Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A
Abstract
:1. Introduction
2. Results
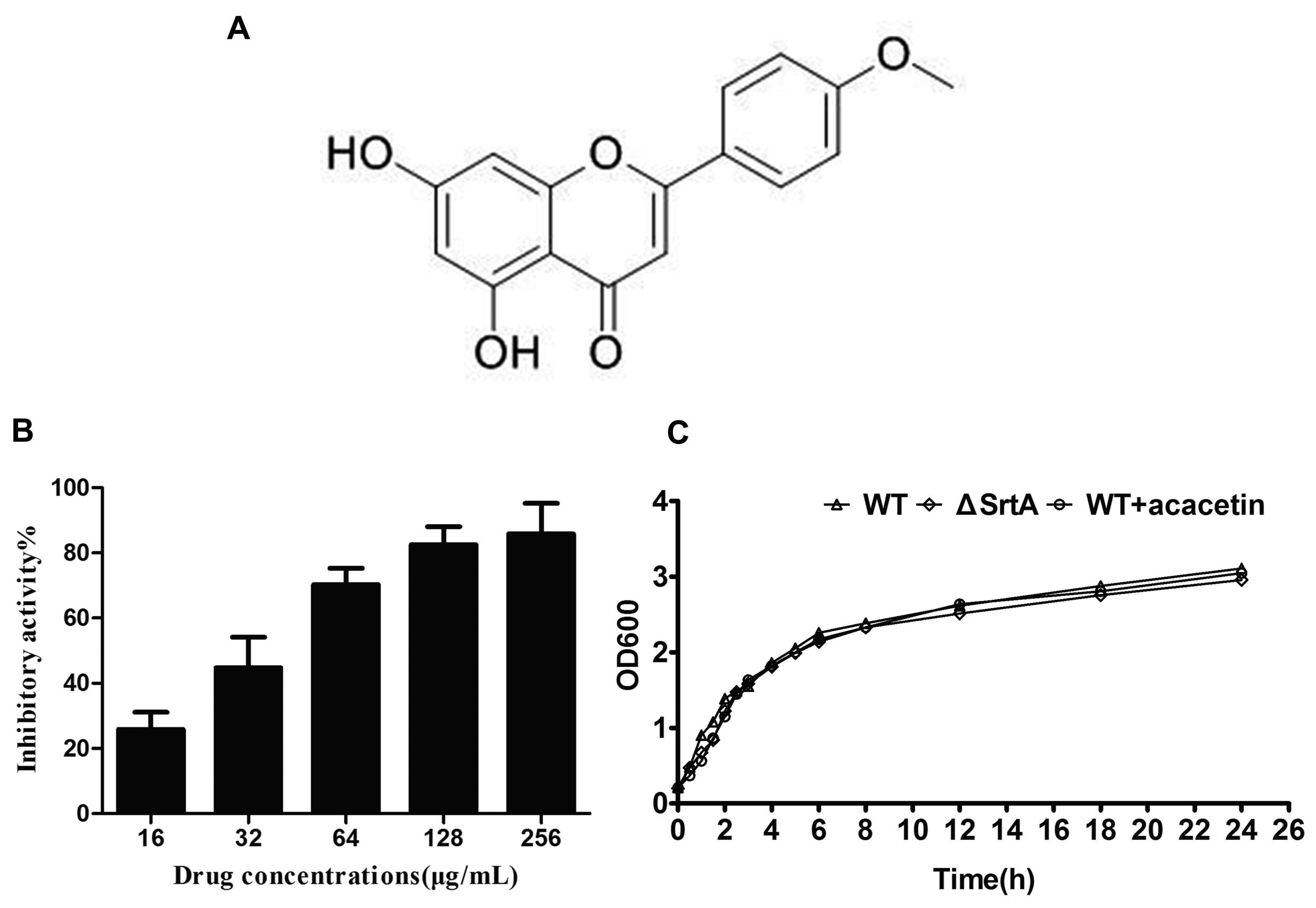
2.1. Acacetin Inhibits SrtA-Catalyzed Transpeptidation
2.2. Acacetin Has No Influence on the Growth of S. aureus
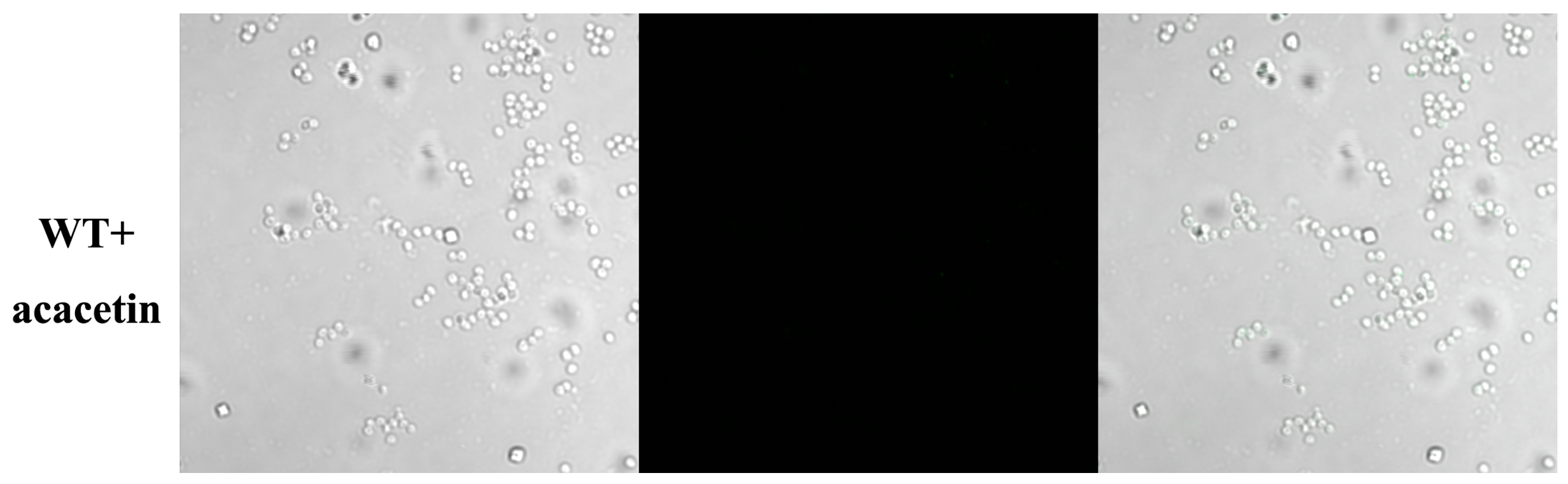
2.3. Acacetin Influences the Assembly of SpA into the Cell Wall
2.4. Acacetin Reduces the Adherence of S. aureus to Fibrinogen
2.5. Determination of the SrtA-Acacetin Binding Mechanism
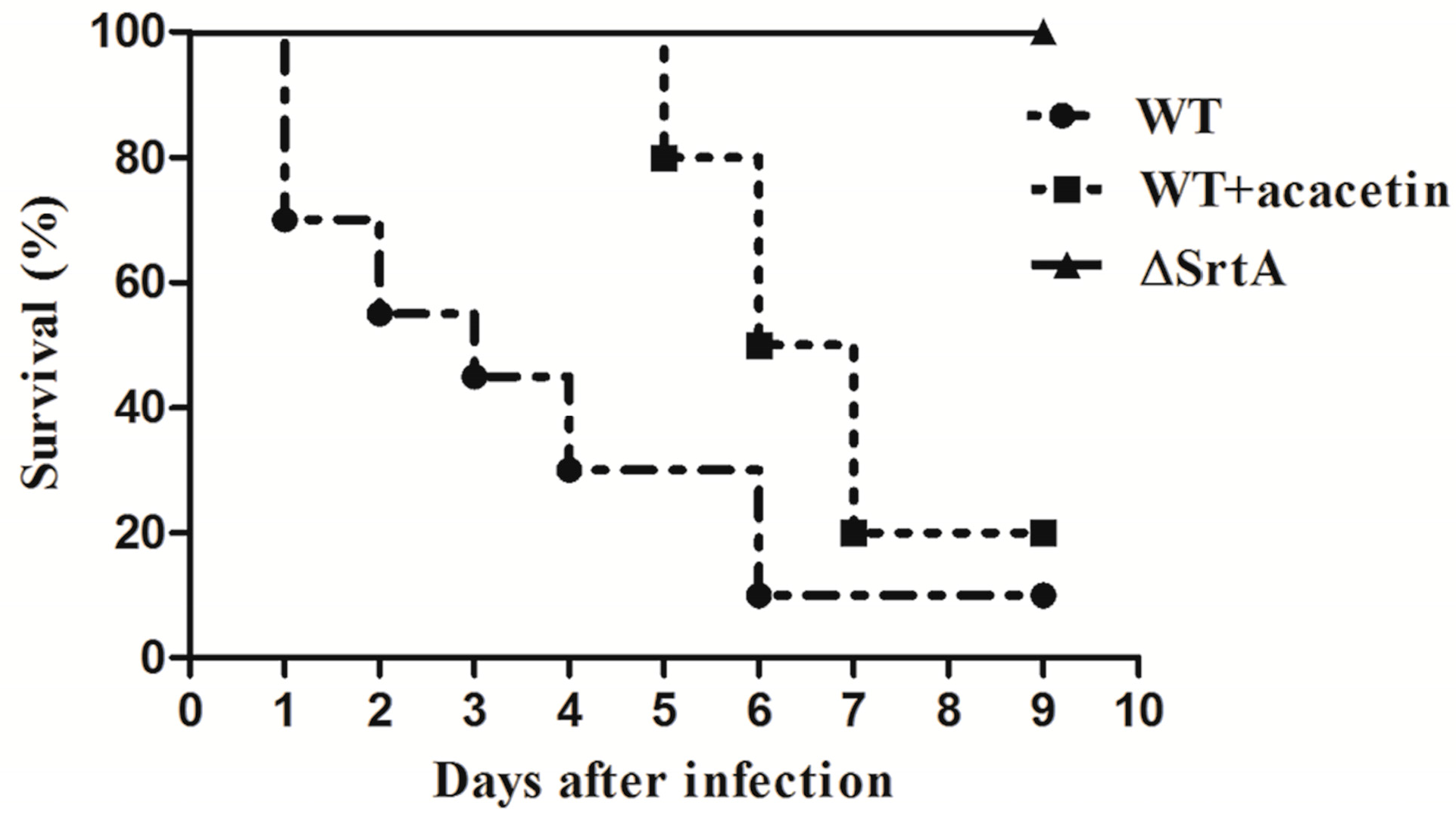
2.6. Acacetin Significantly Increases the Survival Rate of S. aureus-Infected Mice
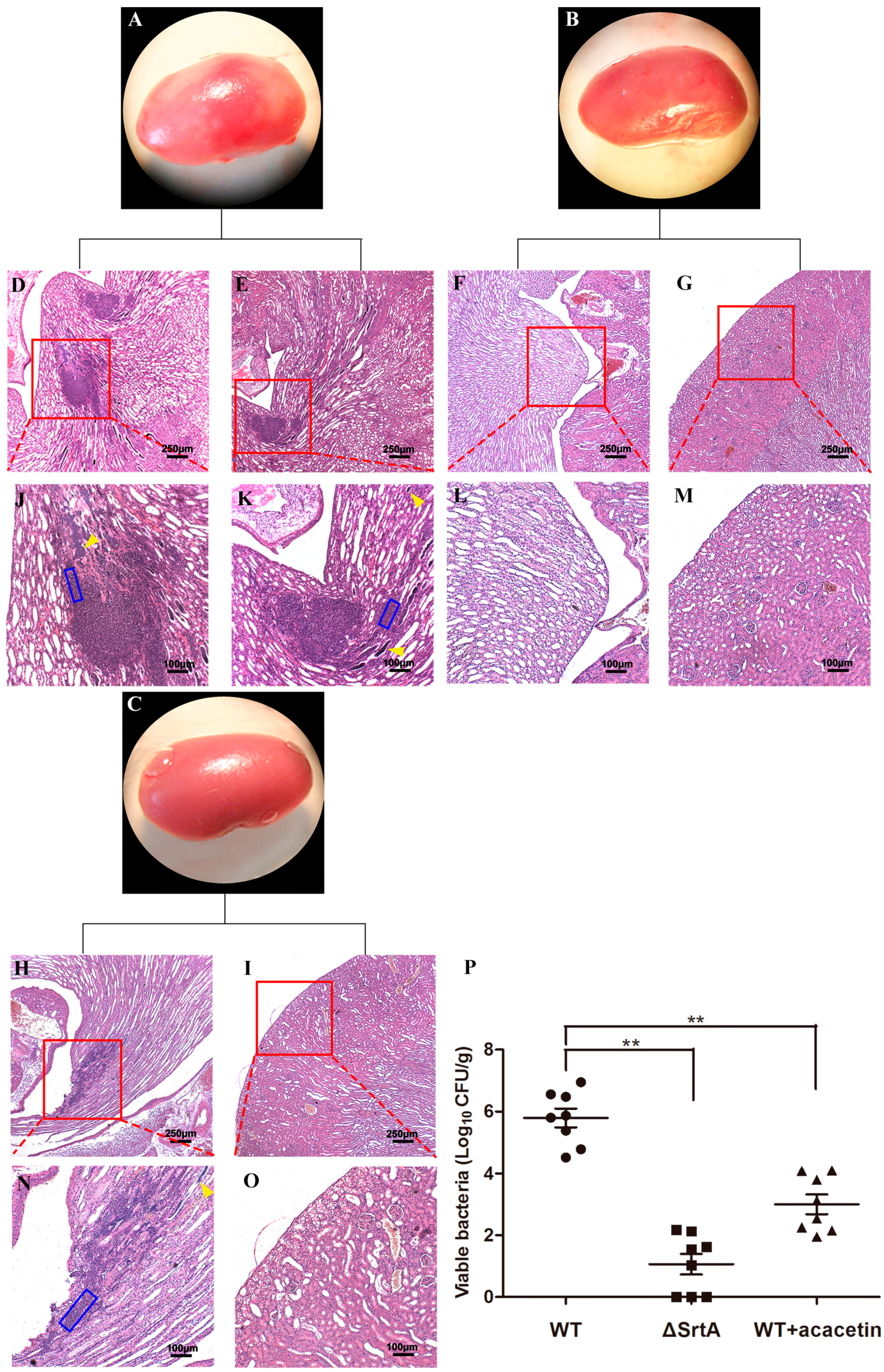
2.7. Acacetin Alleviates the Symptoms of S. aureus-Induced Renal Abscess in Mice
3. Discussion
4. Materials and Methods
4.1. Microbial Strains, Plasmids and Reagents
4.2. Inhibition of Sortase A Activity
4.3. Minimum Inhibitory Concentrations (MIC) and Growth Curves
4.4. Fibrinogen-Binding Assay
4.5. SpA-Related Fluorescence Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fitzgerald-Hughes, D.; Devocelle, M.; Humphreys, H. Beyond conventional antibiotics for the future treatment of methicillin-resistant Staphylococcus aureus infections: Two novel alternatives. FEMS. Immunol. Med. Microbiol. 2012, 65, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Cusimano, M.G.; Schillaci, D. Antiadhesion agents against Gram-positive pathogens. Future Microbiol. 2014, 9, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Paterson, G.K.; Mitchell, T.J. The biology of Grampositive sortase enzymes. Trends Microbiol. 2004, 12, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.R.; Barnett, T.C. Surface proteins of grampositive bacteria and how they get there. Ann. Rev. Microbiol. 2006, 60, 397–423. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.W.; Melvin, J.A.; McCafferty, D.G. Sortase transpeptidases: Insights into mechanism, substrate specificity, and inhibition. Biopolymers 2010, 94, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Ton-That, H.; Marraffini, A.; Schneewind, O. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 2004, 1694, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, G.; Ton-That, H.; Schneewind, O. Staphylococcus aureus Sortase, an enzyme that anchors surface proteins to the cell wall. Science 1999, 285, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Spirig, T.; Weiner, E.M.; Clubb, R.T. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 2011, 82, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, G.; Jensen, E.R.; Lenoy, E.; Schneewind, O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 2000, 97, 5510–5515. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, R.; Nandakumar, M.P.; Marten, M.R.; Ross, J.M. Proteome analysis of membrane and cell wall associated proteins from Staphylococcus aureus. J. Proteome Res. 2005, 4, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Suree, N.; Jung, M.E.; Clubb, R.T. Recent advances towards new anti-infective agents that inhibit cell surface protein anchoring in Staphylococcus aureus and other gram-positive pathogens. Mini Rev. Med. Chem 2007, 7, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Totsika, M.; Schillaci, D. Sortase A: An ideal target for anti-virulence drug development. Microb. Pathog. 2014, 77, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Ton-That, H.; Su, K.; Schneewind, O. An iron-regulated sortase enzyme anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.G.; Kim, H.K.; Burts, M.L.; Krausz, T.; Schneewind, O.; Missiakas, D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009, 23, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- McAdow, M.; Kim, H.K.; Dedent, A.C.; Hendrickx, A.P.; Schneewind, O.; Missiakas, D.M. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011, 7, e1002307. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, C.; Wang, T.; Xiang, H.; Chen, F.; Hu, J.; Liu, B.; Cai, H.; Zhong, X.; Deng, X.; et al. Coagulase-negative and nonhemolytic strain of Staphylococcus aureus for investigating the roles of SrtA in S. aureus-induced bloodstream infection. Pathog. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Raffa, D.; Maggio, B.; Raimondi, M.V.; Schillaci, D.; Daidone, G. Sortase A inhibitors: Recent advances and future perspectives. J. Med. Chem. 2015, 58, 9108–9123. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Jonquieres, R. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc. Natl. Acad. Sci. USA 2000, 97, 5013–5015. [Google Scholar] [CrossRef] [PubMed]
- Maresso, A.W.; Schneewind, O. Sortase as a target of anti-infective therapy. Pharmacol. Rev. 2008, 60, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, F.; Bi, C.; Wang, L.; Zhong, X.; Cai, H.; Deng, X.; Niu, X.; Wang, D. Quercitrin, an inhibitor of Sortase A, interferes with the adhesion of Staphylococcal aureus. Molecules 2015, 20, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Li, X.; Chen, D.Y.; Zhang, N.; Wang, Y.; Shan, Y.; Hu, Y.; Xu, R.A.; Jin, J.; Ge, R.S. Determination of acacetin in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study. J. Chromatogr. B 2015, 986–987, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Valkama, E.; Salminen, J.P.; Koricheva, J.; Pihlaja, K. Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann. Bot. 2004, 94, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Nambiar, D.; Tailor, D.; Pal, A.; Agarwal, R.; Singh, R.P. Acacetin inhibits in vitro and in vivo angiogenesis and downregulates Stat signaling and VEGF expression. Cancer Prev. Res. 2013, 6, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.T.; Lin, S.S.; Wang, C.K.; Lee, Y.B.; Chen, K.S.; Fong, Y.; Shih, Y.W. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38alpha MAPK signaling pathway. Mol. Cell Biochem. 2011, 350, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Hung, S.H.; Yin, L.T.; Huang, C.S.; Chao, C.H.; Liu, C.L.; Shih, Y.W. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol. Cell Biochem. 2010, 333, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kanno, S.; Tomizawa, A.; Yomogida, S.; Ishikawa, M. Acacetin induces apoptosis in human T cell leukemia Jurkat cells via activation of a caspase cascade. Oncol. Rep. 2012, 27, 204–209. [Google Scholar] [PubMed]
- Chen, W.P.; Yang, Z.G.; Hu, P.F.; Bao, J.P.; Wu, L.D. Acacetin inhibits expression of matrix metalloproteinases via a MAPK-dependent mechanism in fibroblast-like synoviocytes. J. Cell Mol. Med. 2015, 19, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Komape, N.P.; Aderogba, M.; Bagla, V.P.; Masoko, P.; Eloff, J.N. Anti-bacterial and anti-oxidant activities of leaf extracts of Combretum vendae (Combretecacea) and the isolation of an anti-bacterial compound. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ton-That, H.; Liu, G.; Mazmanian, S.K.; Faull, K.F.; Schneewind, O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA 1999, 96, 12424–12429. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Betley, M.J.; Hopkins, C.A.; Perez, N.E.; Pier, G.B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J. Infect. Dis. 1987, 156, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.B.; Nam, K.W.; Ahn, H.; Shin, J.; Kim, S.; Mar, W. Therapeutic effect of (Z)-3-(2,5-dimethoxyphenyl)-2-(4-methoxyphenyl) acrylonitrile (DMMA) against Staphylococcus aureus infection in a murine model. Biochem. Biophys. Res. Commun. 2010, 396, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Zhu, K.; Gong, S.; Dramsi, S.; Wang, Y.T.; Li, J.; Chen, F.; Zhang, R.; Zhou, L.; et al. Antiinfective therapy with a small molecule inhibitor of Staphylococcus aureus sortase. Proc. Natl. Acad. Sci. USA 2014, 111, 13517–13522. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, B.; Wang, D.; Wang, L.; Deng, X.; Bi, C.; Xiong, Y.; Wu, Q.; Cui, Y.; Zhang, Y.; et al. Role of sortase A in the pathogenesis of Staphylococcus aureus-induced mastitis in mice. FEMS Microbiol. Lett. 2014, 351, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds screened in this study are available from the authors.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, C.; Dong, X.; Zhong, X.; Cai, H.; Wang, D.; Wang, L. Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A. Molecules 2016, 21, 1285. https://doi.org/10.3390/molecules21101285
Bi C, Dong X, Zhong X, Cai H, Wang D, Wang L. Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A. Molecules. 2016; 21(10):1285. https://doi.org/10.3390/molecules21101285
Chicago/Turabian StyleBi, Chongwei, Xiaoyun Dong, Xiaobo Zhong, Hongjun Cai, Dacheng Wang, and Lin Wang. 2016. "Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A" Molecules 21, no. 10: 1285. https://doi.org/10.3390/molecules21101285
APA StyleBi, C., Dong, X., Zhong, X., Cai, H., Wang, D., & Wang, L. (2016). Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A. Molecules, 21(10), 1285. https://doi.org/10.3390/molecules21101285




