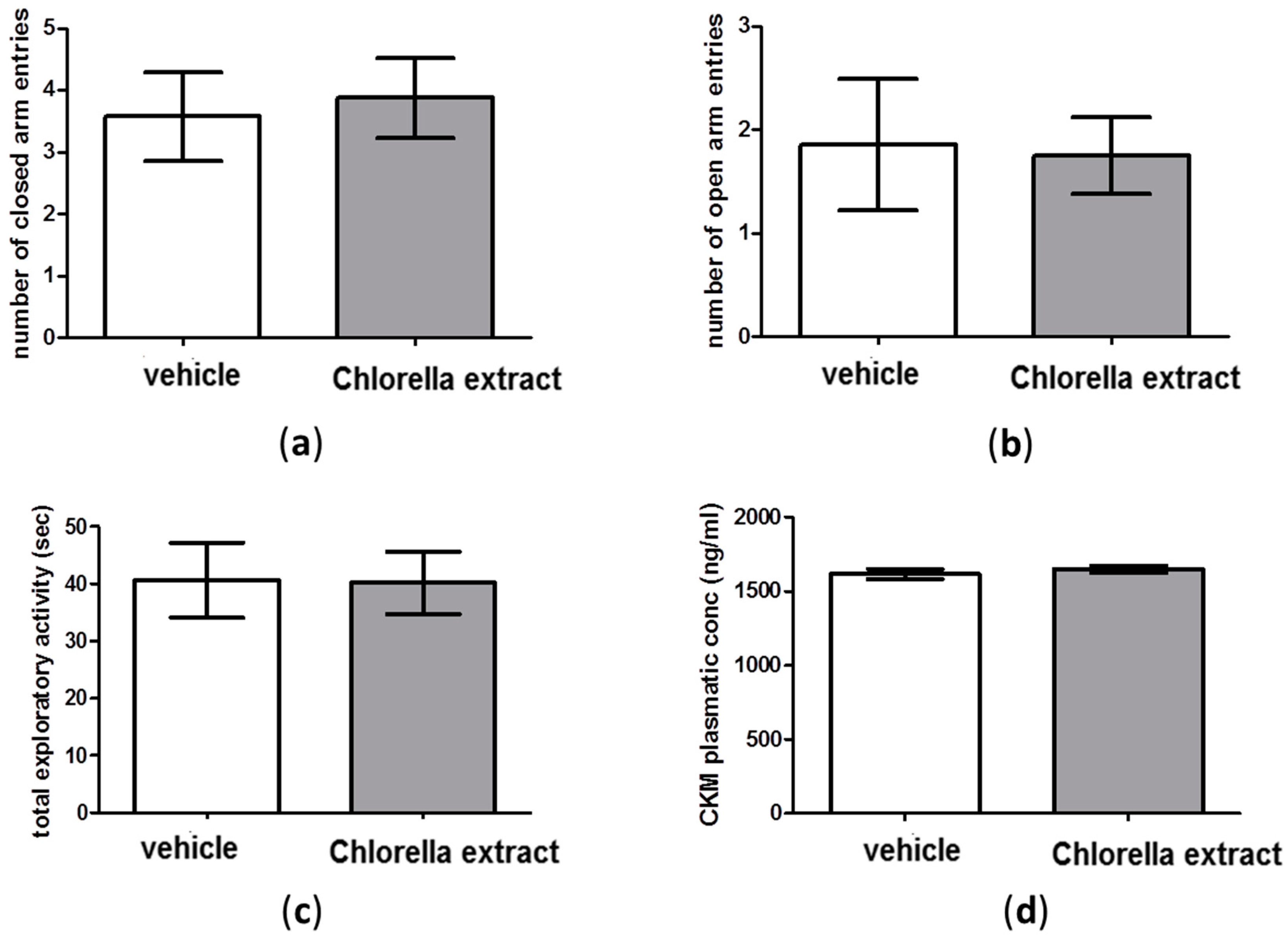
2.3. Effects of Acute Oral Administration of Chlorella Sorokiniana Extract on Locomotion
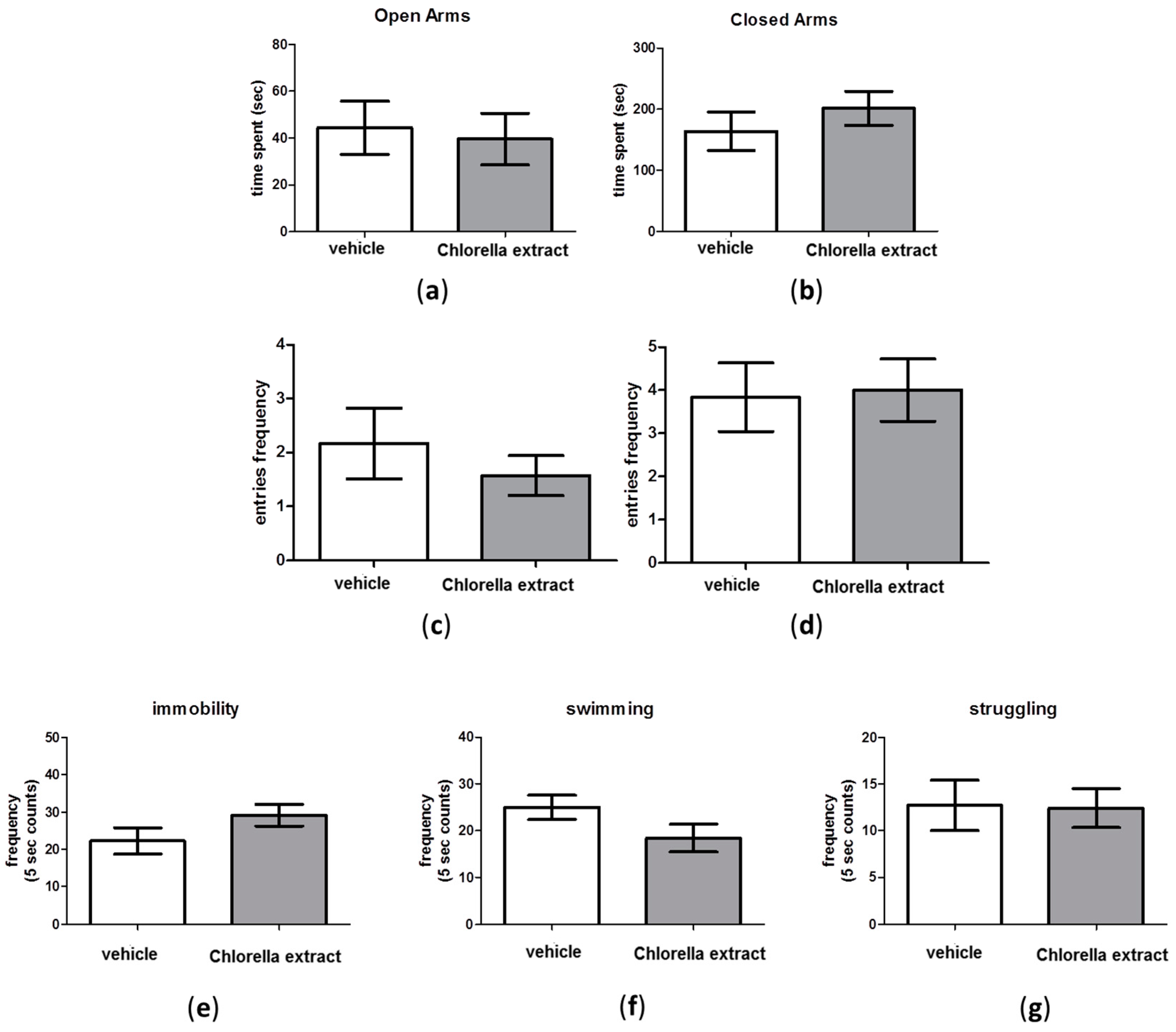
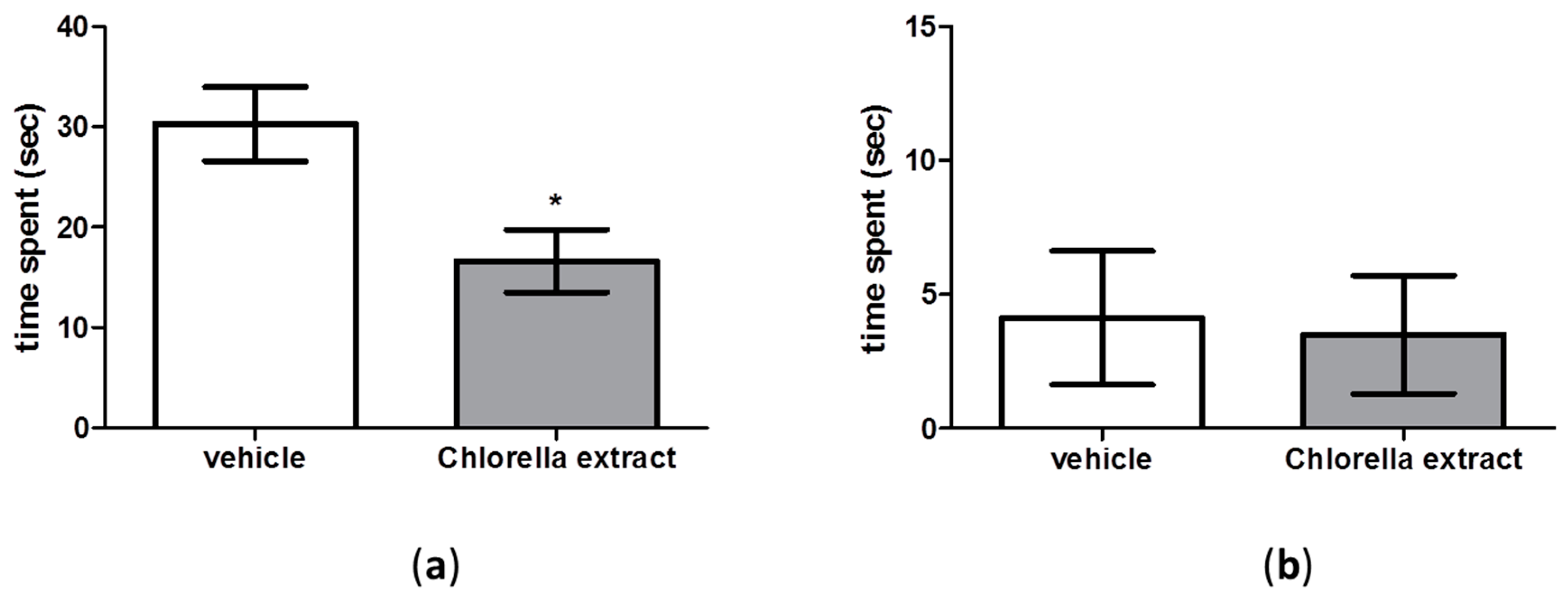
2.4. Effects of Acute Oral Administration of Chlorella sorokiniana Extract on Emotional Profile
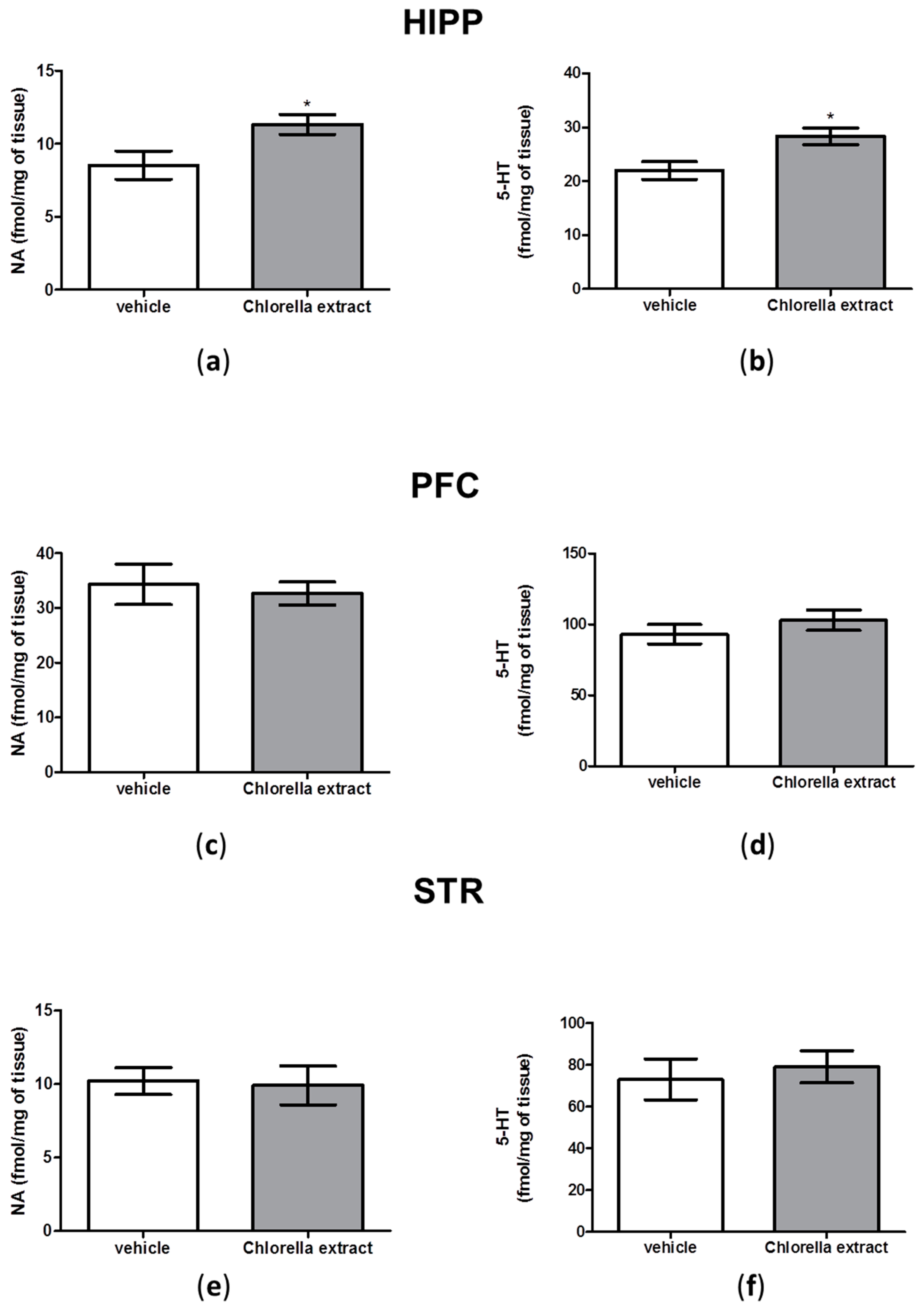
2.5. Effects of Acute Oral Administration of Chlorella sorokiniana Extract on Central Monoamine Content
2.6. Effects of Acute Oral Administration of Chlorella sorokiniana Exctract on Social Behaviour
3. Discussion
4. Experimental Section
4.1. Microalgae Cultivation
4.2. Extract and PUFA Characterization
4.3. Animals and Treatment
4.4. NOR Test
4.5. FST
4.6. Social Interaction Test
4.7. EPM Test
4.8. Post-Mortem Tissue Analysis
4.8.1. Monoamine Quantification
4.8.2. Measurement of Plasma Levels of Creatine-Kinase M-Type
4.9. Blindness of the Study
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Camargo Adel, P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibanez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade approach of red macroalgae gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Anjum, F.M.; Sohaib, M.; Sameen, A. Tackling metabolic syndrome by functional foods. Rev. Endocr. Metab. Disord. 2013, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, M.; Lindstrom, M.E.; Li, J. Fatty acid and lipid profiles with emphasis on n-3 fatty acids and phospholipids from ciona intestinalis. Lipids 2015, 50, 1009–1027. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Fei, C.; Song, J.; Bao, Y.; He, M.; Wang, C. Combining and comparing coalescent, distance and character-based approaches for barcoding microalgaes: A test with Chlorella-like species (chlorophyta). PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Wang, J.; Miao, C.; Zheng, Y.; Chen, S. Two-step in situ biodiesel production from microalgae with high free fatty acid content. Bioresour. Technol. 2013, 136, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. ω-3 Fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of epa and dha. