Biocatalytic Synthesis of Novel Partial Esters of a Bioactive Dihydroxy 4-Methylcoumarin by Rhizopus oryzae Lipase (ROL)
Abstract
:1. Introduction
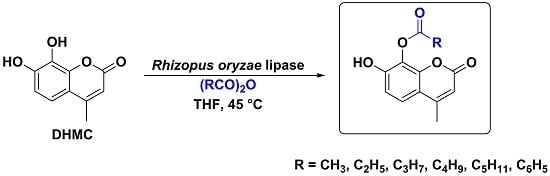
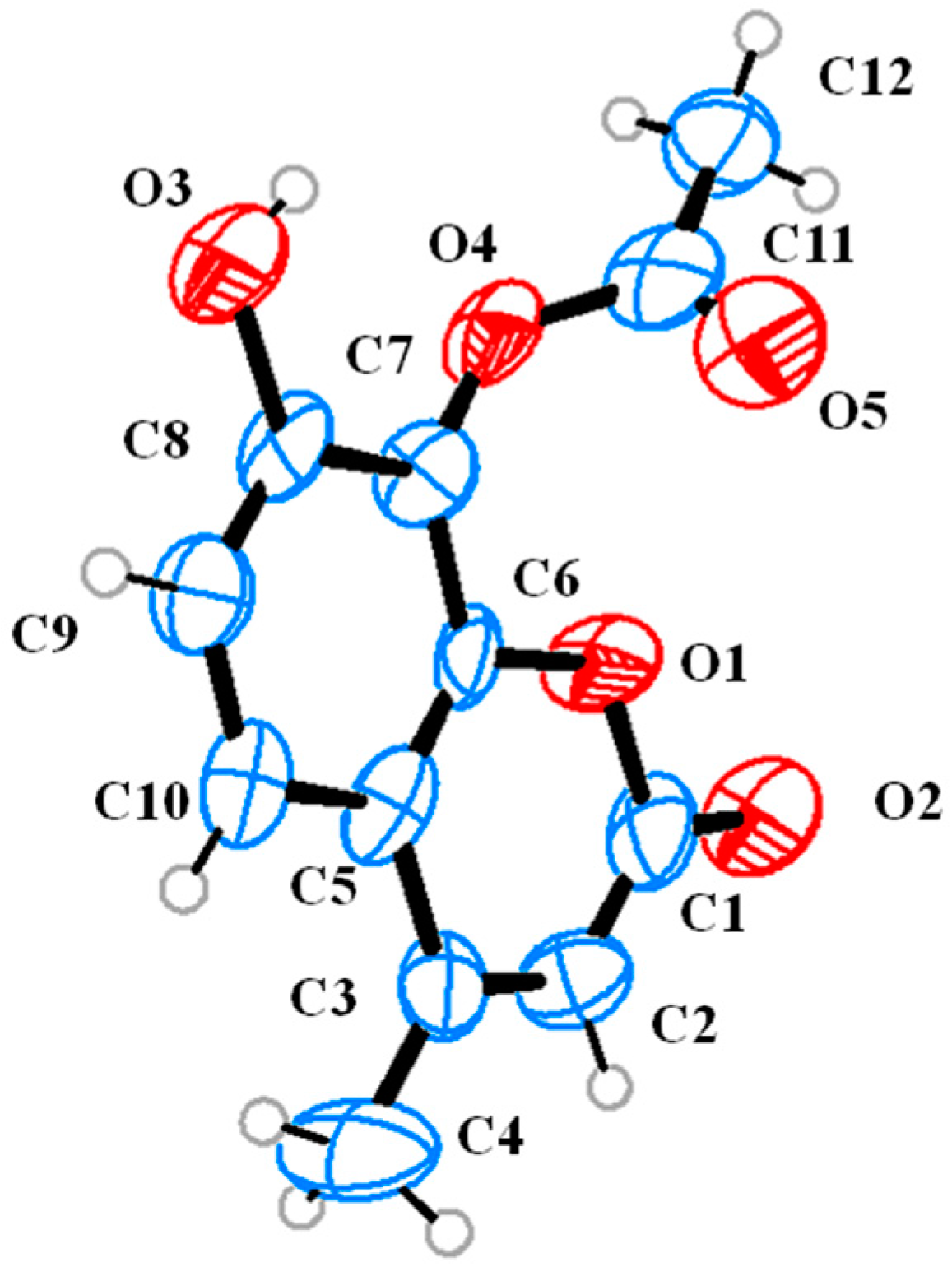
2. Results and Discussion
3. Experimental Section
3.1. Reagents and Solvents
3.2. Lipase Production Conditions
3.3. Determination of Lipase Activity
3.4. Synthesis
Regioselective Rhizopus oryzae Lipase (ROL)-Mediated Acylation Reaction on 7,8-Dihydroxy-4-methylcoumarin (1).
