Catalytic Synthesis of a New Series of Alkyl Uronates and Evaluation of Their Physicochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
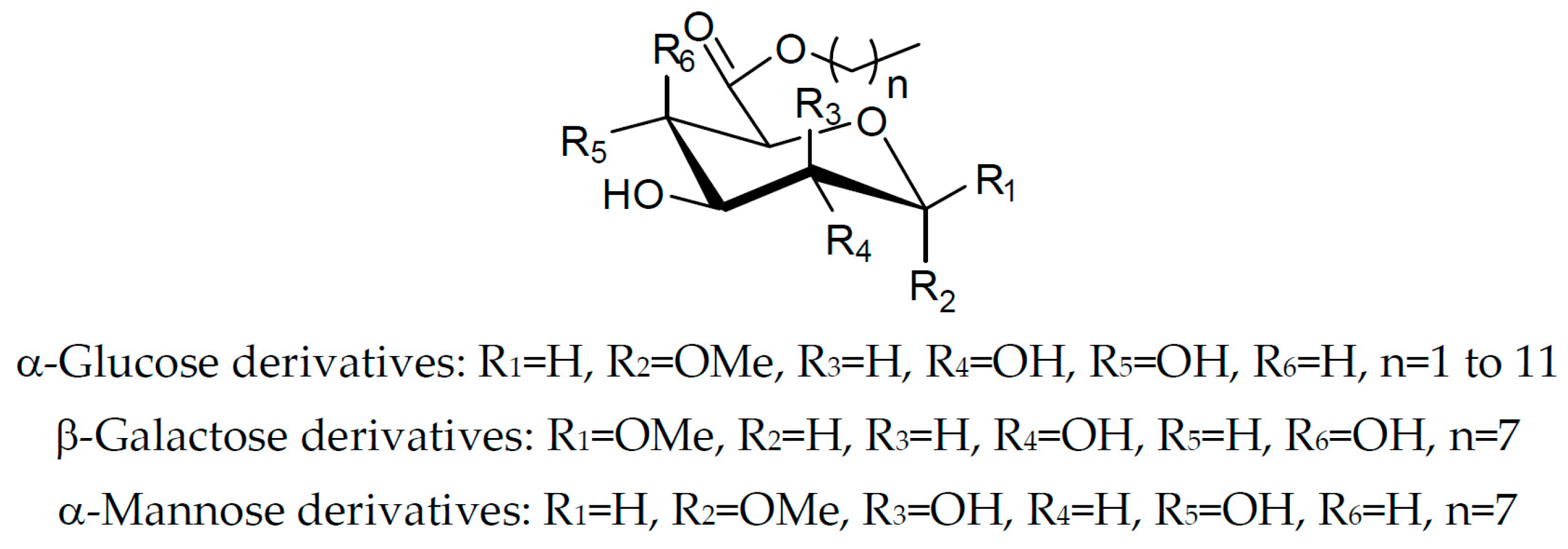
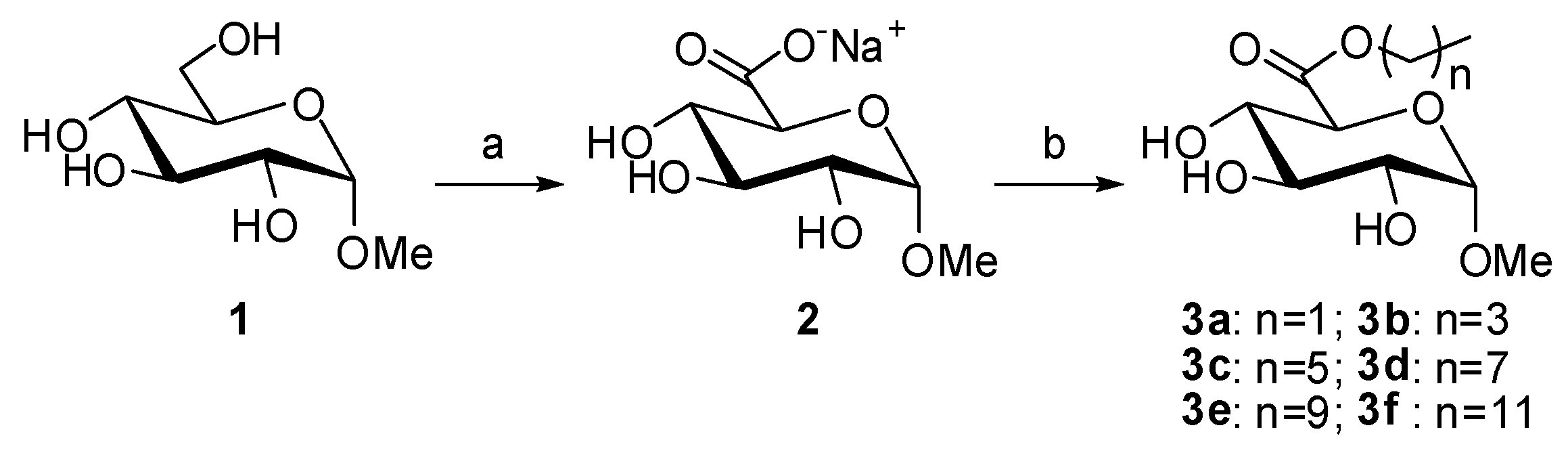
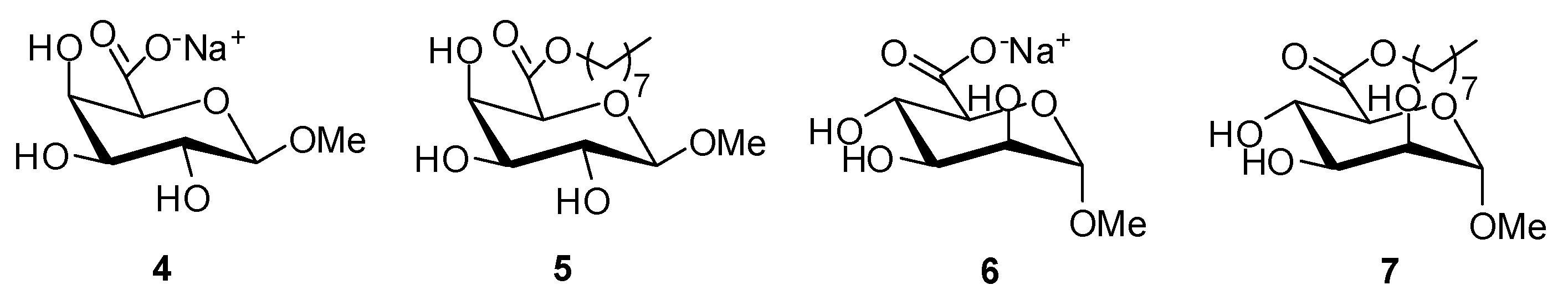
2.1. Synthesis
2.2. Aqueous Solubility
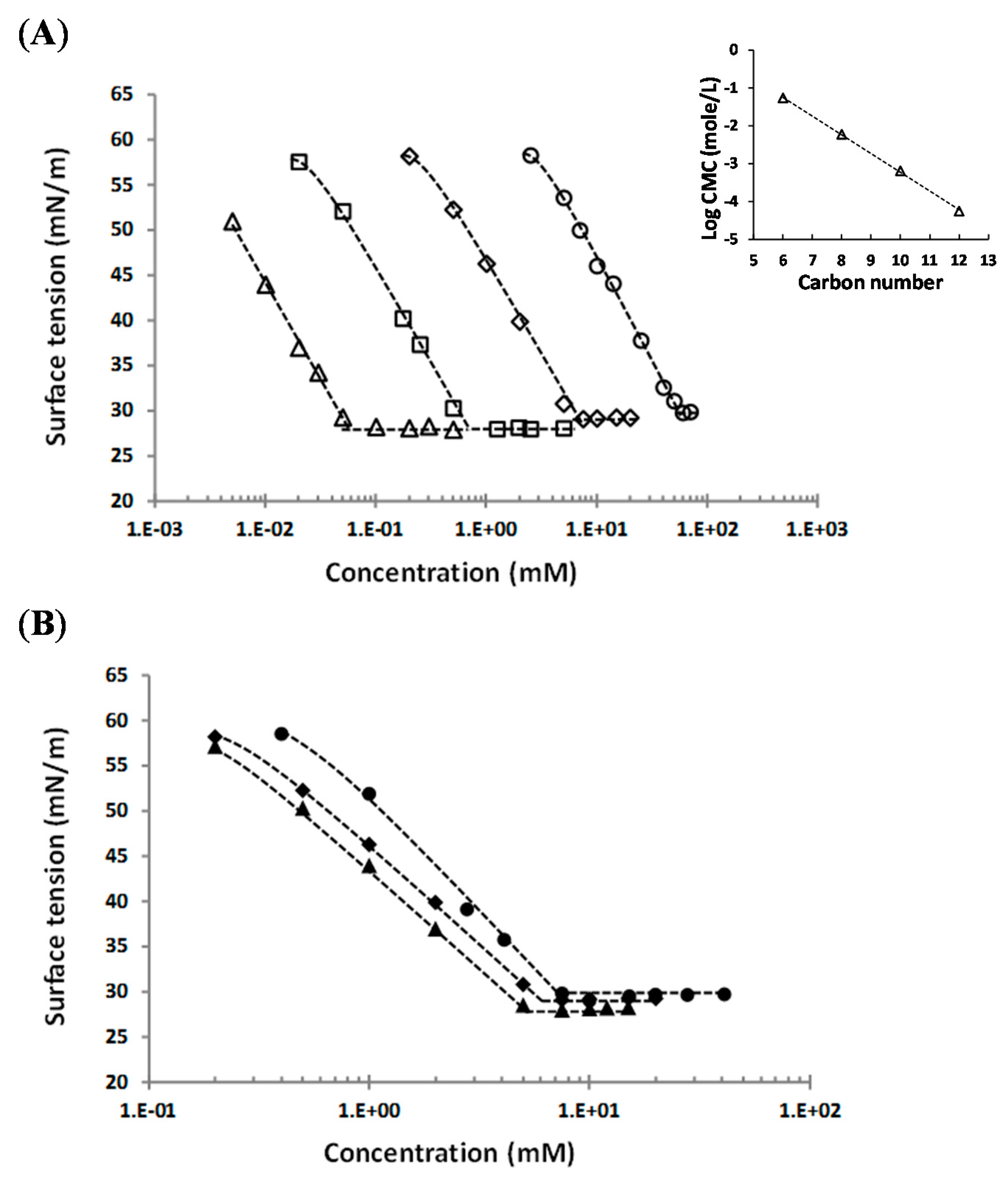
2.3. Surface Activity
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Oxidation
3.3. General Procedure for the Esterification
3.4. Compound Characterization Data
3.5. Determination of Aqueous Solubility
3.6. Determination of the Surface Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Von Rybinski, W.; Hill, K. Alkyl polyglycosides—Properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Ruiz, C.C. Sugar-Based Surfactants: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2008; pp. 245–306. [Google Scholar]
- Garcia, M.T.; Ribosa, L.; Campos, E.; Leal, J.S. Ecological properties of alkylglucosides. Chemosphere 1997, 35, 545–556. [Google Scholar]
- Stubenrauch, C. Sugar surfactants—Aggregation, interfacial, and adsorption phenomena. Curr. Opin. Colloid Int. Sci. 2001, 6, 160–170. [Google Scholar] [CrossRef]
- Matsumura, S.; Imai, K.; Yoshikawa, S.; Kawada, K.; Uchibor, T. Structural activities, biodegradability and antimicrobial properties of n-alkyl glucosides, mannosides and galactosides. J. Am. Oil Chem. Soc. 1990, 67, 996–1001. [Google Scholar] [CrossRef]
- Hill, K.; Rhode, O. Sugar-based surfactants for consumer products and technical applications. Fett-Lipid 1999, 101, 25–33. [Google Scholar] [CrossRef]
- Baker, I.J.A.; Matthews, B.; Suares, H.; Krodkiewska, I.; Furlong, D.N.; Grieser, F.; Drummond, C.J. Sugar fatty acid ester surfactants: structure and ultimate aerobic biodegradability. J. Surf. Det. 2000, 3, 1–11. [Google Scholar] [CrossRef]