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Innis, S.M.; Jacobson, K. Dietary lipids in early development and intestinal inflammatory disease. Nutr. Rev. 2007, 65, S188–S193. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Bazinet, R.P. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Investig. Drugs 2008, 9, 735–743. [Google Scholar] [PubMed]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Gregoire, S.; Bretillon, L.; Laye, S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M. Essential fatty acids, dha and human brain. Indian J. Pediatr. 2005, 72, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional aspects of depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Morgese, M.G.; Tucci, P.; Mhillaj, E.; Bove, M.; Schiavone, S.; Trabace, L.; Cuomo, V. Lifelong nutritional ω-3 deficiency evokes depressive-like state through soluble β amyloid. Mol. Neurobiol. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, I.G.; Bird, S.S.; Marur, V.R.; Baranov, S.V.; Greenberg, H.K.; Porter, C.L.; Kristal, B.S. Dietary ω-3 fatty acids do not change resistance of rat brain or liver mitochondria to Ca2+ and/or prooxidants. J. Lipids 2012, 2012, 797105. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Harada, T.; Kami, H.; Yano, T.; Imada, K.; Mizuguchi, K. Effects of eicosapentaenoic acid on synaptic plasticity, fatty acid profile and phosphoinositide 3-kinase signaling in rat hippocampus and differentiated PC12 cells. J. Nutr. Biochem. 2010, 21, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; Schultzberg, M.; Basun, H.; Cederholm, T.; et al. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s disease patients during oral ω-3 fatty acid supplementation: The omegad study. J. Alzheimers Dis. 2015, 48, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Vedin, I.; Hjorth, E.; Basun, H.; Faxen Irving, G.; Schultzberg, M.; Eriksdotter, M.; Palmblad, J.; Vessby, B.; Wahlund, L.O.; et al. Effects of supplementation with ω-3 fatty acids on oxidative stress and inflammation in patients with alzheimer's disease: The omegad study. J. Alzheimers Dis. 2014, 42, 823–831. [Google Scholar] [PubMed]