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macáková, K.; Řeháková, Z.; Mladěnka, P.; Karlíčková, J.; Filipský, T.; Říha, M.; Prasad, A.K.; Parmar, V.S.; Jahodář, L.; Pávek, P.; et al. In vitro platelet antiaggregatory properties of 4-methylcoumarins. Biochimie 2012, 12, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; García, A.G.T.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.C.; Tagliapietra, S.; Martina, K.; Palmisano, G.; Cravotto, G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv. 2016, 6, 46394–46405. [Google Scholar] [CrossRef]
- Arya, A.; Kumar, V.; Mathur, D.; Singh, S.; Brahma, R.; Singh, R.; Singh, S.; Sharma, G.L.; Parmar, V.S.; Prasad, A.K. Synthesis of potential bioactive novel 7-[2-hydroxy-3-(1,2,3-triazol-1-yl)propyloxy]-3-alkyl-4-methylcoumarins. J. Heterocycl. Chem. 2015, 52. [Google Scholar] [CrossRef]
- Mathur, D.; Prasad, A.K.; Cameron, T.S.; Jha, A. Novel approach to 3,3-dimethyl-4-morpholino-3,4-dihydrocoumarins via hetero-Diels-Alder reaction. Tetrahedron 2014, 70, 5608–5618. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, A.; Manral, S.; Sinha, R.; Arora, S.; Sharma, A.; Goel, S.; Kalra, N.; Chatterji, S.; Dwarakanath, B.S.; et al. Calreticulin transacetylase mediated upregulation of thioredoxin by 7,8-diacetoxy-4-methylcoumarin enhances the antioxidant potential and the expression of vascular endothelial growth factor in peripheral blood mononuclear cells. Chem. Biol. Interact. 2013, 206, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, B.K.; Sharma, N.K.; Gyanda, K.; Jain, S.K.; Tyagi, Y.K.; Baghel, A.S.; Pandey, M.; Sharma, S.K.; Prasad, A.K.; et al. Specificities of acetoxy derivatives of coumarins, biscoumarins, chromones, flavones, isoflavones and xanthones for acetoxy drug: Protein transacetylase. Eur. J. Med. Chem. 2006, 42, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, G.; Rani, U.; Parmar, V.S. Coumarins from Fraxinus floribunda leaves. Phytochemistry 1980, 19, 2494–2495. [Google Scholar] [CrossRef]
- Khurana, S.K.; Krishnamoorthy, V.; Parmar, V.S.; Sanduja, R.; Chawla, H.L. 3,4,7-Trimethylcoumarin from Trigonella foenum-graecum stems. Phytochemistry 1982, 21, 2145–2146. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jha, H.N.; Sanduja, S.K.; Sanduja, R. Trigocoumarin—A new coumarin from Trigonella foenumgraecum. Z. Naturforsch 1981, 37B, 521–523. [Google Scholar] [CrossRef]