- Ducret, A.; Giroux, A.; Trani, M.; Lortie, R. Enzymatic preparation of biosurfactants from sugars or sugar alcohols and fatty acids in organic media under reduced pressure. Biotechnol. Bioeng. 1995, 48, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Skagerlind, P.; Larsson, K.; Barfoed, M.; Hult, K. Glucoside ester synthesis in microemulsions catalyzed by Candida antartica component B lipase. J. Am. Oil Chem. Soc. 1997, 74, 39–42. [Google Scholar] [CrossRef]
- Blecker, C.; Piccicuto, S.; Lognay, G.; Deroanne, C.; Marlier, M.; Paquot, M. Enzymatically prepared n-alkyl esters of glucuronic acid: The Effect of hydrophobic chain length on surface properties. J. Colloid Int. Sci. 2002, 247, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Moreau, B.; Lognay, G.C.; Blecker, C.; Brohée, J.C.; Chéry, F.; Rollin, P.; Paquot, M.; Marlier, M. Synthesis of novel d-glucuronic acid fatty esters using Candida antarctica lipase in tert-butanol. Biotechnol. Lett. 2004, 26, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Fukada, K.; Kawasaki, M.; Seimiya, T.; Abe, Y.; Fujiwara, M.; Ohbu, K. Stereochemical aspects of micellar properties of esterified glucoside surfactants in water: apparent molar volume, adiabatic compressibility, and aggregation number. Colloid Polym. Sci. 2000, 278, 576–580. [Google Scholar] [CrossRef]
- Dupuy, C.; Auvray, X.; Petipas, C.; Rico-Lattes, I.; Lattes, A. Anomeric effects on the structure of micelles of alkyl maltosides in water. Langmuir 1997, 13, 3965–3967. [Google Scholar] [CrossRef]
- Razafindralambo, H.; Blecker, C.; Mezdour, S.; Deroanne, C.; Crowet, J.M.; Brasseur, R.; Lins, L.; Paquot, M. Impacts of the carbonyl group location of ester bond on interfacial properties of sugar-based surfactants: Experimental and computational evidences. J. Phys. Chem. B 2009, 113, 8872–8877. [Google Scholar] [CrossRef] [PubMed]
- Hato, M.; Minamikawa, H.; Tamada, K.; Baba, T.; Tanabe, Y. Self-assembly of synthetic glycolipid/water systems. Adv. Colloid Int. Sci. 1999, 80, 233–270. [Google Scholar] [CrossRef]
- Becerra, N.; Toro, C.; Zanacco, A.L.; Lemp, E.; Gunther, G. Characterization of micelles formed by sucrose 6-O-monoesters. Colloids Surf. A 2008, 327, 134–139. [Google Scholar] [CrossRef]
