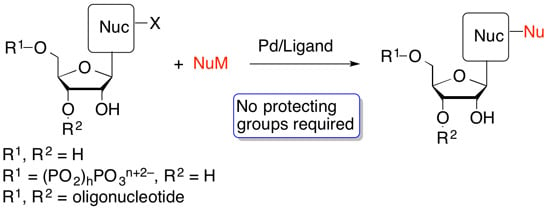
Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides
Abstract
:1. Introduction
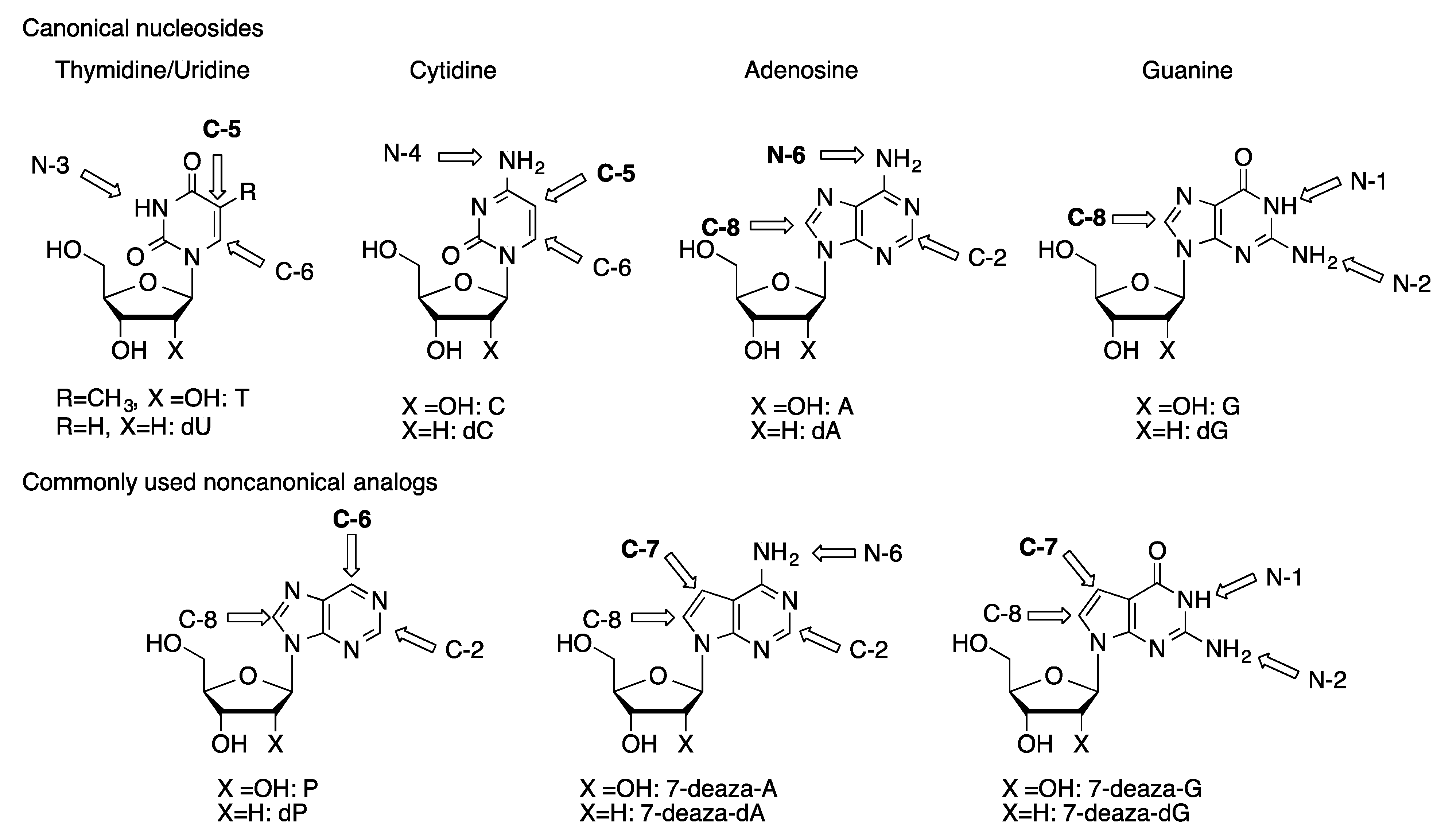
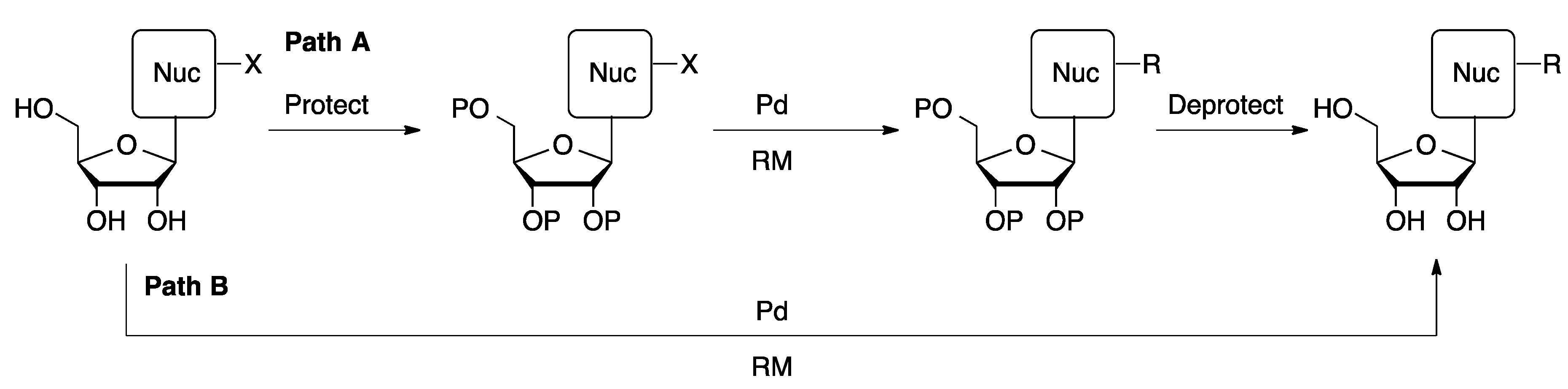
2. Cross-Coupling of Unprotected Nucleosides with Ligand-Free Palladium Catalysts
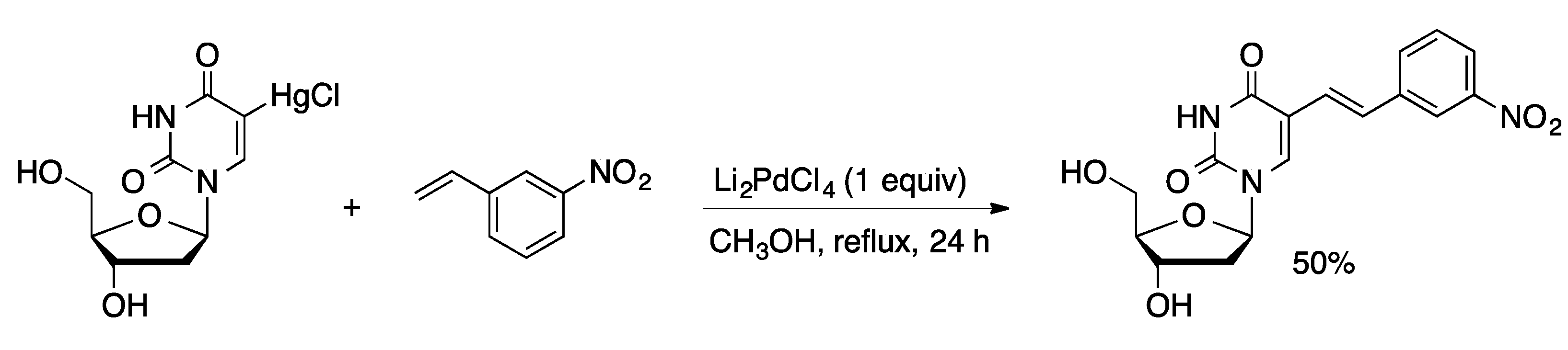