- Parmar, V.S.; Rathore, J.S.; Singh, S.; Jain, A.K.; Gupta, S.R. Troupin, a 4-methylcoumarin from Tamarix troupii. Phytochemistry 1985, 24, 871–872. [Google Scholar] [CrossRef]
- Allis, C.D.; Chicoine, L.G.; Richman, R.; Schulman, G. Deposition related histone acetylation in micronuclei of conjugation tetrahymena. Proc. Natl. Acad. Sci. USA 1985, 82, 8048–8052. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. (New Biol.) 1971, 231, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Raj, H.G.; Parmar, V.S.; Jain, S.C.; Kohli, E.; Tyagi, Y.K.; Wengel, J.; Olsen, C.E. Assay and characterization of 7,8-diacetoxy-4-methylcoumarin-protein transacetylase from rat liver microsomes based on the irreversible inhibition of cytosolic Glutathione S-transferase. Bioorg. Med. Chem. 2000, 7, 1707–1712. [Google Scholar] [CrossRef]
- Singh, I.; Kohli, E.; Raj, H.G.; Gyanda, K.; Jain, S.K.; Tyagi, Y.K.; Gupta, G.; Kumari, R.; Kumar, A.; Pal, G.; et al. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part 9: Comparison of acetoxy 4-methylcoumarins and other polyphenolic acetates reveal the specificity to acetoxy drug: Protein transacetylase for pyran carbonyl group in proximity to the oxygen heteroatom. Bioorg. Med. Chem. 2002, 10, 4103–4111. [Google Scholar] [PubMed]
- Khurana, P.; Kumari, R.; Vohra, P.; Kumar, A.; Seema; Gupta, G.; Raj, H.G.; Dwarakanath, B.S.; Parmar, V.S.; Saluja, D.; et al. Acetoxy drug: Protein transacetylase catalyzed activation of human platelet nitric oxide synthase by polyphenolic peracetates. Bioorg. Med. Chem. 2006, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Raj, H.G.; Parmar, V.S.; Jain, S.C.; Goel, S.; Singh, A.; Tyagi, Y.K.; Jha, H.N.; Olsen, C.E.; Wengel, J. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part 4. Hyperbolic activation of rat liver microsomal NADPH cytochrome C reductase by the novel acetylator 7,8-diacetoxy-4-methylcoumarin. Bioorg. Med. Chem. 1999, 7, 369–373. [Google Scholar] [CrossRef]
- Raj, H.G.; Parmar, V.S.; Jain, S.C.; Goel, S.; Singh, A.; Gupta, K.; Rohil, V.; Tyagi, Y.K.; Jha, H.N.; Olsen, C.E.; et al. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-one. Part II: Mechanism-based inhibition of rat liver microsome-mediated aflatoxin B1-DNA binding by the candidate antimutagen 7,8-diacetoxy-4-methylcoumarin. Bioorg. Med. Chem. 1998, 6, 1895–1904. [Google Scholar] [CrossRef]
