Microwave-Assisted Synthesis of Cinnamyl Long Chain Aroma Esters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Cinnamyl Long Chain Esters
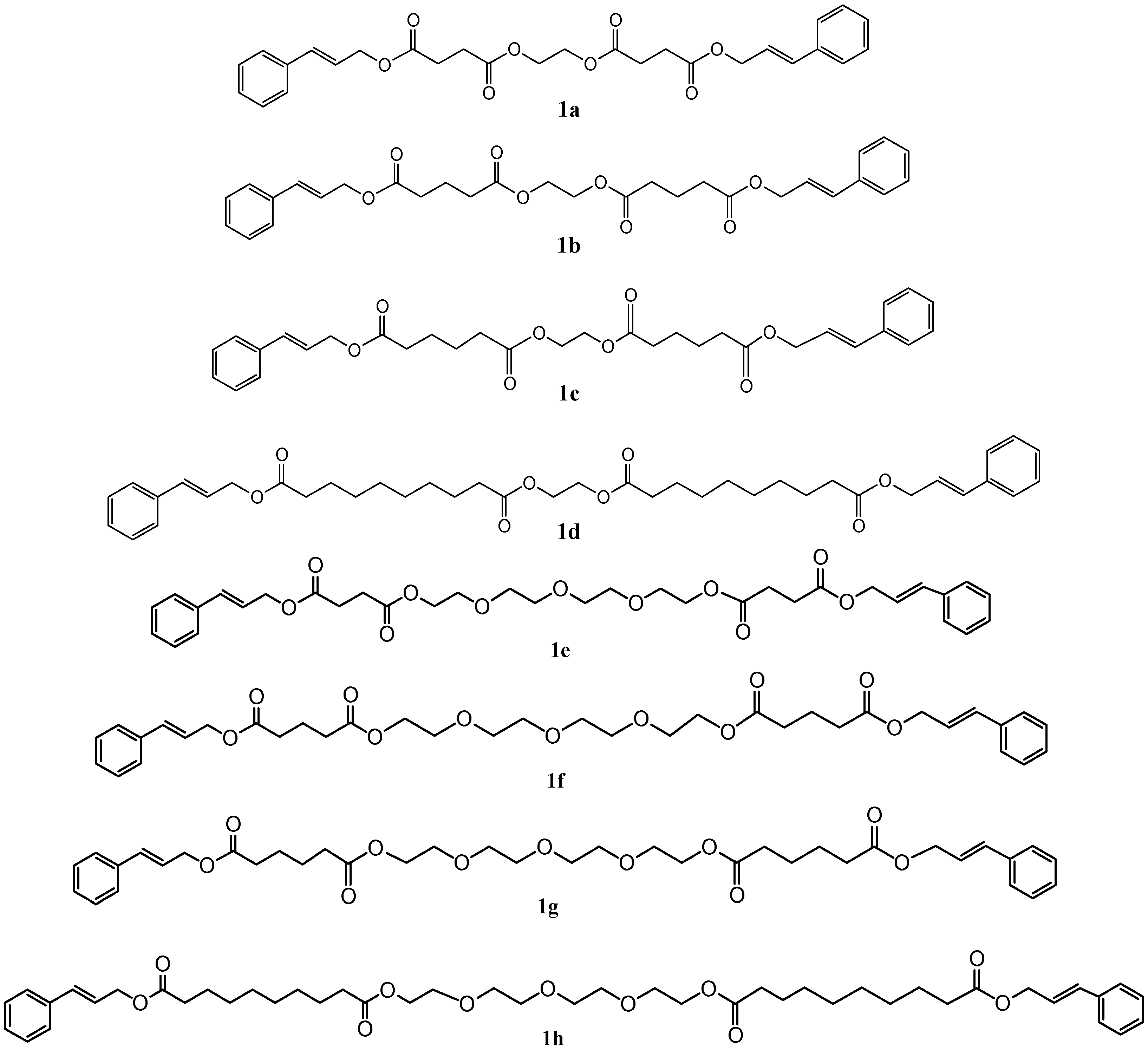

| Compound | Reaction Time | Reaction Yield (%) | ||
|---|---|---|---|---|
| Conventional Method | Microwave Assisted Method | Conventional Method | Microwave Assisted Method | |
| 1a | 30 h | 10 min | 90 | 90 |
| 1b | 37 h | 12 min | 91 | 90 |
| 1c | 42 h | 14 min | 91 | 92 |
| 1d | 51 h | 17 min | 92 | 92 |
| 1e | 39 h | 12 min | 90 | 91 |
| 1f | 46 h | 15 min | 91 | 92 |
| 1g | 58 h | 18 min | 92 | 90 |
| 1h | 65 h | 20 min | 93 | 93 |
2.2. Thermal Properties
| Compound | T1%/°C | Tmax/°C | ||
|---|---|---|---|---|
| Inert Conditions | Oxidative Conditions | Inert Conditions | Oxidative Conditions | |
| 1a | 225 | 218 | 367 | 353/546 |
| 1b | 230 | 220 | 371 | 361/532 |
| 1c | 242 | 232 | 384 | 375/530 |
| 1d | 252 | 244 | 402 | 384/533 |
| 1e | 215 | 200 | 360 | 350/527 |
| 1f | 220 | 208 | 367 | 352/538 |
| 1g | 225 | 219 | 380 | 365/530 |
| 1h | 230 | 225 | 417 | 379/524 |
3. Experimental Section
3.1. General Information
3.2. Materials
3.3. General Synthesis of Esters under Conventional and Microwave Conditions
4. Conclusions
Conflicts of Interest
References
- Croteau, R. Fragrance and Flavor Substances; D & PS Verlag: Pattensen, Germany, 1980. [Google Scholar]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrances and Flavor Materials: Preparation, Properties and Uses; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Armstrong, D.W.; Gillies, B.; Yamazaki, H. Flavor Chemistry, Trends and Development; Charalambous, G., Ed.; Elsevier Science Publisher: New York, NY, USA, 1989. [Google Scholar]
- Gildemeister, E.; Hoffmann, F.R. The Volatile Oils; Wiley: New York, NY, USA, 1913. [Google Scholar]
- Bedoukian, P.Z.; Geraniol and nerol. Allured Publishing Corporation: Weaton, IL, USA, 1986.
- Qin, G.; Tao, S.; Zhang, H.; Huang, W.; Wu, J.; Xu, Y.; Zhang, S. Evolution of the aroma volatiles of pear fruits supplemented with fatty acid metabolic precursors. Molecules 2014, 19, 20183–20196. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [PubMed]