- Garofalakis, G.; Murray, B.S.; Sarney, D.B. Surface activity and critical aggregation concentration of pure sugar esters with different sugar headgroups. J. Colloid Int. Sci. 2000, 229, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Bhagwat, S.S.; Penfield, K.W.; Aikens, P.; Shah, D.O. On the measurement of critical micelle concentrations of pure and technical-grade nonionic surfactants. J. Surf. Det. 2000, 3, 53–58. [Google Scholar] [CrossRef]
- Wadouachi, A.; Kovensky, J. Synthesis of glycosides of glucuronic, galacturonic and mannuronic acids: An overview. Molecules 2011, 16, 3933–3968. [Google Scholar] [CrossRef]
- Lebedev, O.L.; Khidekel, E.G.; Razuvaev, G.A. Isotopic analysis of nitrogen by electron paramagnetic resonance method. Doklady Akademii Nauk SSR 1961, 140, 1327–1329. [Google Scholar]
- Anelli, P. L.; Biffi, C.; Montanari, F.; Quici, S. Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 1987, 52, 2559–2562. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Mano, E.; Song, Z.; Tschaen, D.M.; Grabowski, E.J.J.; Reider, P.J. Oxidation of primary alcohols to carboxylic acids with sodium chlorite catalyzed by TEMPO and bleach. J. Org. Chem. 1999, 64, 2564–2566. [Google Scholar] [CrossRef]
- Epp, J.B.; Widlanski, T. S. Facile preparation of nucleoside-5′-carboxylic acids. J. Org. Chem. 1999, 64, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Richel, A.; Laurent, P.; Wathelet, B.; Wathelet, J.P.; Paquot, M. Microwave-assisted synthesis of d-glucuronic acid derivatives using cost-effective solid acid catalysts. Tetrahedron Lett. 2010, 51, 1356–1360. [Google Scholar] [CrossRef]
- Abel, S.; Dupradeau, F.Y.; Raman, E.P.; MacKerell, A.D.; Marchi, M. Molecular simulations of dodecyl-β-maltoside micelles in water: Influence of the headgroup conformation and force field parameters. J. Phys. Chem. B 2011, 115, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, L.; Somasundaran, P. Study of mixtures of n-dodecyl-β-d-maltoside with anionic, cationic, and nonionic surfactant in aqueous solutions using surface tension and fluorescence techniques. J. Colloid