- Sullivan, E.L.; Riper, K.M.; Lockard, R.; Valleau, J.C. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm Behav. 2015, 76, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.; Motasaddi Zarandy, M.; Hassanzadeh, G.; Shidfar, F.; Jalaie, S.; Rahimi, V. The effect of low ω-3/ω-6 ratio on auditory nerve conduction in rat pups. Acta Med. Iran. 2015, 53, 346–350. [Google Scholar] [PubMed]
- Chou, Y.C.; Prakash, E.; Huang, C.F.; Lien, T.W.; Chen, X.; Su, I.J.; Chao, Y.S.; Hsieh, H.P.; Hsu, J.T. Bioassay-guided purification and identification of PPARα/γ agonists from Chlorella sorokiniana. Phytother. Res. 2008, 22, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.E.; Yoo, K.H.; Seo, W.H.; Won, N.H.; Hong, Y.S.; Lee, J.W. Acute tubulointerstitial nephritis following ingestion of chlorella tablets. Pediatr. Nephrol. 2007, 22, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Takehara, I.; Masuzawa, T.; Saito, T.; Naoki, Y. Nutrigenomic studies of effects of chlorella on subjects with high-risk factors for lifestyle-related disease. J. Med. Food 2008, 11, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Singh, H.; Ramaswamy, S. Chlorella-induced psychosis. Psychosomatics 2013, 54, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Parsaeimehr, A.; Sun, Z.; Dou, X.; Chen, Y.F. Simultaneous improvement in production of microalgal biodiesel and high-value α-linolenic acid by a single regulator acetylcholine. Biotechnol. Biofuels 2015, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Ngangkham, M.; Ratha, S.K.; Prasanna, R.; Saxena, A.K.; Dhar, D.W.; Sarika, C.; Prasad, R.B. Biochemical modulation of growth, lipid quality and productivity in mixotrophic cultures of Chlorella sorokiniana. Springerplus 2012, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Chang, H.Y. Lipid production of microalga Chlorella sorokiniana cy1 is improved by light source arrangement, bioreactor operation mode and deep-sea water supplements. Biotechnol. J. 2016, 11, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P.; Mhillaj, E.; Morgese, M.G.; Colaianna, M.; Zotti, M.; Schiavone, S.; Cicerale, M.; Trezza, V.; Campolongo, P.; Cuomo, V.; et al. Memantine prevents memory consolidation failure induced by soluble α amyloid in rats. Front. Behav. Neurosci. 