- Goretti, M.; Turchetti, B.; Cramarossa, M.R.; Forti, L.; Buzzini, P. Production of flavours and fragrances via bioreduction of (4R)-(−)-carvone and (1R)-(−)-myrtenal by mon-conventional yeast whole-cells. Molecules 2013, 18, 5736–5748. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Liu, J.B.; Yang, Z.M.; Song, H.L.; Liu, Y.; Zou, T.T. Aroma-active compounds in jinhua ham produced with different fermentation periods. Molecules 2014, 19, 19097–19113. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, M.; Bajgrowicz, J.A.; Kraft, P. Fragrance chemistry-milestones and perspectives. Chimia 2001, 55, 379–387. [Google Scholar]
- Welsh, W.W.; Murray, M.D.; Williams, R.E. Microbiological and enzymatic production of flavor and fragrance chemicals. Crit. Rev. Biotechnol. 1989, 9, 105–169. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Yin, D.; Sun, B. Synthesis and odor evaluation of five new sulfur-containing ester flavor compounds from 4-ethyloctanoic acid. Molecules 2010, 15, 5104–5111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, Y.; Li, N.G.; Zhu, Y.; Duan, J.A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 2014, 19, 16458–16476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.; Wen, H.M.; Zhu, H.H.; Wang, T.L.; Cheng, D.; Yang, K.D.; Chen, Y.Q. Synthesis and biological evaluation of liguzinediol mono- and dual ester prodrugs as promising inotropic agents. Molecules 2014, 19, 18057–18072. [Google Scholar] [CrossRef] [PubMed]
- Mattarei, A.; Carraro, M.; Azzolini, M.; Paradisi, C.; Zoratti, M.; Biasutto, L. New water-soluble carbamate ester derivatives of resveratrol. Molecules 2014, 19, 15900–15917. [Google Scholar] [CrossRef] [PubMed]
- Wisniak, J.; Benajahu, H. Sulfur bromination of jojoba oil. Ind. Eng. Chem. Prod. Res. Dev. 1978, 17, 335–342. [Google Scholar] [CrossRef]
- Hong, S.G.; Hsu, H.W.; Ye, M.T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Idzik, K.R.; Nödler, K.; Maier, F.; Licha, T. Efficient synthesis and reaction kinetics of readily water soluble esters containing sulfonic groups. Molecules 2014, 19, 21022–21033. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, Y.; Sang, Z.; Sun, C.; Dai, Y.; Deng, Y. Synthesis, characterization, antibacterial and antifungal evaluation of novel monosaccharide esters. Molecules 2012, 17, 8661–8673. [Google Scholar] [CrossRef] [PubMed]
- March, J. Advanced Organic Chemistry; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Ishihara, K.; Ohara, S.; Yamamoto, H. Direct condensation of carboxylic acids with alcohols catalyzed by hafnium (IV) salts. Science 2000, 290, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Worzakowska, M.; Ścigalski, P. Synthesis and thermal behavior of linear neryl diesters in inert and oxidative atmospheres. J. Therm. Anal. Calorim. 2014, 115, 783–792. [Google Scholar] [CrossRef]