Interface Sci. 2004, 278, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Drummond, C.J.; Krodkiewska, I.; Grieser, F. How chain length, headgroup polymerization, and anomeric configuration govern the thermotropic and lyotropic liquid crystalline phase behavior and the air−water interfacial adsorption of glucose-based surfactants. Langmuir 2000, 16, 7359–7367. [Google Scholar] [CrossRef]
- Savelli, M.P.; van Roekeghem, P.; Douillet, O.; Cave, G.; Gode, P.; Ronco, G.; Villa, P. Effects of tail alkyl chain length (n), head group structure and junction (Z) on amphiphilic properties of 1-Z-R-d,l-xylitol compounds (R = CnH2n+1). Int. J. Pharm. 1999, 182, 221–236. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Jiao, J.; Zhang, H.; Wang, R.; Chen, H. Aggregation behavior of COOH-functionalized imidazolium-based surface active ionic liquids in aqueous solution. J. Mol. Liq. 2012, 173, 103–107. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004; p. 444. [Google Scholar]
- Schmidt-Lassen, J.; Lindhorst, T.K. Exploring the meaning of sugar configuration in a supramolecular environment: Comparison of six octyl glycoside micelles by ITC and NMR spectroscopy. MedChemComm 2014, 5, 1218–1226. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lin, Y.Y.; Chen, E.M.; Hsu, C.T.; Kwan, C.C. A study of the equilibrium surface tension and the critical micelle concentration of mixed surfactant solutions. Langmuir 1999, 15, 4370–4376. [Google Scholar] [CrossRef]
- Klammt, C.; Schwarz, D.; Lehnet, I.; Sobhanifar, S.; Loehr, F.; Zeelen, J.; Glaubitz, C.; Doetsch, V.; Bernhard, F. Cell-free expression of integral membrane proteins for structural studies. In Cell-Free Protein Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Darmstadt, Germany, 2008; pp. 141–164. [Google Scholar]
- Klammt, C.; Schwarz, D.; Fendler, K.; Haase, W.; Doetsch, V.; Bernhard, F. Evaluation of detergents for the soluble expression of α-helical and β-barrel-type integral membrane proteins by a preparative scale individual cell-free expression system. FEBS J. 2005, 272, 6024–6038. [Google Scholar] [CrossRef] [PubMed]