2.1. Cross-Coupling with Organosilanes and Organoboranes
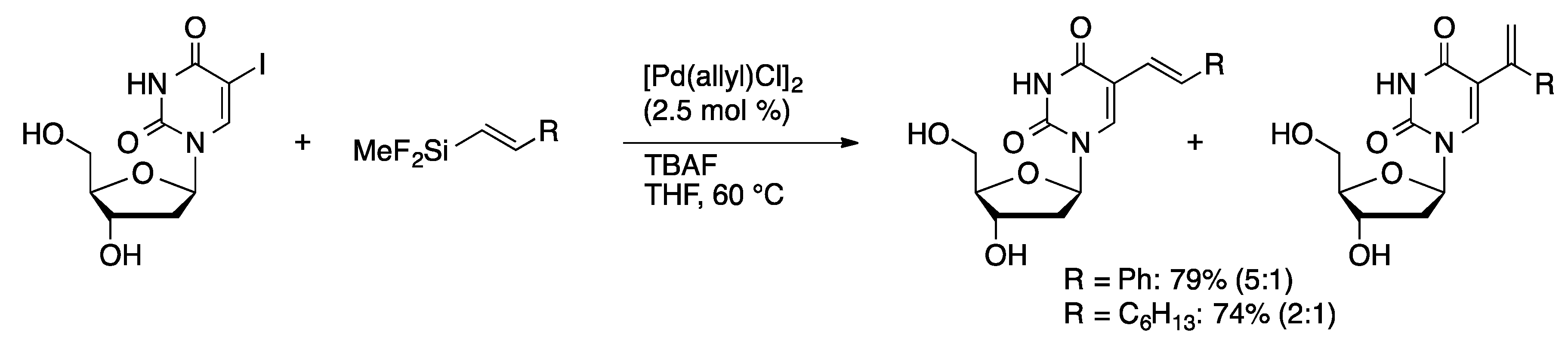

2.2. Heck Coupling

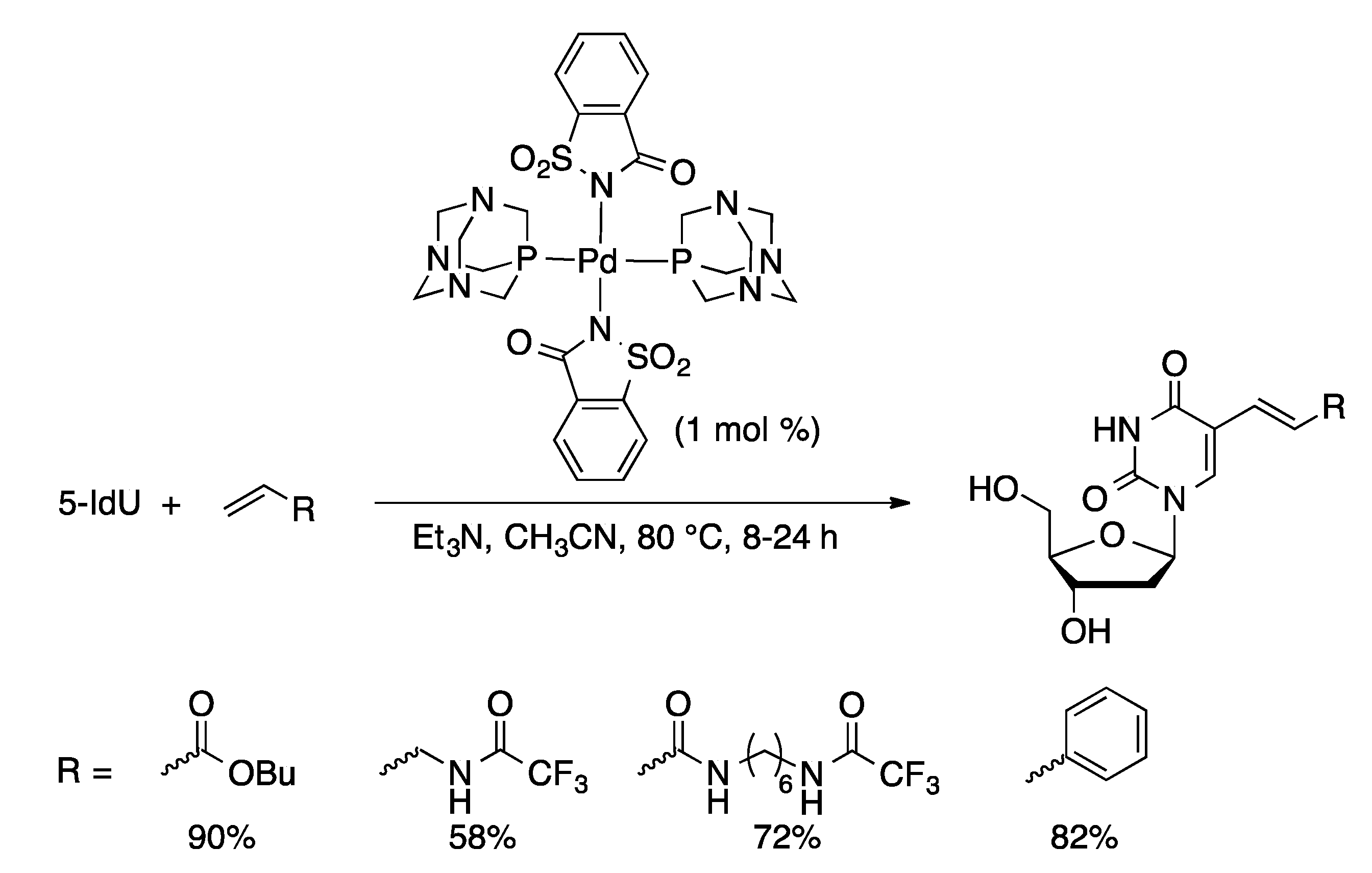
2.3. Direct Arylation via C-H Activation
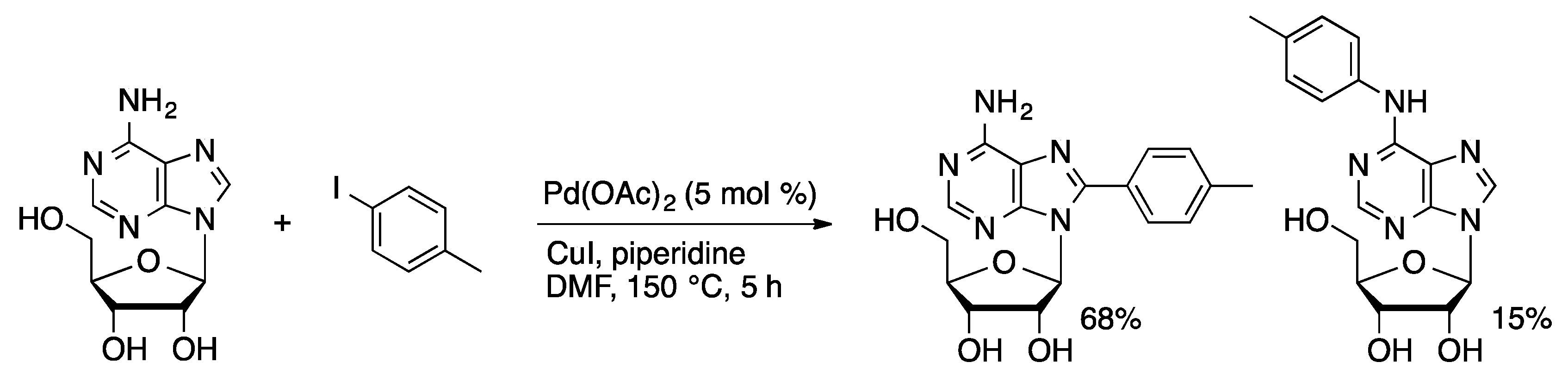

3. Cross-Coupling of Unprotected Nucleosides with Palladium Complexes of Hydrophobic Ligands
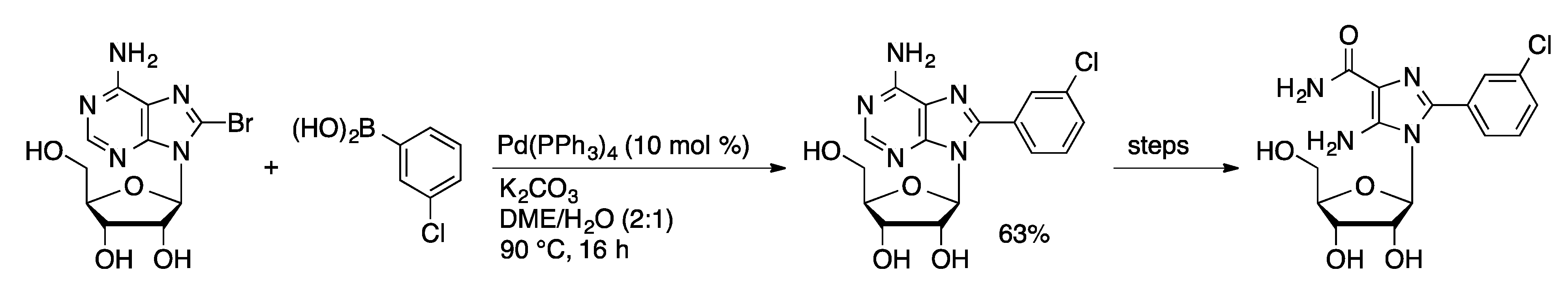
3.1. Suzuki Couplings
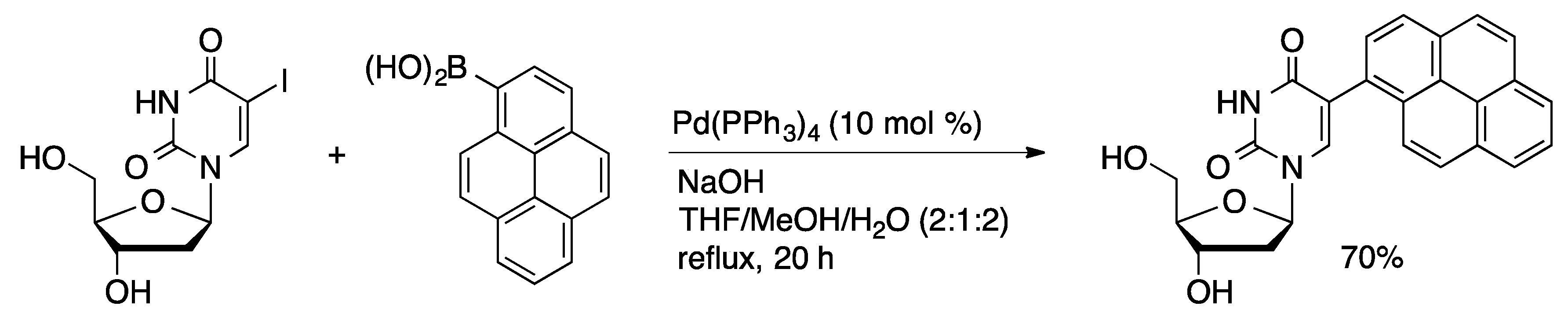
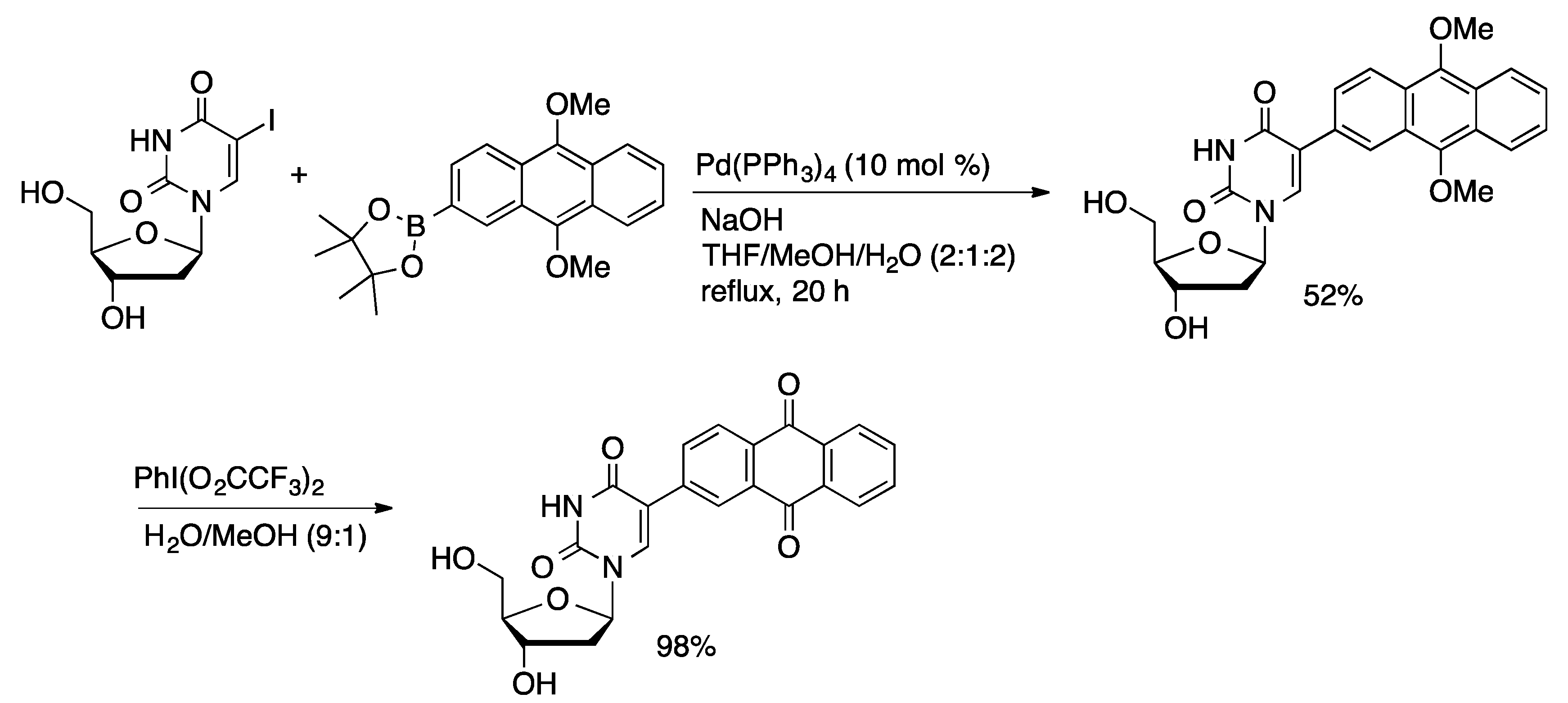
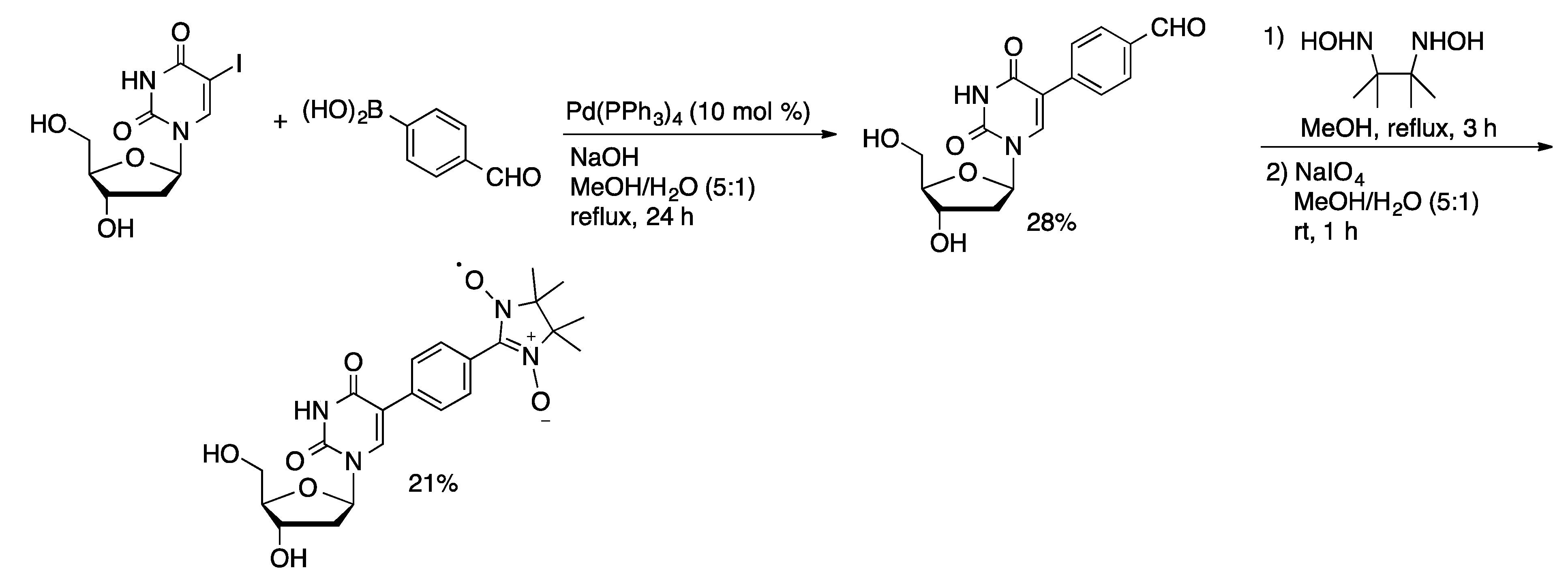