2014, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Xuan, A.G.; Chen, Y.; Long, D.H.; Zhang, M.; Ji, W.D.; Zhang, W.J.; Liu, J.H.; Hong, L.P.; He, X.S.; Chen, W.L. PPARα agonist fenofibrate ameliorates learning and memory deficits in rats following global cerebral ischemia. Mol. Neurobiol. 2015, 52, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kariharan, T.; Nanayakkara, G.; Parameshwaran, K.; Bagasrawala, I.; Ahuja, M.; Abdel-Rahman, E.; Amin, A.T.; Dhanasekaran, M.; Suppiramaniam, V.; Amin, R.H. Central activation of PPAR-γ ameliorates diabetes induced cognitive dysfunction and improves bdnf expression. Neurobiol. Aging 2015, 36, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, T.; Li, G.; Wu, C.; Wang, C.; Li, L.; Sha, S.; Chen, L.; Liu, G.; Chen, L. Activation of PPARγ ameliorates spatial cognitive deficits through restoring expression of ampa receptors in seipin knock-out mice. J. Neurosci. 2016, 36, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. α-Linolenic acid: An ω-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? Biomed. Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef] [PubMed]
- Basiouni, S.; Stockel, K.; Fuhrmann, H.; Schumann, J. Polyunsaturated fatty acid supplements modulate mast cell membrane microdomain composition. Cell. Immunol. 2012, 275, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Besshoh, S.; Chen, S.; Brown, I.R.; Gurd, J.W. Developmental changes in the association of nmda receptors with lipid rafts. J. Neurosci. Res. 2007, 85, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.M.; Rabin, S.J.; Lipsky, R.H.; Mocchetti, I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-d-aspartate. J. Biol. Chem. 1998, 273, 29394–29399. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N.; Widmann, C.; Lazdunski, M.; Heurteaux, C. Activation of the nuclear factor-kappab is a key event in brain tolerance. J. Neurosci. 2001, 21, 4668–4677. [Google Scholar] [PubMed]