- Hait, S.K.; Moulik, S.P. Determination of critical micelle concentration (CMC) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. J. Surf. Det. 2001, 4, 303–309. [Google Scholar] [CrossRef]
- Lu, B.; Vayssade, M.; Miao, Y.; Chagnault, V.; Grand, E.; Wadouachi, A.; Postel, D.; Drelich, A.; Egles, C.; Pezron, I. Physico-chemical properties and cytotoxic effects of sugar-based surfactants: Impact of structural variations. Colloid. Surf. B Biointerface. 2016, 145, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, H.; Shinoda, K. Krafft points, critical micelle concentrations, surface tension, and solubilizing power of aqueous solutions of fluorinated surfactants. J. Phys. Chem. 1976, 80, 2468–2470. [Google Scholar] [CrossRef]
- Mazer, N.A.; Benedek, G.B.; Carey, M.C. An investigation of the micellar phase of sodium dodecyl sulfate in aqueous sodium chloride solutions using quasielastic light scattering spectroscopy. J. Phys. Chem. 1976, 80, 1075–1085. [Google Scholar] [CrossRef]
- Manet, S.; Karpichev, Y.; Dedovets, D.; Oda, R. Effect of Hofmeister and alkylcarboxylate anionic counterions on the Krafft temperature and melting temperature of cationic gemini surfactants. Langmuir 2013, 29, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Pestman, J.M.; Terpstra, K.R.; Stuart, M.C.A.; van Doren, H.A.; Brisson, A.; Kellogg, R.M.; Engberts, J.B.F.N. Nonionic bolaamphiphiles and gemini surfactants based on carbohydrates. Langmuir 1997, 13, 6857–6860. [Google Scholar] [CrossRef]
- Matsuki, H.; Ichikawa, R.; Kaneshina, S.; Kamaya, H.; Ueda, I. Differential Scanning Calorimetric Study on the Krafft Phenomenon of Local Anesthetics. J. Colloid Interface Sci. 1996, 181, 362–369. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
 ) and H-6 of methyl α-glucoside (
) and H-6 of methyl α-glucoside (  ) allows the evaluation of the conversion. From (B) to (C), the H-5 signal shifted from 4.1 to 3.9 ppm because of the basic pH (sodium carboxylate instead of carboxylic acid).