3.2. Stille Coupling


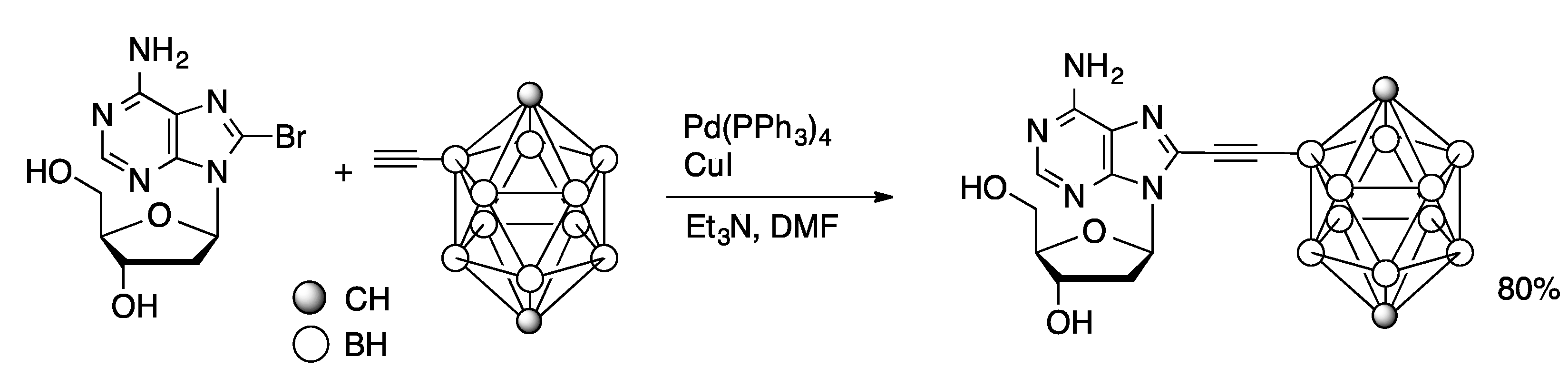
3.3. Sonogashira Coupling


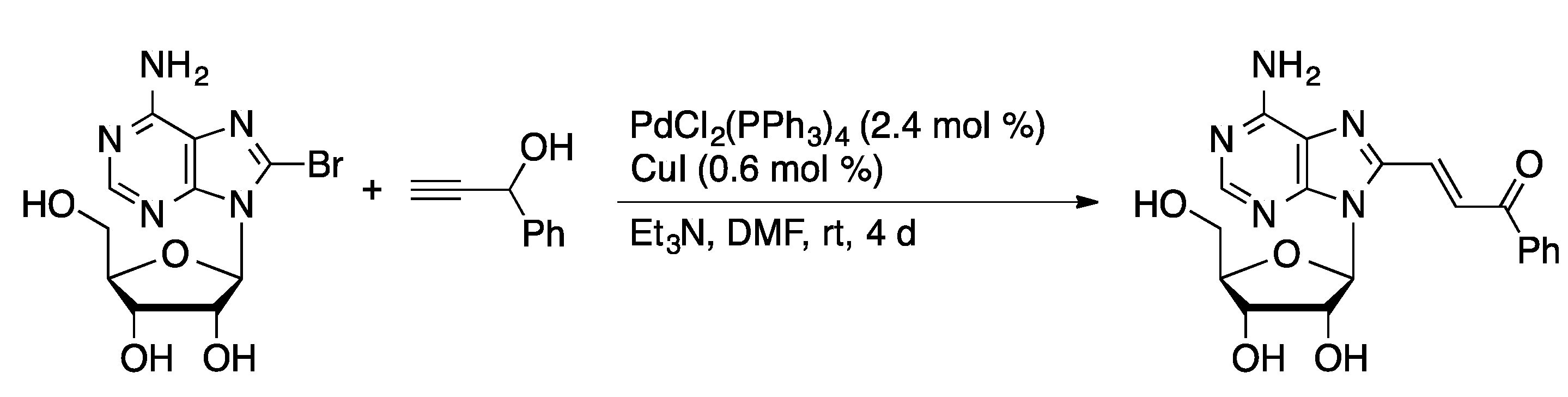

3.4. Heck Couplings
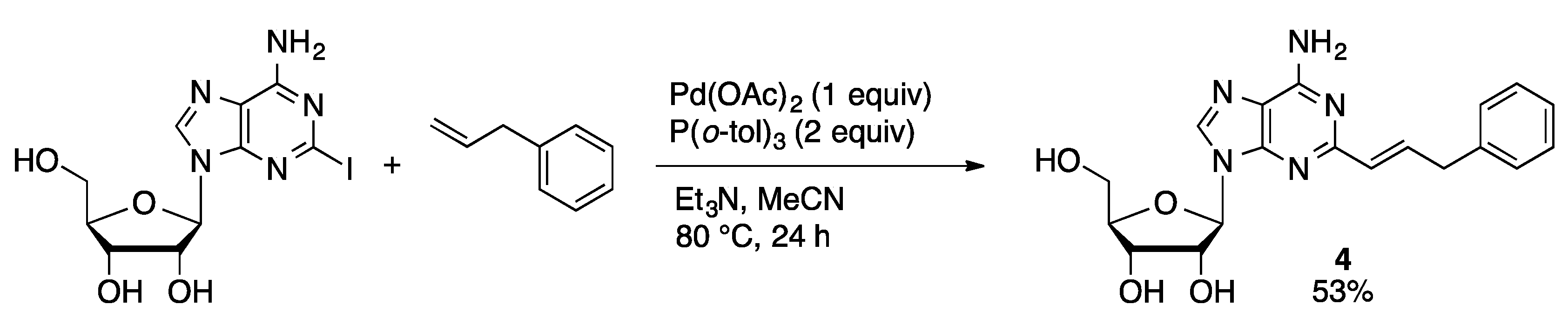
4. Aqueous-Phase Cross-Coupling of Nucleosides, Nucleotides, and Oligonucleotides Using Hydrophilic Ligand-Supported Catalysts
4.1. Nucleosides
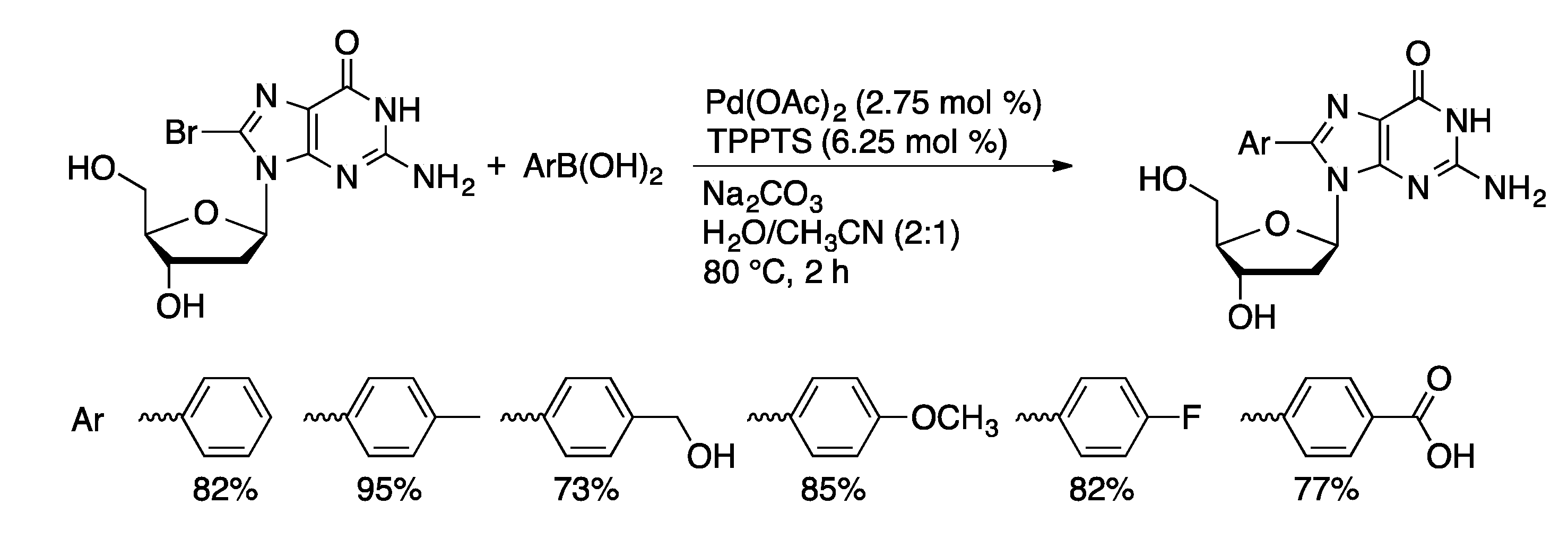
4.1.1. Suzuki Coupling