- Baghela, A.S.; Tandona, R.; Gupta, G.; Kumar, A.; Sharma, R.K.; Aggarwal, N.; Kathuria, A.; Saini, N.K.; Bose, M.; Prasad, A.K.; et al. Characterization of protein acyltransferase function of recombinant purified GlnA1 from Mycobacterium tuberculosis: A moon lighting property. Microbiol. Res. 2011, 166, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Raj, H.G.; Kumari, R.; Seema; Muralidhar, K.M.; Dwarkanath, B.S.; Rastogi, R.C.; Prasad, A.K.; Watterson, A.C.; Parmar, V.S. Novel function of calreticulin: Characterization of calreticulin as transacetylase-mediating protein acetylation independent of acetyl CoA using PA. Pure Appl. Chem. 2006, 78, 985–992. [Google Scholar] [CrossRef]
- Seema; Kumari, R.; Gupta, G.; Saluja, D.; Kumar, A.; Goel, S.; Tyagi, Y.K.; Gulati, R.; Vinocha, A.; Muralidhar, K.M.; et al. Characterization of protein transacetylase from human placenta as a signalling molecule calreticulin using polyphenolic peracetates as acetyl donors. Cell Biochem. Biophys. 2007, 47, 53–64. [Google Scholar] [CrossRef]
- Bansal, S.; Gaspari, M.; Raj, H.G.; Kumar, A.; Cuda, G.; Verheij, E.; Tyagi, Y.K.; Ponnan, P.; Rastogi, R.C.; Parmar, V.S. Calreticulin transacetylase mediates the acetylation of nitric oxide synthase by polyphenolic acetates. Appl. Biochem. Biotechnol. 2008, 144, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Kumar, A.; Sinha, R.; Manral, S.; Arora, S.; Ram, S.; Mishra, R.K.; Gupta, P.; Bansal, S.K.; Prasad, A.K.; et al. Calreticulin transacetylase catalyzed modification of the TNF-α mediated pathway in the human peripheral blood mononuclear cells by PA. Chem. Biol. Interact. 2010, 185, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kohli, E.; Gaspari, M.; Raj, H.G.; Parmar, V.S.; van der Greef, J.; Gupta, G.; Kumari, R.; Prasad, A.K.; Goel, S.; Pal, G.; et al. Establishment of the enzymatic protein acetylation independent of acetyl CoA: Recombinant glutathione S-transferase 3–3 is acetylated by a novel membrane-bound transacetylase using 7,8-diacetoxy-4-methylcoumarin as acetyl donor. FEBS Lett. 2002, 530, 139–142. [Google Scholar] [CrossRef]
- Raj, H.G.; Kohli, E.; Goswami, R.; Goel, S.; Rastogi, R.C.; Jain, S.C.; Wengal, J.; Olsen, C.E.; Parmar, V.S. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part-8: Acetoxycoumarin: Protein transacetylase specificity for aromatic nuclear acetoxy groups in proximity to the oxygen heteroatom. Bioorg. Med. Chem. 2001, 9, 1085–1089. [Google Scholar] [CrossRef]