) allows the evaluation of the conversion. From (B) to (C), the H-5 signal shifted from 4.1 to 3.9 ppm because of the basic pH (sodium carboxylate instead of carboxylic acid).
 ) and H-6 of methyl α-glucoside (
) and H-6 of methyl α-glucoside (  ) allows the evaluation of the conversion. From (B) to (C), the H-5 signal shifted from 4.1 to 3.9 ppm because of the basic pH (sodium carboxylate instead of carboxylic acid).
) allows the evaluation of the conversion. From (B) to (C), the H-5 signal shifted from 4.1 to 3.9 ppm because of the basic pH (sodium carboxylate instead of carboxylic acid).
| Molecule | Tk a (°C) | [C]max at ≈25 °C (mM) | State |
|---|---|---|---|
| Hexyl (1-α-methyl)glucuronate 3c | undetected | 50–60 | wax |
| Octyl (1-α-methyl)glucuronate 3d | undetected | 15–20 | wax |
| Decyl (1-α-methyl)glucuronate 3e | undetected | 0.5–0.75 | powder |
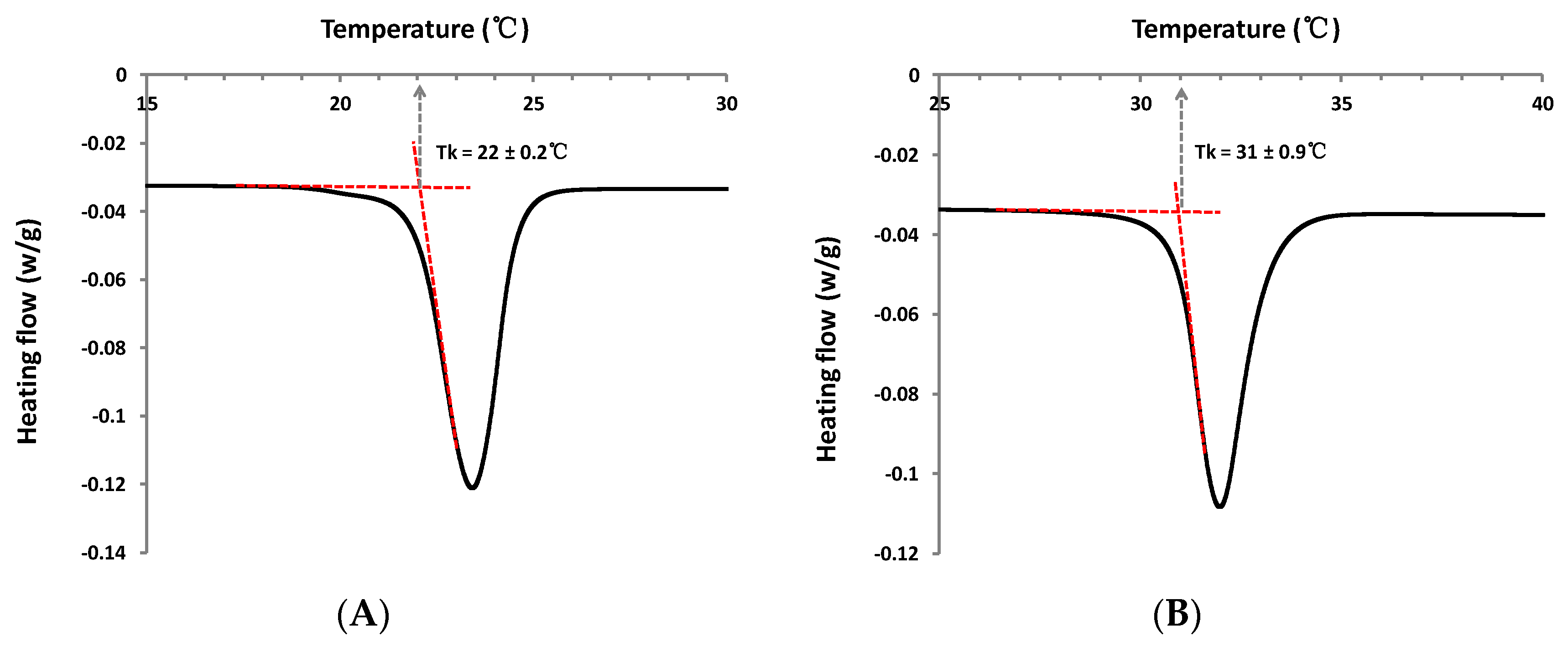
| Dodecyl (1-α-methyl)glucuronate 3f | 22 ± 0.2 | 0.03–0.05 | powder |
| Octyl (1-β-methyl)galacturonate 5 | 31 ± 0.9 | insoluble at ≈ 25 °C; >100 at T > Tk | powder |
| Octyl (1-α-methyl)mannuronate 7 | undetected | 5.0–6.2 | oil |
| Molecule | CMC (mM) | [C]max (mM) | γcmc (mN·m−1) | Amin (Å2/molecule) | T (°C) |
|---|---|---|---|---|---|
| Hexyl (1-α-methyl)glucuronate 3c | 55 ± 5 | 50–60 | 30.8 ± 0.8 | 39.2 ± 2.9 | 25 |