- Lipsky, R.H.; Xu, K.; Zhu, D.; Kelly, C.; Terhakopian, A.; Novelli, A.; Marini, A.M. Nuclear factor kappab is a critical determinant in N-methyl-d-aspartate receptor-mediated neuroprotection. J. Neurochem. 2001, 78, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tian, F.; Mearow, K.; Okagaki, P.; Lipsky, R.H.; Marini, A.M. The excitoprotective effect of N-methyl-d-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J. Neurochem. 2005, 94, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Sopian, N.F.; Ajat, M.; Shafie, N.I.; Noor, M.H.; Ebrahimi, M.; Rajion, M.A.; Meng, G.Y.; Ahmad, H. Does short-term dietary ω-3 fatty acid supplementation influence brain hippocampus gene expression of zinc transporter-3? Int. J. Mol. Sci. 2015, 16, 15800–15810. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, C.; Jeannotte, A.; Sidhu, A. Attenuation of the norepinephrine transporter activity and trafficking via interactions with α-synuclein. Eur. J. Neurosci. 2006, 24, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Jeannotte, A.M.; Sidhu, A. Regulation of the norepinephrine transporter by α-synuclein-mediated interactions with microtubules. Eur. J. Neurosci. 2007, 26, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C. Synergistic benefits of serotonin and noradrenaline reuptake inhibition. Depress. Anxiety 1998, 7 (Suppl. 1), 5–6. [Google Scholar] [CrossRef]
- Smythies, J. Section III. The norepinephrine system. Int. Rev. Neurobiol. 2005, 64, 173–211. [Google Scholar] [PubMed]
- Pittaluga, A.; Raiteri, M. N-methyl-d-aspartic acid (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. I. Location on axon terminals and pharmacological characterization. J. Pharmacol. Exp. Ther. 1992, 260, 232–237. [Google Scholar] [PubMed]
- Zimmer, L.; Delpal, S.; Guilloteau, D.; Aioun, J.; Durand, G.; Chalon, S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci. Lett. 2000, 284, 25–28. [Google Scholar] [CrossRef]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Munchow, A.H.; Rios, L.M.; Zhang, G.; Asgeirsdottir, H.N.; Stackman, R.W., Jr. The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. 2013, 23, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Takano, M. Vasculitis of the retina. It’s treatment and light-coagulation for the hemorrhage of retinal vein obstruction. Nihon Ganka Kiyo 1971, 22, 889–897. [Google Scholar] [PubMed]