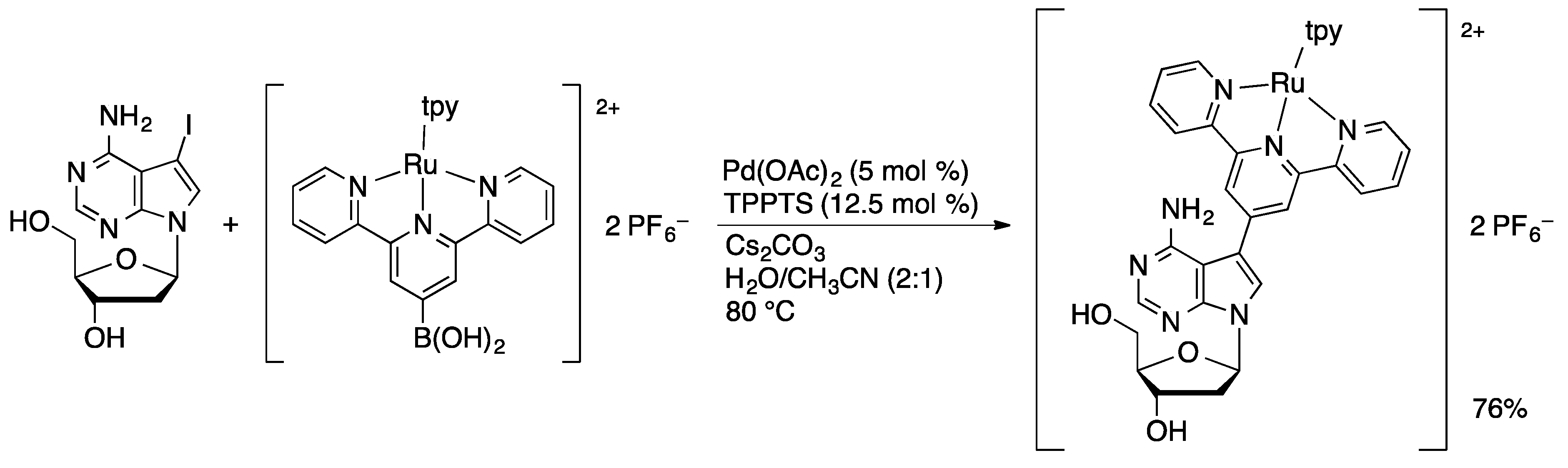

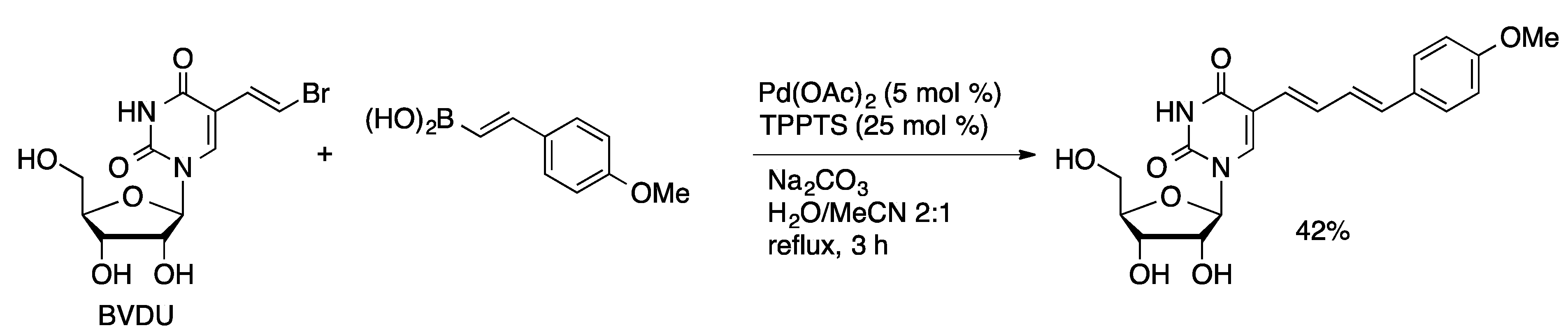

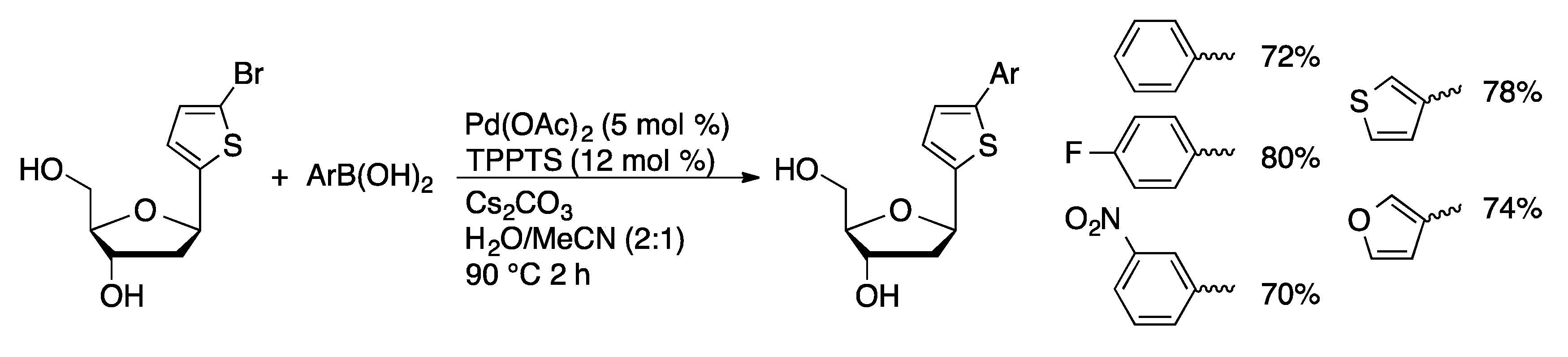
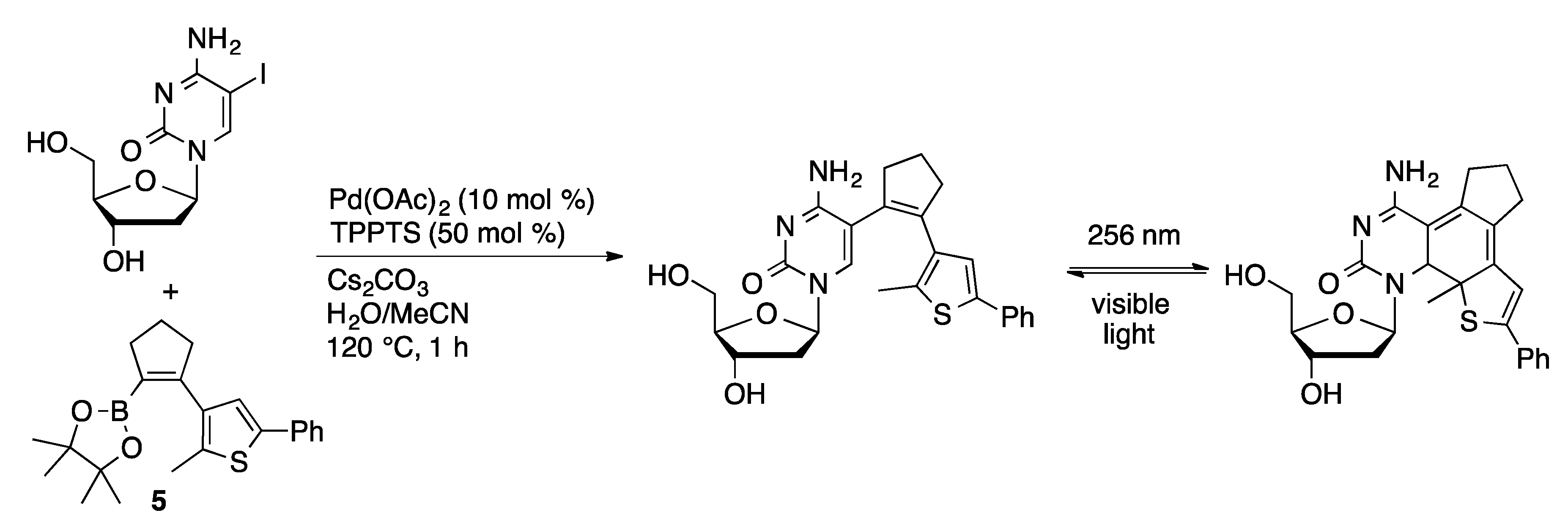
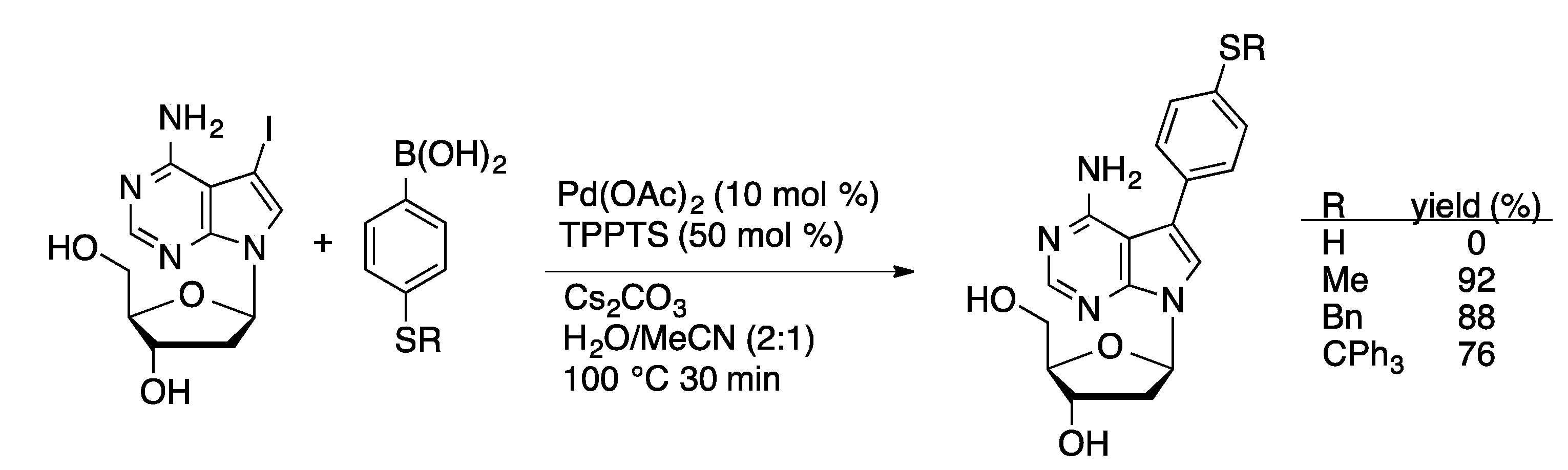