- Kohli, E.; Gaspari, M.; Raj, H.G.; Parmar, V.S.; Sharma, S.K.; van der Greef, J.; Kumari, R.; Gupta, G.; Seema; Khurana, P.; et al. Acetoxy drug: Protein transacetylase of buffaloe liver: Characterization and mass spectrometry of the acetylated protein product. Biochim. Biophys. Acta 2004, 169, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.; Plou, F.J. Regioselective lipase-catalyzed synthesis of 3-O-acyl derivatives of resveratrol and study of their antioxidant properties. J. Agric. Food Chem. 2010, 58, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, G.; Piattelli, M.; Sanfilippo, C. Acetylation of phenols in organic solvent catalyzed by lipase from Chromobacterium viscosum. Tetrahedron 1992, 48, 2477–2482. [Google Scholar] [CrossRef]
- Miyazawa, T.; Hamada, M.; Morimoto, R. Candida antarctica lipase B-catalyzed regioselective deacylation of dihydroxybenzenes acylated at both phenolic hydroxy groups. Can. J. Chem. 2016, 94, 44–49. [Google Scholar] [CrossRef]
- Ciuffreda, P.; Casati, S.; Santaniello, E. Regioselective hydrolysis of diacetoxynaphthalenes catalyzed by Pseudomonas sp. lipase in an organic solvent. Tetrahedron 2000, 56, 317–321. [Google Scholar] [CrossRef]
- Lambusta, D.; Nicolosi, G.; Patti, A.; Piatelli, M. Enzyme-mediated regioprotection-deprotection of hydroxyl groups in (+)-catechin. Synthesis 1993, 11, 1155–1158. [Google Scholar] [CrossRef]
- Kumar, R.; Azim, A.; Kumar, V.; Sharma, S.K.; Prasad, A.K.; Howarth, O.W.; Olsen, C.E.; Jain, S.C.; Parmar, V.S. Lipase-catalyzed chemo- and enantioselective acetylation of 2-alkyl/aryl-3-hydroxypropiophenones. Bioorg. Med. Chem. 2001, 9, 2643–2652. [Google Scholar] [CrossRef]
- Kumar, G.; Dhawan, A.; Singh, B.K.; Sharma, N.K.; Sharma, S.K.; Prasad, A.K.; Van der Eycken, E.V.; Len, C.; Watterson, A.C.; Parmar, V.S. Highly selective biocatalytic transesterification reactions on aryl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoates. Catal. Lett. 2015, 145, 919–929. [Google Scholar] [CrossRef]
- Mathur, D.; Bohra, K.; Bhatia, S.; Kumar, M.; Verma, P.; Saxena, R.K.; Parmar, V.S.; Prasad, A.K. Diastereoselective acetylation studies on 4-C-hydroxymethyl-1,2-O-isopropylidene-3-O-alkyl-β-l-threo-pentofuranose: Key precursor for biocompatible sugar-PEG copolymers. Trends Carbohydr. Res. 2011, 3, 42–50. [Google Scholar]