- Worzakowska, M.; Ścigalski, P. TG/DSC/FTIR characterization of linear geranyl diesters. J. Therm. Anal. Calorim. 2013, 113, 53–60. [Google Scholar] [CrossRef]
- Worzakowska, M. Thermal properties of citronelly diesters. J. Therm. Anal. Calorim. 2014, 118, 299–309. [Google Scholar] [CrossRef]
- Worzakowska, M.; Ścigalski, P. Thermal behavior of cinnamyl diesters studied by the TG/FTIR/QMS in inert atmosphere. J. Anal. Appl. Pyrol. 2014, 106, 48–56. [Google Scholar] [CrossRef]
- Worzakowska, M. Synthesis, characterization, and thermal properties of new flavor compounds. J. Therm. Anal. Calorim. 2014, 116, 727–736. [Google Scholar] [CrossRef]
- Worzakowska, M. TG/FTIR/QMS studies of long chain esters of geraniol. J. Anal. Appl. Pyrol. 2014, 110, 181–193. [Google Scholar] [CrossRef]
- Worzakowska, M. Thermal properties of neryl long chain esters obtained under microwave irradiation. J. Therm. Anal. Calorim. 2015. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Loupy, A. Microwave in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Leadbeater, N.E. Microwave Heating as a Tool for Sustainable Chemistry; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Arnold, R.T.; Smith, G.G.; Dodson, R.M. Mechanism of the pyrolysis of esters. J Org. Chem. 1950, 15, 1256–1260. [Google Scholar] [CrossRef]
- Bailey, W.J.; Barclay, R., Jr. Pyrolysis of esters. V. Mechanism of 1,4-elimination. J Org. Chem. 1956, 21, 328–331. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1a–1h are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worzakowska, M. Microwave-Assisted Synthesis of Cinnamyl Long Chain Aroma Esters. Molecules 2015, 20, 10594-10603. https://doi.org/10.3390/molecules200610594
Worzakowska M. Microwave-Assisted Synthesis of Cinnamyl Long Chain Aroma Esters. Molecules. 2015; 20(6):10594-10603. https://doi.org/10.3390/molecules200610594
Chicago/Turabian StyleWorzakowska, Marta. 2015. "Microwave-Assisted Synthesis of Cinnamyl Long Chain Aroma Esters" Molecules 20, no. 6: 10594-10603. https://doi.org/10.3390/molecules200610594
APA StyleWorzakowska, M. (2015). Microwave-Assisted Synthesis of Cinnamyl Long Chain Aroma Esters. Molecules, 20(6), 10594-10603. https://doi.org/10.3390/molecules200610594