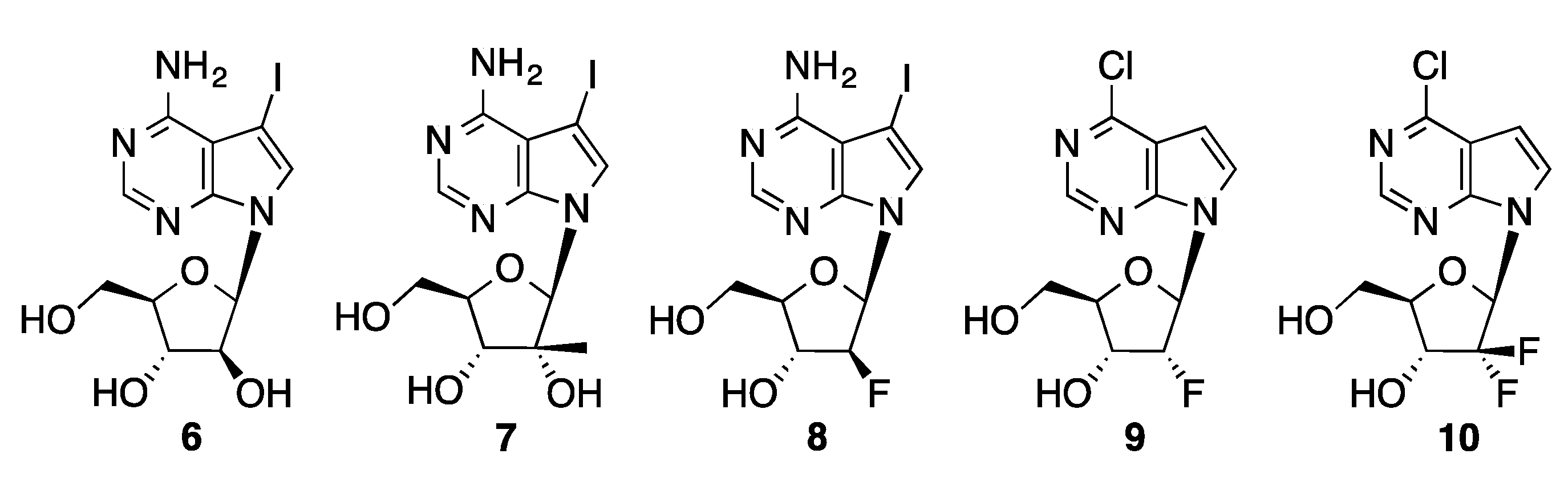

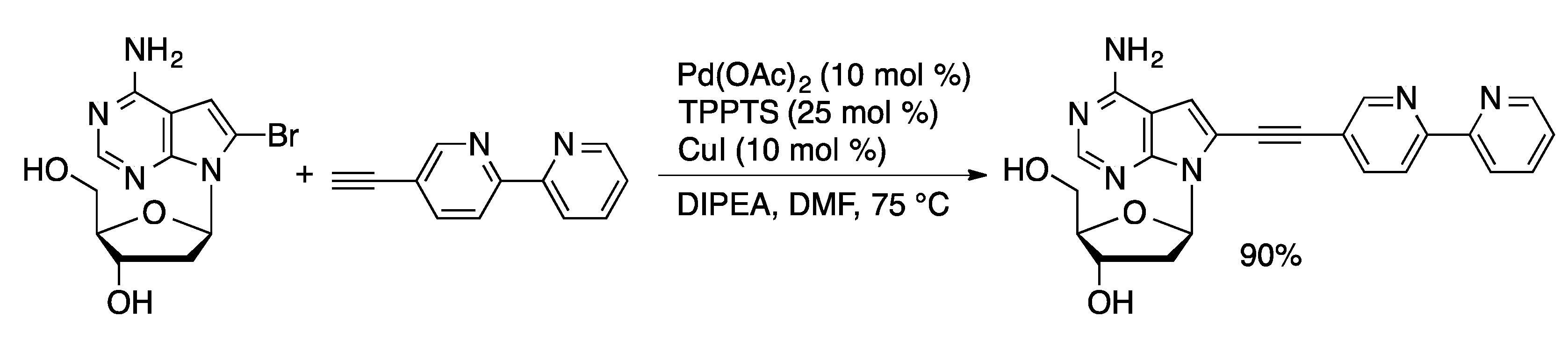
4.1.2. Sonogashira Coupling
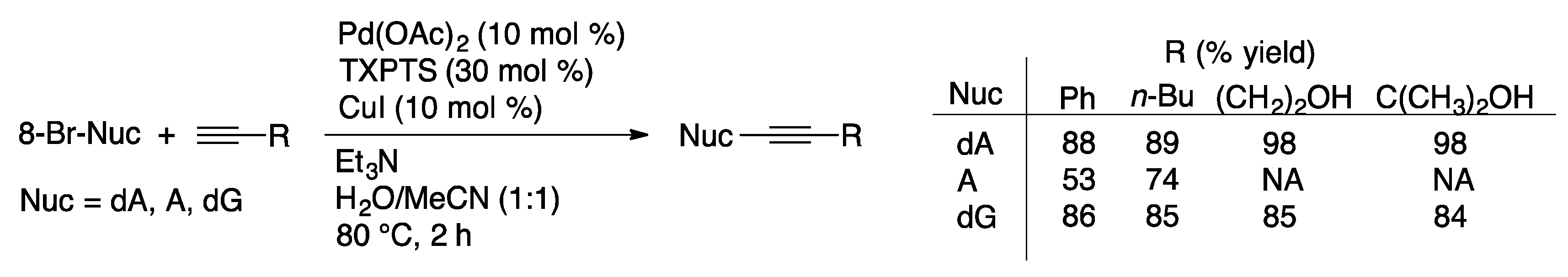

4.1.3. Heck Couplings
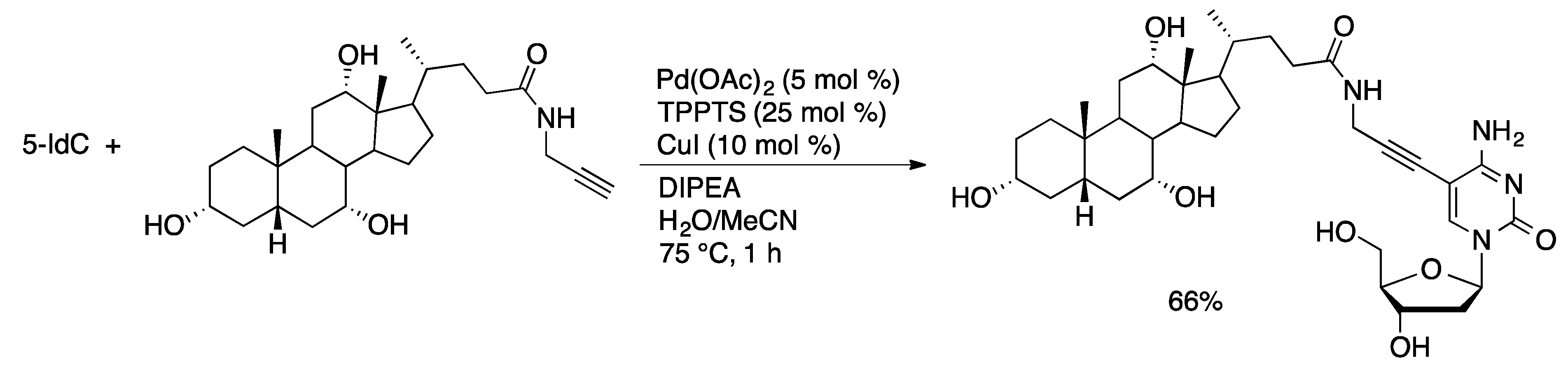
4.2. Nucleotides
4.2.1. Suzuki Coupling
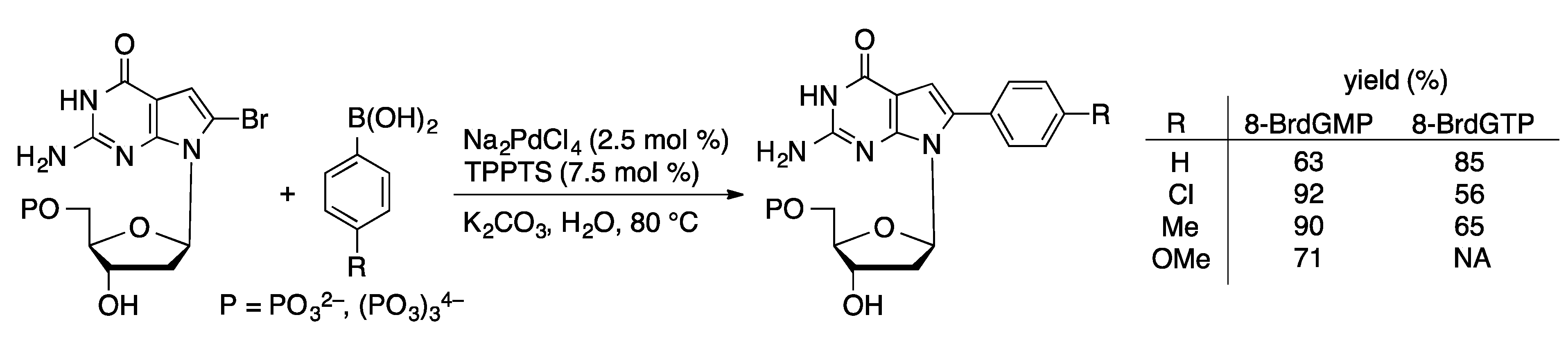
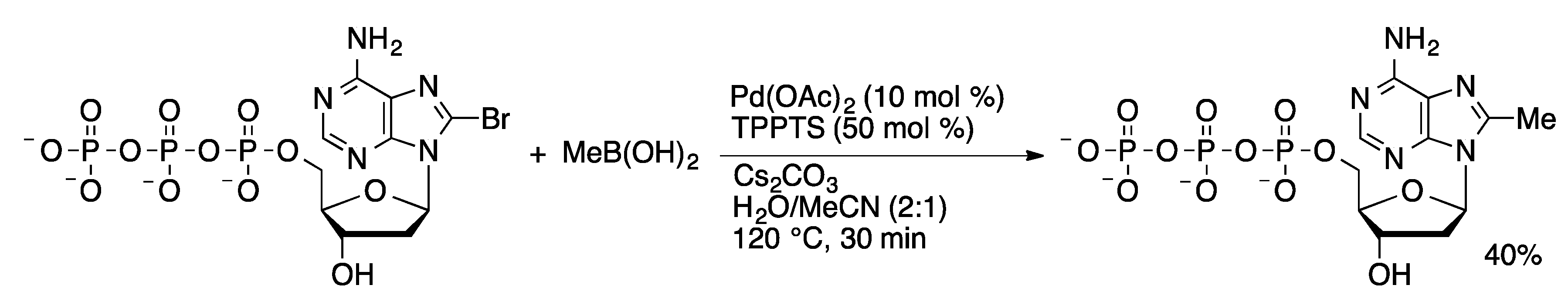