- Malhotra, S.; Calderón, M.; Prasad, A.K.; Parmar, V.S.; Haag, R. Novel chemoenzymatic methodology for the regioselective glycine loading on polyhydroxy compounds. Org. Biomol. Chem. 2010, 8, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, H.V.; Duisberg, C. Ueber die Verbindungen der Phenole mit Acetessigäther. Ber. Dtsch. Chem. Ges. 1883, 16, 2119–2122. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.H.; Xu, C.J.; Lin, G.C. Purification and partial characterization of a lipase from Antrodia cinnamomea. Process Biochem. 2006, 41, 734–738. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glucogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds 1, 2a and 3a–3f are available with the authors.




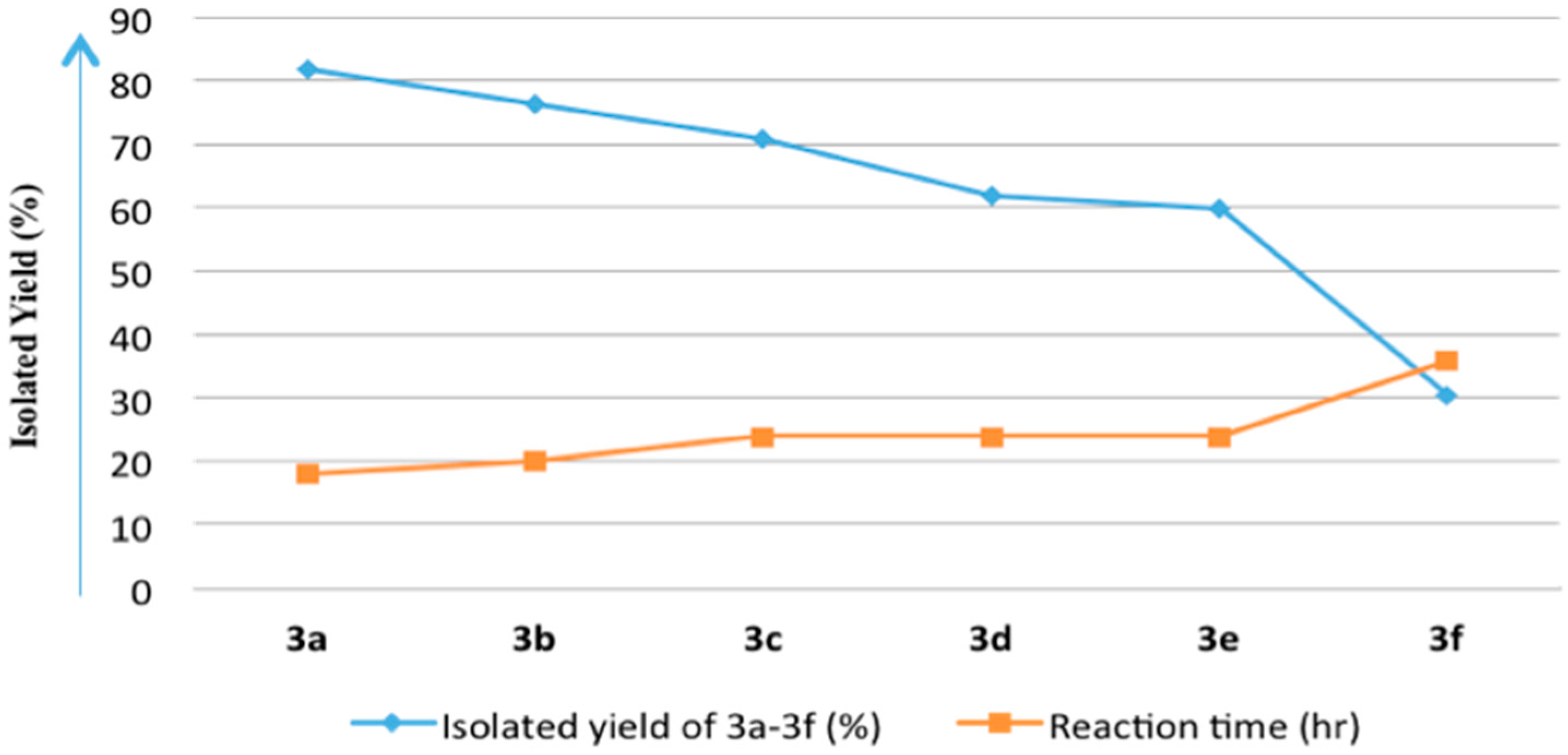
| Entry | Acid Anhydride (4a–4f) | Isolated Yield of 8-Acyloxy-7-hydroxy-4-methylcoumarin (3a–3f) a (%) | 7,8-Diacyloxy-4-methylcoumarin (2a–2f) | Reaction Time (h) |
|---|---|---|---|---|
| 1 | Acetic anhydride (4a) | 3a/82.0 | 2a/Traces b | 18 |
| 2 | Propanoic anhydride (4b) | 3b/76.5 | 2b/<10% b | 20 |
| 3 | Butanoic anhydride (4c) | 3c/71.0 | 2c/<10% b | 24 |
| 4 | Pentanoic anhydride (4d) | 3d/62.0 | 2d/<20% b | 24 |
| 5 | Hexanoic anhydride (4e) | 3e/60.0 | 2e/<20% b | 24 |
| 6 | Benzoic anhydride (4f) | 3f/30.5 | 2f/59.0% | 36 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Mathur, D.; Srivastava, S.; Malhotra, S.; Rana, N.; Singh, S.K.; Singh, B.K.; Prasad, A.K.; Varma, A.J.; Len, C.; et al. Biocatalytic Synthesis of Novel Partial Esters of a Bioactive Dihydroxy 4-Methylcoumarin by Rhizopus oryzae Lipase (ROL). Molecules 2016, 21, 1499. https://doi.org/10.3390/molecules21111499
Kumar V, Mathur D, Srivastava S, Malhotra S, Rana N, Singh SK, Singh BK, Prasad AK, Varma AJ, Len C, et al. Biocatalytic Synthesis of Novel Partial Esters of a Bioactive Dihydroxy 4-Methylcoumarin by Rhizopus oryzae Lipase (ROL). Molecules. 2016; 21(11):1499. https://doi.org/10.3390/molecules21111499
Chicago/Turabian StyleKumar, Vinod, Divya Mathur, Smriti Srivastava, Shashwat Malhotra, Neha Rana, Suraj K. Singh, Brajendra K. Singh, Ashok K. Prasad, Anjani J. Varma, Christophe Len, and et al. 2016. "Biocatalytic Synthesis of Novel Partial Esters of a Bioactive Dihydroxy 4-Methylcoumarin by Rhizopus oryzae Lipase (ROL)" Molecules 21, no. 11: 1499. https://doi.org/10.3390/molecules21111499