- O’Dell, T.J.; Connor, S.A.; Guglietta, R.; Nguyen, P.V. β-adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 2015, 22, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Hagena, H.; Hansen, N.; Manahan-Vaughan, D. β-adrenergic control of hippocampal function: Subserving the choreography of synaptic information storage and memory. Cereb. Cortex 2016, 26, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Stanton, P.K.; Sarvey, J.M. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J. Neurosci. 1985, 5, 2169–2176. [Google Scholar] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Glikmann-Johnston, Y.; Saling, M.M.; Reutens, D.C.; Stout, J.C. Hippocampal 5-HT1A receptor and spatial learning and memory. Front. Pharmacol. 2015, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARγ as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Neyrinck, A.M.; Vlaeminck, B.; Fievez, V.; Cani, P.D.; Delzenne, N.M. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS ONE 2014, 9, e87560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Badeli, Z.; Sahebkar, A. A randomized controlled trial of 6-week chlorella vulgaris supplementation in patients with major depressive disorder. Complement. Ther. Med. 2015, 23, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl.) 1995, 121, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Cambon, K.; Dos-Santos Coura, R.; Groc, L.; Carbon, A.; Weissmann, D.; Changeux, J.P.; Pujol, J.F.; Granon, S. Aggressive behavior during social interaction in mice is controlled by the modulation of tyrosine hydroxylase expression in the prefrontal cortex. Neuroscience 2010, 171, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Tonissaar, M.; Philips, M.A.; Eller, M.; Harro, J. Sociability trait and serotonin metabolism in the rat social interaction test. Neurosci. Lett. 2004, 367, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Duxon, M.S.; Starr, K.R.; Upton, N. Latency to paroxetine-induced anxiolysis in the rat is reduced by co-administration of the 5-HT1A receptor antagonist way100635. Br. J. Pharmacol. 