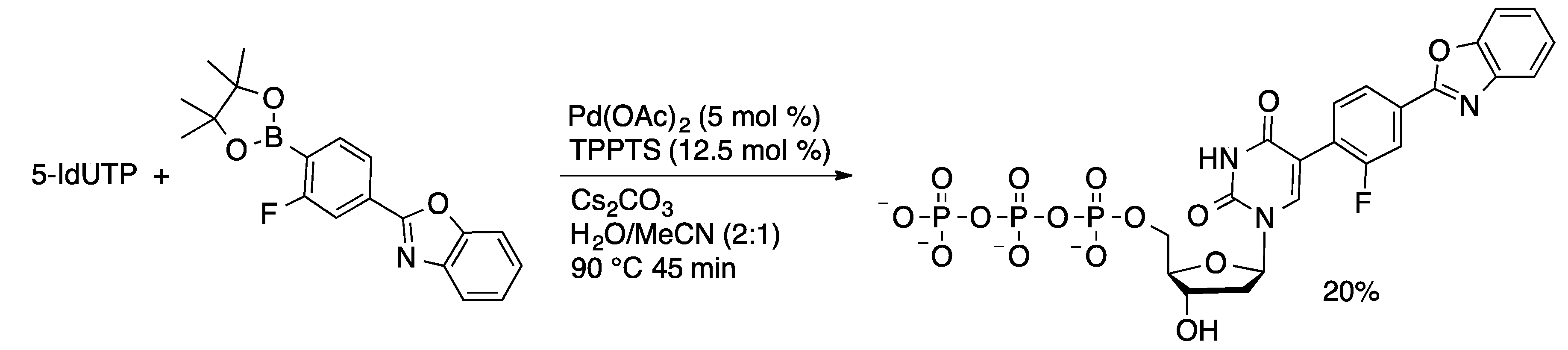

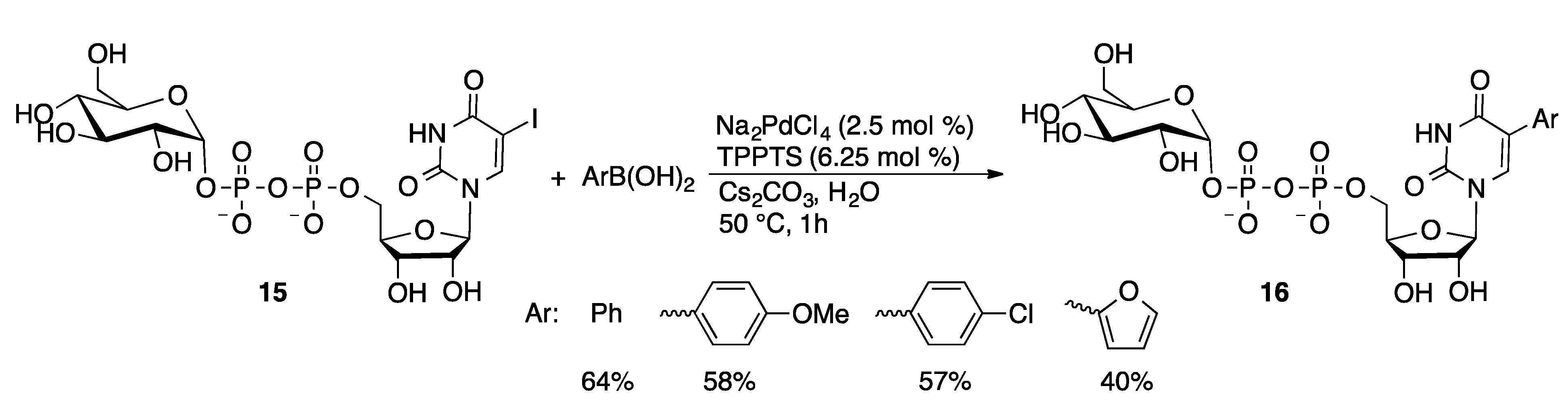

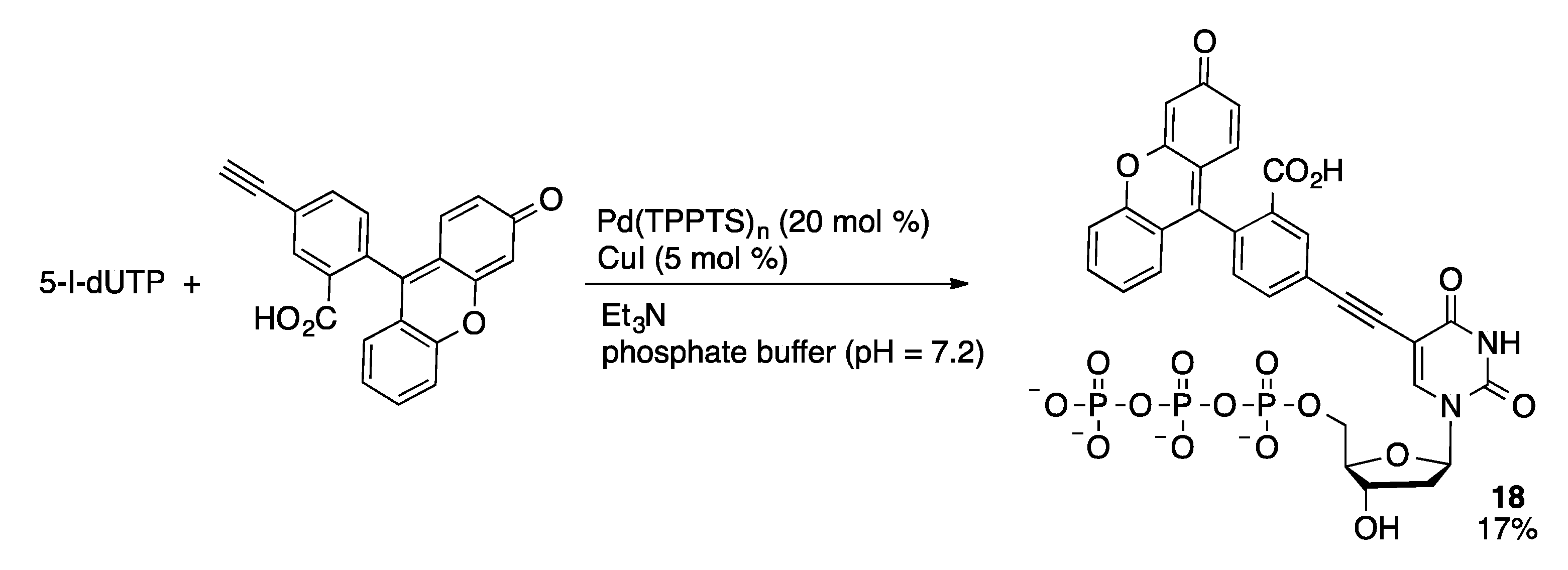
4.2.2. Sonogashira Coupling

4.2.3. Heck Coupling
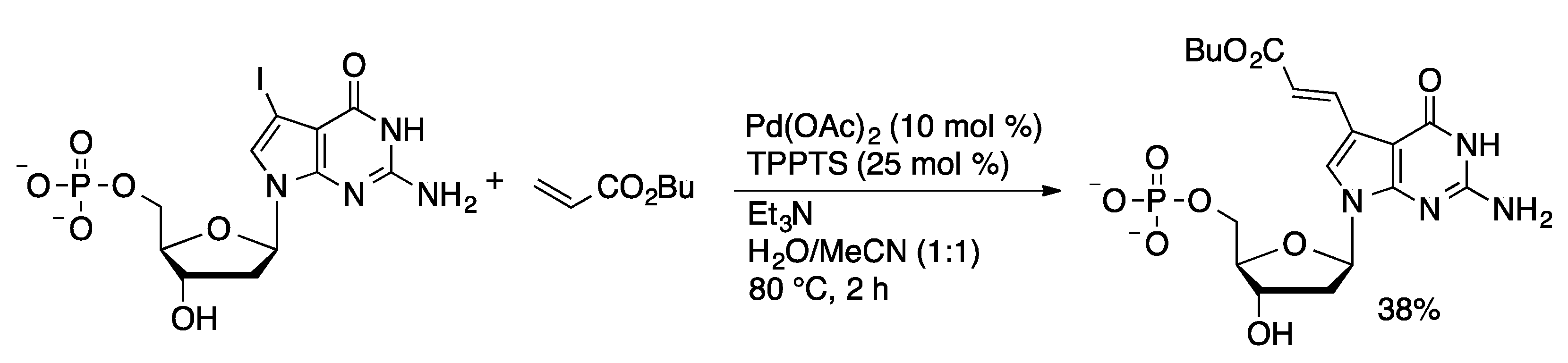
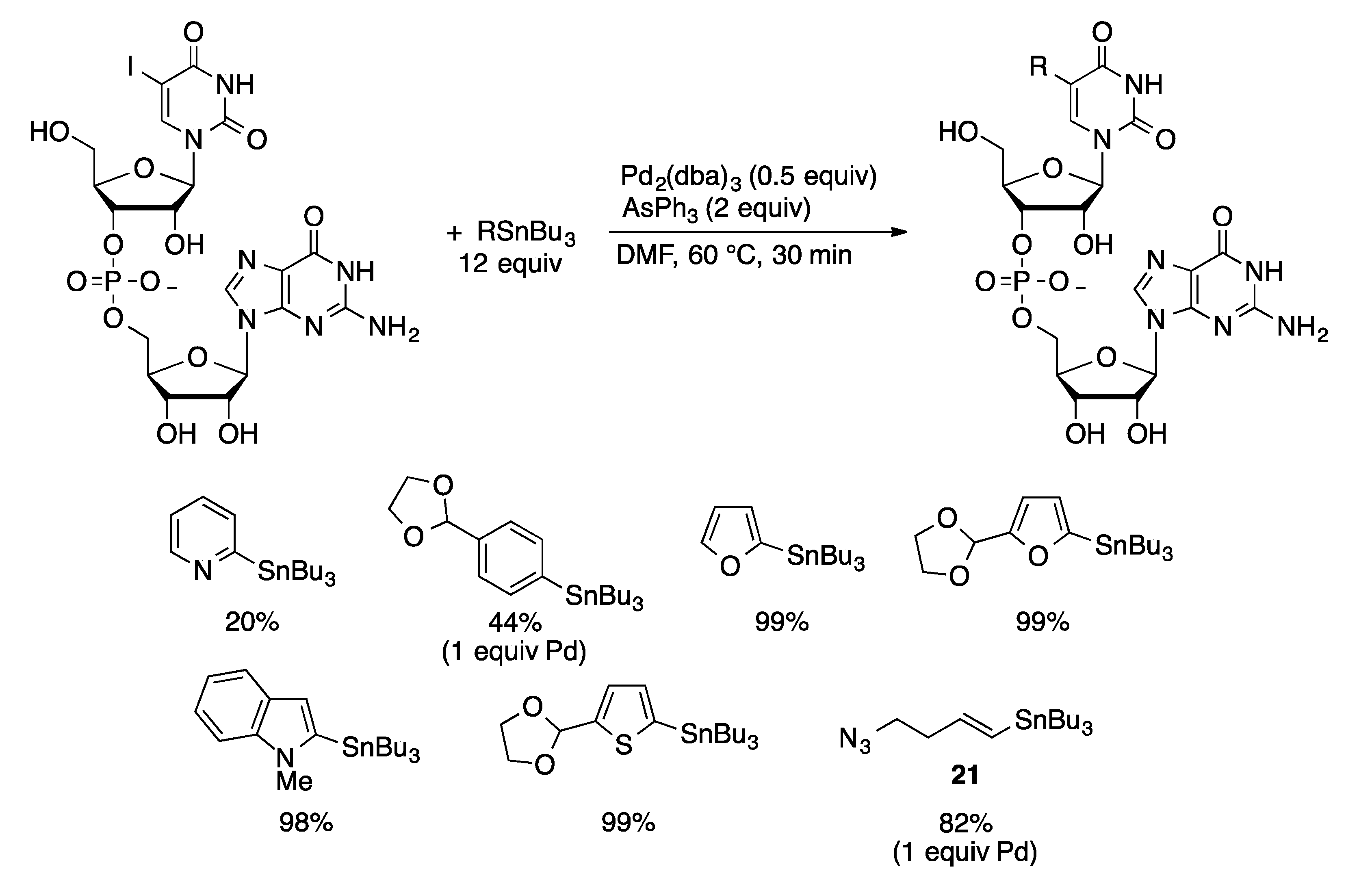
4.3. Oligonucleotides


5. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- De Clercq, E. The unabated synthesis of new nucleoside analogs with antiviral potential: A tribute to Morris J. Robins. Nucleos. Nucleot. Nucl. Acids 2009, 28, 586–600. [Google Scholar] [CrossRef]
- Robak, T. New Purine Nucleoside Analogs for Acute Lymphoblastic Leukemia. Clin. Cancer Drugs 2014, 1, 2–10. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Sofia, M.J.; Chang, W.; Furman, P.A.; Mosley, R.T.; Ross, B.S. Nucleoside, Nucleotide, and Non-Nucleoside Inhibitors of Hepatitis C Virus NS5B RNA-Dependent RNA-Polymerase. J. Med. Chem. 2012, 55, 2481–2531. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Xu, D.; Lim, K.; Harvey, R.G. Efficient Syntheses of C8-Aryl Adducts of Adenine and Guanine Formed by Reaction of Radical Cation Metabolites of Carcinogenic Polycyclic Aromatic Hydrocarbons with DNA. J. Org. Chem. 2007, 72, 4856–4863. [Google Scholar] [CrossRef] [PubMed]
- Champeil, E.; Pradhan, P.; Lakshman, M.K. Palladium-catalyzed synthesis of nucleoside adducts from bay-and fjord-region diol epoxides. J. Org. Chem. 2007, 72, 5035–5045. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, M.K.; Gunda, P. Palladium-catalyzed synthesis of carcinogenic polycyclic aromatic hydrocarbon epoxide-nucleoside adducts: The first amination of a chloro nucleoside. Org. Lett. 2003, 5, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Tanpure, A.A.; Pawar, M.G.; Srivatsan, S.G. Fluorescent Nucleoside Analogs: Probes for Investigating Nucleic Acid Structure and Function. Isr. J. Chem. 2013, 53, 366–378. [Google Scholar] [CrossRef]
- Toseland, C.P.; Webb, M.R. Fluorescent nucleoside triphosphates for single-molecule enzymology. In Single Molecule Enzymology: Methods and Protocols; Mashanov, G.I., Batters, C., Eds.; Spring Science+Business Media, LLC: New York, NY, USA, 2011; Volume 778, pp. 161–174. [Google Scholar]
- Matarazzo, A.; Hudson, R.H.E. Fluorescent adenosine analogs: A comprehensive survey. Tetrahedron 2015, 71, 1627–1657. [Google Scholar] [CrossRef]
- Dodd, D.W.; Hudson, R.H.E. Intrinsically fluorescent base-discriminating nucleoside analogs. Mini-Rev. Org. Chem. 2009, 6, 378–391. [Google Scholar] [CrossRef]
- Thomsen, N.M.; Vongsutilers, V.; Gannett, P.M. Synthesis of C8-Aryl Purines, Nucleosides, and Phosphoramidites. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.R.; Charles, I. Recent developments in the synthesis and applications of C5-substituted pyrimidine nucleosides and nucleotides. Curr. Org. Chem. 2012, 16, 1996–2013. [Google Scholar] [CrossRef]
- Kore, A.R.; Yang, B.; Srinivasan, B. Recent Developments in the Synthesis of Substituted Purine Nucleosides and Nucleotides. Curr. Org. Chem. 2014, 18, 2072–2107. [Google Scholar] [CrossRef]
- Colacot, T.J. New Trends in Cross-Coupling: Theory and Applications; Royal Society of Chemistry: London, UK, 2015; p. 864. [Google Scholar]
- Agrofoglio, L.A.; Gillaizeau, I.; Saito, Y. Palladium-assisted routes to nucleosides. Chem. Rev. 2003, 103, 1875–1916. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, M.K. Synthesis of biologically important nucleoside analogs by palladium-catalyzed C–N bond formation. Curr. Org. Synth. 2005, 2, 83–112. [Google Scholar] [CrossRef]
- Hocek, M.; Fojta, M. Cross-coupling reactions of nucleoside triphosphates followed by polymerase incorporation. Construction and applications of base-functionalized nucleic acids. Org. Biomol. Chem. 2008, 6, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- De Ornellas, S.; Williams, T.J.; Baumann, C.G.; Fairlamb, I.J.S. Catalytic C-H/C-X bond functionalisation of nucleosides, nucleotides, nucleic acids, amino acids, peptides and proteins. In C-H and C-X Bond Functionalization; Ribas, X., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 11, pp. 409–447. [Google Scholar]
- Hervé, G.; Sartori, G.; Enderlin, G.; MacKenzie, G.; Len, C. Palladium-catalyzed Suzuki reaction in aqueous solvents applied to unprotected nucleosides and nucleotides. RSC Adv. 2014, 4, 18558–18594. [Google Scholar] [CrossRef]
- Hervé, G.; Len, C. Heck and Sonogashira couplings in aqueous media—Application to unprotected nucleosides and nucleotides. Sust. Chem. Proc. 2015, 3, 1–14. [Google Scholar] [CrossRef]
- Hervé, G.; Len, C. Aqueous microwave-assisted cross-coupling reactions applied to unprotected nucleosides. Front. Chem. 2015, 3. [Google Scholar] [CrossRef]
- Bigge, C.F.; Kalaritis, P.; Deck, J. R.; Mertes, M.P. Palladium-catalyzed coupling reactions of uracil nucleosides and nucleotides. J. Am. Chem. Soc. 1980, 102, 2033–2038. [Google Scholar] [CrossRef]
- Hirota, K.; Isobe, Y.; Kitade, Y.; Maki, Y. A simple synthesis of 5-(1-alkenyl)uracil derivatives by palladium catalyzed oxidative coupling of uracils with olefins. Synthesis 1987, 5, 495–496. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. “Homeopathic” Palladium Nanoparticle Catalysis of Carbon–Carbon Coupling Reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, H.; Hatanaka, Y.; Kuroboshi, M.; Hiyama, T. Synthesis of 5-substituted pyrimidine nucleosides through a palladium-catalyzed cross-coupling of alkylhalosilanes. Heterocycles 1996, 42, 375–384. [Google Scholar] [CrossRef]
- Gallagher-Duval, S.; Hervé, G.; Sartori, G.; Enderlin, G.; Len, C. Improved microwave-assisted ligand-free Suzuki-Miyaura cross-coupling of 5-iodo-2′-deoxyuridine in pure water. New J. Chem. 2013, 37, 1989–1995. [Google Scholar] [CrossRef]
- Kumar, P.; Hornum, M.; Nielsen, L.J.; Enderlin, G.; Andersen, N.K.; Len, C.; Hervé, G.; Sartori, G.; Nielsen, P. High-Affinity RNA Targeting by Oligonucleotides Displaying Aromatic Stacking and Amino Groups in the Major Groove. Comparison of Triazoles and Phenyl Substituents. J. Org. Chem. 2014, 79, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Enderlin, G.; Sartori, G.; Hervé, G.; Len, C. Synthesis of 6-aryluridines via Suzuki-Miyaura cross-coupling reaction at room temperature under aerobic ligand-free conditions in neat water. Tetrahedron Lett. 2013, 54, 3374–3377. [Google Scholar] [CrossRef]
- De Vries, J.G. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans. 2006, 421–429. [Google Scholar]
- Sakthivel, K.; Barbas, C.F., III. Expanding the Potential of DNA for Binding and Catalysis: Highly Functionalized dUTP Derivatives That Are Substrates for Thermostable DNA Polymerases. Angew. Chem. Int. Ed. 1998, 37, 2872–2875. [Google Scholar] [CrossRef]
- Ding, H.; Greenberg, M.M. Hole Migration is the Major Pathway Involved in Alkali-Labile Lesion Formation in DNA by the Direct Effect of Ionizing Radiation. J. Am. Chem. Soc. 2007, 129, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Hervé, G.; Len, C. First ligand-free, microwave-assisted, Heck cross-coupling reaction in pure water on a nucleoside—Application to the synthesis of antiviral BVDU. RSC Adv. 2014, 4, 46926–46929. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T. Synthesis of Biaryls through Aromatic C–H Bond Activation: A Review of Recent Developments. Adv. Synth. Catal. 2014, 356, 1661–1696. [Google Scholar] [CrossRef]
- Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C. Cross-Coupling of Heteroarenes by C–H Functionalization: Recent Progress towards Direct Arylation and Heteroarylation Reactions Involving Heteroarenes Containing One Heteroatom. Adv. Synth. Catal. 2014, 356, 17–117. [Google Scholar] [CrossRef]
- Čerňa, I.; Pohl, R.; Hocek, M. The first direct C–H arylation of purine nucleosides. Chem. Commun. 2007, 45, 4729–4730. [Google Scholar] [CrossRef]
- Storr, T.E.; Firth, A.G.; Wilson, K.; Darley, K.; Baumann, C.G.; Fairlamb, I.J.S. Site-selective direct arylation of unprotected adenine nucleosides mediated by palladium and copper: Insights into the reaction mechanism. Tetrahedron 2008, 64, 6125–6137. [Google Scholar] [CrossRef]
- Storr, T.E.; Baumann, C.G.; Thatcher, R.J.; De Ornellas, S.; Whitwood, A.C.; Fairlamb, I.J.S. Pd(0)/Cu(I)-Mediated Direct Arylation of 2′-Deoxyadenosines: Mechanistic Role of Cu(I) and Reactivity Comparisons with Related Purine Nucleosides. J. Org. Chem. 2009, 74, 5810–5821. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gloudeman, J.; Wnuk, S.F. Palladium-Catalyzed Direct Arylation of 5-Halouracils and 5-Halouracil Nucleosides with Arenes and Heteroarenes Promoted by TBAF. J. Org. Chem. 2014, 79, 4094–4103. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Fokin, V.V. Organic Synthesis “On Water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Amann, N.; Pandurski, E.; Fiebig, T.; Wagenknecht, H.A. Electron injection into DNA: Synthesis and spectrscopic properties of pyrenyl-modified oligonucleotides. Chem. Eur. J. 2002, 8, 4877–4883. [Google Scholar] [CrossRef] [PubMed]
- Amann, N.; Wagenknecht, H.A. Preparation of pyrenyl-modified nucleosides via Suzuki-Miyaura cross-coupling reactions. Synlett 2002, 5, 687–691. [Google Scholar] [CrossRef]
- Jacobsen, M.F.; Ferapontova, E.E.; Gothelf, K.V. Synthesis and electrochemical studies of an anthraquinone-conjugated nucleoside and derived oligonucleotides. Org. Biomol. Chem. 2009, 7, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Inasaki, T.; Saito, I. Synthesis and ESR studies of nitronyl nitroxide-tethered oligodeoxynucleotides. Tetrahedron Lett. 2005, 46, 791–795. [Google Scholar] [CrossRef]
- Kögler, M.; Vanderhoydonck, B.; De Jonghe, S.; Rozenski, J.; Van Belle, K.; Herman, J.; Louat, T.; Parchina, A.; Sibley, C.; Lescrinier, E.; et al. Synthesis and Evaluation of 5-Substituted 2′-deoxyuridine Monophosphate Analogs As Inhibitors of Flavin-Dependent Thymidylate Synthase in Mycobacterium tuberculosis. J. Med. Chem. 2011, 54, 4847–4862. [Google Scholar] [CrossRef] [PubMed]
- Fresneau, N.; Hiebel, M.A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient synthesis of unprotected C-5-aryl/heteroaryl-2′-deoxyuridine via a Suzuki-Miyaura reaction in aqueous media. Molecules 2012, 17, 14409–14417. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.; Valis, L.; Huber, R.; Amann, N.; Wagenknecht, H.A. Preparation of pyrene-modified purine and pyrimidine nucleosides via Suzuki-Miyaura cross-couplings and characterization of their fluorescent properties. Synthesis 2003, 15, 2335–2340. [Google Scholar]
- Valis, L.; Wagenknecht, H.A. Synthesis and optical properties of the C-8 adduct of Benzo[a]pyrene and deoxyguanosine. Synlett 2005, 15, 2281–2284. [Google Scholar]
- Kohyama, N.; Katashima, T.; Yamamoto, Y. Synthesis of novel 2-aryl AICAR derivatives. Synthesis 2004, 17, 2799–2804. [Google Scholar]
- Ogasawara, S.; Saito, I.; Maeda, M. Synthesis and reversible photoisomerization of photo-switchable nucleoside, 8-styryl-2′-deoxyguanosine. Tetrahedron Lett. 2008, 49, 2479–2482. [Google Scholar] [CrossRef]
- Wagner, C.; Wagenknecht, H.A. Reductive electron transfer in phenothiazine-modified DNA is dependent on the base sequence. Chem. Eur. J. 2005, 11, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Wanninger-Weiß, C.; Wagenknecht, H.A. Synthesis of 5-(2-pyrenyl)-2′-deoxyuridine as a DNA modification for electron-transfer studies: The critical role of the position of the chromophore attachment. Eur. J. Org. Chem. 2008, 2008, 64–71. [Google Scholar] [CrossRef]