| Octyl (1-α-methyl)glucuronate 3d | 6.0 ± 0.5 | 15–20 | 29.2 ± 0.4 | 41.0 ± 1 | 25 |
| Decyl (1-α-methyl)glucuronate 3e | 0.65 ± 0.05 | 0.5–0.75 | 28.1 ± 0.1 | 42.4 ± 2 | 25 |
| Dodecyl (1-α-methyl)glucuronate 3f | 0.056 ± 0.004 | 0.03–0.05 | 28.3 ± 0.3 | 45.6 ± 5 | 25 |
| Octyl (1-β-methyl)galacturonate 5 | 6.9 ± 0.4 | >100 | 29.4 ± 0.4 | 42.9 ± 3.9 | 40 |
| Octyl (1-α-methyl)mannuronate 7 | 5.5 ± 0.5 | 5.0–6.2 | 28.3 ± 0.3 | 42.8 ± 2 | 25 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Drelich, A.; Omri, M.; Pezron, I.; Wadouachi, A.; Pourceau, G. Catalytic Synthesis of a New Series of Alkyl Uronates and Evaluation of Their Physicochemical Properties. Molecules 2016, 21, 1301. https://doi.org/10.3390/molecules21101301
Lu H, Drelich A, Omri M, Pezron I, Wadouachi A, Pourceau G. Catalytic Synthesis of a New Series of Alkyl Uronates and Evaluation of Their Physicochemical Properties. Molecules. 2016; 21(10):1301. https://doi.org/10.3390/molecules21101301
Chicago/Turabian StyleLu, Huiling, Audrey Drelich, Mehdi Omri, Isabelle Pezron, Anne Wadouachi, and Gwladys Pourceau. 2016. "Catalytic Synthesis of a New Series of Alkyl Uronates and Evaluation of Their Physicochemical Properties" Molecules 21, no. 10: 1301. https://doi.org/10.3390/molecules21101301