2000, 130, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.J.; Cheeta, S.; File, S.E. Anxiogenic effects of nicotine in the dorsal hippocampus are mediated by 5-HT1A and not by muscarinic m1 receptors. Neuropharmacology 2000, 39, 300–307. [Google Scholar] [CrossRef]
- File, S.E.; Zangrossi, H., Jr.; Andrews, N. Social interaction and elevated plus-maze tests: Changes in release and uptake of 5-HT and GABA. Neuropharmacology 1993, 32, 217–221. [Google Scholar] [CrossRef]
- Francavilla, M.; Trotta, P.; Luque, R. Phytosterols from dunaliella tertiolecta and dunaliella salina: A potentially novel industrial application. Bioresour. Technol. 2010, 101, 4144–4150. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Colaianna, M.; Zotti, M.; Morgese, M.G.; Trotta, P.; Tucci, P.; Schiavone, S.; Cuomo, V.; Trabace, L. Extraction, characterization and in vivo neuromodulatory activity of phytosterols from microalga dunaliella tertiolecta. Curr. Med. Chem. 2012, 19, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Giustino, A.; Beckett, S.; Ballard, T.; Cuomo, V.; Marsden, C.A. Perinatal cocaine reduces responsiveness to cocaine and causes alterations in exploratory behavior and visual discrimination in young-adult rats. Brain Res. 1996, 728, 149–156. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Bartolini, L.; Kopf, S.R.; Pepeu, G. Acetylcholine release from the frontal cortex during exploratory activity. Brain Res. 1998, 784, 218–227. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- File, S.E. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods 1980, 2, 219–238. [Google Scholar] [CrossRef]
- Tucci, P.; Morgese, M.G.; Colaianna, M.; Zotti, M.; Schiavone, S.; Cuomo, V.; Trabace, L. Neurochemical consequence of steroid abuse: Stanozolol-induced monoaminergic changes. Steroids 2012, 77, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Sample Availability: Not available.
| Compound | UF (mg·g−1 TL) |
|---|---|
| 10-Heneicosene (c,t) | |
| 2-Dexyl-1-decanol | 3.24 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol acetate (isomer) | 0.43 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol acetate (isomer) | 0.22 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol acetate (isomer) | 0.32 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 64.26 |
| Squalene | 1.23 |
| Not Identified | 8.55 |
| α-Tocopherol | 2.72 |
| (22E)-Ergosta-5,7,9(11),22-tetraen-3-ol | 6.23 |
| (3β,22E)-Ergosta-5,8,22-trien-3-ol | 9.16 |
| Ergosterol | 43.36 |
| (3β)-Ergosta-5,8-dien-3-ol | 7.12 |
| (3β,5α)-Ergost-7-en-3-ol | 21.56 |
| Compound | FAMEs (mg·g−1 TL) |
|---|---|
| Omega 3 | 169.53 |
| (Z,Z,Z)-7,10,13-Hexadecatrienoic acid methyl ester | 69.24 |
| α-Linolenic acid methyl ester | 100.29 |
| Omega 6 | 116.66 |
| (Z,Z)-7,10-Hexadecadienoic acid methyl ester | 35.56 |
| (Z,Z)-9,12-Heptadecadienoic acid methyl ester | 2.06 |
| Linoleic acid methyl ester | 79.04 |
| Saturated | 58.60 |
| Dodecanoic acid methyl ester | 0.02 |
| Tridecanoic acid methyl ester | 0.02 |
| Tetradecanoic acid methyl ester | 1.27 |
| Methyl 13-methyltetradecanoate | 0.35 |
| Pentadecanoic acid methyl ester | 0.60 |
| Hexadecanoic acid methyl ester | 43.88 |
| 15-Methylhexadecanoic acid methyl ester | 1.68 |
| Heptadecanoic acid methyl ester | 1.84 |
| Methyl stearate | 6.17 |
| Eicosanoic acid methyl ester | 1.53 |
| Heneicosanoic acid methyl ester | 0.16 |
| Docosanoic acid methyl ester | 0.36 |
| Tricosanoic acid methyl ester | 0.07 |
| Tetracosanoic acid methyl ester | 0.15 |
| Pentacosanoic acid methyl ester | 0.12 |
| Hexacosanoic acid methyl ester | 0.38 |
| Monounsaturated | 54.29 |
| Methyl myristoleate | 0.25 |
| cis-7-Tetradecenoic acid methyl ester | 0.72 |
| (Z-13-Methyltetradec-9-enoic acid methyl ester | 0.37 |
| Methyl palmitoleate | 20.50 |
| (Z)-7-Hexadecenoic acid methyl ester | 2.03 |
| (E)-9-Hexadecenoic acid methyl ester | 2.88 |
| (Z)-9-Heptadecenoic acid methyl ester | 2.95 |
| Oleic acid methyl ester | 24.47 |
| Methyl 9-eicosenoate | 0.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgese, M.G.; Mhillaj, E.; Francavilla, M.; Bove, M.; Morgano, L.; Tucci, P.; Trabace, L.; Schiavone, S. Chlorella sorokiniana Extract Improves Short-Term Memory in Rats. Molecules 2016, 21, 1311. https://doi.org/10.3390/molecules21101311
Morgese MG, Mhillaj E, Francavilla M, Bove M, Morgano L, Tucci P, Trabace L, Schiavone S. Chlorella sorokiniana Extract Improves Short-Term Memory in Rats. Molecules. 2016; 21(10):1311. https://doi.org/10.3390/molecules21101311
Chicago/Turabian StyleMorgese, Maria Grazia, Emanuela Mhillaj, Matteo Francavilla, Maria Bove, Lucia Morgano, Paolo Tucci, Luigia Trabace, and Stefania Schiavone. 2016. "Chlorella sorokiniana Extract Improves Short-Term Memory in Rats" Molecules 21, no. 10: 1311. https://doi.org/10.3390/molecules21101311