- Ehrenschwender, T.; Wagenknecht, H.A. Synthesis and spectroscopic characterization of BODIPY-modified uridines as potential fluorescent probes for nucleic acids. Synthesis 2008, 2008, 3657–3662. [Google Scholar] [CrossRef]
- Hassan, M.E. Palladium-catalyzed cross-coupling reaction of organostannanes with nucleoside halides. Coll. Czech. Chem. Commun. 1991, 56, 1944–1947. [Google Scholar] [CrossRef]
- Holzberger, B.; Strohmeier, J.; Siegmund, V.; Diederichsen, U.; Marx, A. Enzymatic synthesis of 8-vinyl- and 8-styryl-2′-deoxyguanosine modified DNA—Novel fluorescent molecular probes. Bioorg. Med. Chem. Lett. 2012, 22, 3136–3139. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Satoh, K.; Tanaka, H.; Miyasaka, T. Introduction of carbon substituents at C-2 position of purine nucleosides. Nucleic Acids Symp. Ser. 1983, 12, 5–8. [Google Scholar] [PubMed]
- Jäger, S.; Rasched, G.; Komreich-Leshem, H.; Engesser, M.; Thum, O.; Famulok, M. A versatile toolbox for variable DNA functionalization at high density. J. Am. Chem. Soc. 2005, 127, 15071–15072. [Google Scholar] [CrossRef] [PubMed]
- Sági, G.; Ötvös, L.; Ikeda, S.; Andrei, G.; Snoeck, R.; De Clercq, E. Synthesis and antiviral activities of 8-alkynyl-, 8-alkenyl-, and 8-alkyl-2′-deoxyadenosine analogs. J. Med. Chem. 1994, 37, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Volpini, R.; Costanzi, S.; Lambertucci, C.; Vittori, S.; Klotz, K.N.; Lorenzen, A.; Cristalli, G. Introduction of Alkynyl Chains on C-8 of Adenosine Led to Very Selective Antagonists of the A3 Adenosine Receptor. Bioorg. Med. Chem. Lett. 2001, 11, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, A.; Wojtczak, B.; Lesnikowski, Z.J. 2′-Deoxyadenosine Bearing Hydrophobic Carborane Pharmacophore. Nucleos. Nucleot. Nucl. Acids 2007, 26, 1611–1613. [Google Scholar] [CrossRef]
- Seela, F.; Ingale, S.A. “Double Click” Reaction on 7-Deazaguanine DNA: Synthesis and Excimer Fluorescence of Nucleosides and Oligonucleotides with Branched Side Chains Decorated with Proximal Pyrenes. J. Org. Chem. 2010, 75, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Baranger, A.M. Design of an adenosine analogue that selectively improves the affinity of a mutant U1A protein for RNA. J. Am. Chem. Soc. 2003, 125, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Ardhapure, A.V.; Sanghvi, Y.S.; Kapdi, A.R.; García, J.; Sanchez, G.; Lozano, P.; Serrano, J.L. Pd-imidate complexes as recyclable catalysts for the synthesis of C5-alkenylated pyrimidine nucleosides via Heck cross-coupling reaction. RSC Adv. 2015, 5, 24558–24563. [Google Scholar] [CrossRef]
- Li, C.J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: A decade update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, K.H. Beyond TPPTS: New approaches to the development of efficient palladium-catalyzed aqueous-phase cross-coupling reactions. Eur. J. Org. Chem. 2006, 2006, 1827–1835. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. Suzuki-Miyaura Cross-Coupling Reactions in Aqueous Media: Green and Sustainable Syntheses of Biaryls. ChemSusChem 2010, 3, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, K.H. Greener approaches to cross-coupling. In New Trends in Cross-Coupling: Theory and Application; Colacot, T.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 645–696. [Google Scholar]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifiations in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Casalnuovo, A.L.; Calabrese, J.C. Palladium-catalyzed alkylation in aqueous media. J. Am. Chem. Soc. 1990, 112, 4324–4330. [Google Scholar] [CrossRef]
- Western, E.C.; Daft, J.R.; Johnson, E.M., II; Gannett, P.M.; Shaughnessy, K.H. Efficient, one-step Suzuki arylation of unprotected halonucleosides using water-soluble palladium catalysts. J. Org. Chem. 2003, 68, 6767–6774. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, K.H.; Booth, R.S. Sterically demanding, water-soluble alkylphosphines as ligands for high activity Suzuki coupling of aryl bromides in aqueous solvents. Org. Lett. 2001, 3, 2757–2759. [Google Scholar] [CrossRef] [PubMed]
- Hobley, G.; Gubala, V.; Rivera-Sánchez, M.D.C.; Rivera, J.M. Synthesis of 8-heteroaryl-2′-deoxyguanosine derivatives. Synlett 2008, 2008, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Western, E.C.; Shaughnessy, K.H. Inhibitory effects of the guanine moiety on the Suzuki couplings of unprotected halonucleosides in aqueous media. J. Org. Chem. 2005, 70, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Both ligands are available from STREM Chemical Co: TPPTS (15–8007, $79/mmol), TXPTS (15–7860, $149/mmol).
- Collier, A.; Wagner, G.K. Suzuki-Miyaura cross-coupling of unprotected halopurine nucleosides in water-influence of catalyst and cosolvent. Synth. Commun. 2006, 36, 3713–3721. [Google Scholar] [CrossRef]
- Sartori, G.; Enderlin, G.; Hervé, G.; Len, C. Highly effective synthesis of C-5-substituted 2′-deoxyuridine using Suzuki-Miyaura cross-coupling in water. Synthesis 2012, 44, 767–772. [Google Scholar] [CrossRef]
- Sartori, G.; Hervé, G.; Enderlin, G.; Len, C. New, efficient approach for the ligand-free Suzuki-Miyaura reaction of 5-iodo-2′-deoxyuridine in water. Synthesis 2013, 45, 330–333. [Google Scholar] [CrossRef]
- Kapdi, A.; Gayakhe, V.; Sanghvi, Y.S.; García, J.; Lozano, P.; da Silva, I.; Pérez, J.; Serrano, J.L. New water soluble Pd-imidate complexes as highly efficient catalysts for the synthesis of C5-arylated pyrimidine nucleosides. RSC Adv. 2014, 4, 17567–17572. [Google Scholar] [CrossRef]
- Lercher, L.; McGouran, J.F.; Kessler, B.M.; Schofield, C.J.; Davis, B.G. DNA Modification under Mild Conditions by Suzuki-Miyaura Cross-Coupling for the Generation of Functional Probes. Angew. Chem. Int. Ed. 2013, 52, 10553–10558. [Google Scholar] [CrossRef]
- Čapek, P.; Hocek, M. Efficient one-step synthesis of optically pure (adenin-8-yl)phenylalanine nucleosides. Synlett 2005, 19, 3005–3007. [Google Scholar]
- Čapek, P.; Pohl, R.; Hocek, M. Cross-coupling reactions of unprotected halopurine bases, nucleosides, and nucleoside triphosphates with 4-boronophenylalanine in water. Synthesis of (purin-8-yl)- and (purin-6-yl)phenylalanines. Org. Biomol. Chem. 2006, 4, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Vrábel, M.; Pohl, R.; Klepetářová, B.; Votruba, I.; Hocek, M. Synthesis of 2′-deoxyadenosine nucleosides bearing bipyridine-type ligands and their Ru-complexes in position 8 through cross-coupling reactions. Org. Biomol. Chem. 2007, 5, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Vrábel, M.; Pohl, R.; Votruba, I.; Sajadi, M.; Kovalenko, S.A.; Ernsting, N.P.; Hocek, M. Synthesis and photophysical properties of 7-deaza-2′-deoxyadenosines bearing bipyridine ligands and their Ru(II)-complexes in position 7. Org. Biomol. Chem. 2008, 6, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Kalachova, L.; Pohl, R.; Hocek, M. Synthesis of 2′-deoxyuridine and 2′-deoxycytidine nucleosides bearing bipyridine and terpyridine ligands at position 5. Synthesis 2009, 2009, 105–112. [Google Scholar] [CrossRef]
- Rankin, K.M.; Sproviero, M.; Rankin, K.; Sharma, P.; Wetmore, S.D.; Manderville, R.A. C8-Heteroaryl-2′-deoxyguanosine Adducts as Conformational Fluorescent Probes in the NarI Recognition Sequence. J. Org. Chem. 2012, 77, 10498–10508. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, K.M.; Millen, A.L.; Wetmore, S.D.; Manderville, R.A. An indole-linked C8-deoxyguanosine nucleoside acts as a fluorescent reporter of Watson-Crick versus Hoogsteen base pairing. Org. Biomol. Chem. 2011, 9, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Manderville, R.A.; Omumi, A.; Rankin, K.M.; Wilson, K.A.; Millen, A.L.; Wetmore, S.D. Fluorescent C-linked C8-aryl-guanine probe for distinguishing syn from anti structures in duplex DNA. Chem. Res. Toxicol. 2012, 25, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Mineo, R.; Tamamushi, R.; Mizuta, M.; Ohkubo, A.; Taguchi, H.; Seio, K.; Santa, T.; Sekine, M. Synthesis and Fluorescent Properties of Bi- and Tricyclic 4-N-Carbamoyldeoxycytidine Derivatives. J. Org. Chem. 2007, 77, 102–108. [Google Scholar] [CrossRef]
- Mizuta, M.; Seio, K.; Miyata, K.; Sekine, M. Fluorescent Pyrimidopyrimidoindole Nucleosides: Control of Photophysical Characterizations by Substituent Effects. J. Org. Chem. 2007, 72, 5046–5055. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Fischer, B. Analogs of uracil nucleosides with intrinsic fluorescence (NIF-analogs): Synthesis and photophysical properties. Org. Biomol. Chem. 2012, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Storr, T.E.; Strohmeier, J.A.; Baumann, C.G.; Fairlamb, I.J.S. A sequential direct arylation/Suzuki-Miyaura cross-coupling transformation of unprotected 2′-deoxyadenosine affords a novel class of fluorescent analogs. Chem. Commun. 2010, 46, 6470–6472. [Google Scholar] [CrossRef] [Green Version]
- Bárta, J.; Pohl, R.; Klepetářová, B.; Ernsting, N.P.; Hocek, M. Modular Synthesis of 5-Substituted Thiophen-2-yl C-2′-Deoxyribonucleosides. J. Org. Chem. 2008, 73, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Cahová, H.; Jäschke, A. Nucleoside-Based Diarylethene Photoswitches and Their Facile Incorporation into Photoswitchable DNA. Angew. Chem. Int. Ed. 2013, 52, 3186–3190. [Google Scholar] [CrossRef]
- Macíčková-Cahová, H.; Pohl, R.; Horáková, P.; Havran, L.; Špaček, J.; Fojta, M.; Hocek, M. Alkylsulfanylphenyl Derivatives of Cytosine and 7-Deazaadenine Nucleosides, Nucleotides and Nucleoside Triphosphates: Synthesis, Polymerase Incorporation to DNA and Electrochemical Study. Chem. Eur. J. 2011, 17, 5833–5841. [Google Scholar] [CrossRef] [PubMed]
- Gubala, V.; Betancourt, J.E.; Rivera, J.M. Expanding the Hoogsteen Edge of 2′-Deoxyguanosine: Consequences for G-Quadruplex Formation. Org. Lett. 2004, 6, 4735–4738. [Google Scholar] [CrossRef] [PubMed]
- Vongsutilers, V.; Phillips, D.J.; Train, B.C.; McKelvey, G.R.; Thomsen, N.M.; Shaughnessy, K.H.; Lewis, J.P.; Gannett, P.M. The conformational effect of para-substituted C8-arylguanine adducts on the B/Z-DNA equilibrium. Biophys. Chem. 2011, 154, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nauš, P.; Kuchař, M.; Hocek, M. Cytostatic and antiviral 6-arylpurine ribonucleosides IX. synthesis and evaluation of 6-substituted 3-deazapurine ribonucleosides. Coll. Czech. Chem. Comm. 2008, 73, 665–678. [Google Scholar] [CrossRef]
- Nauš, P.; Pohl, R.; Votruba, I.; Džubák, P.; Hajdúch, M.; Ameral, R.; Birkuš, G.; Wang, T.; Ray, A.S.; Mackman, R.; et al. 6-(Het)aryl-7-Deazapurine Ribonucleosides as Novel Potent Cytostatic Agents. J. Med. Chem. 2010, 53, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Bourderioux, A.; Nauš, P.; Perlíková, P.; Pohl, R.; Pichová, I.; Votruba, I.; Džubák, P.; Konečný, P.; Hajdúch, M.; Stray, K.M.; et al. Synthesis and Significant Cytostatic Activity of 7-Hetaryl-7-deazaadenosines. J. Med. Chem. 2011, 54, 5498–5507. [Google Scholar] [CrossRef] [PubMed]
- Amiable, C.; Paoletti, J.; Haouz, A.; Padilla, A.; Labesse, G.; Kaminski, P.A.; Pochet, S. 6-(Hetero)Arylpurine nucleotides as inhibitors of the oncogenic target DNPH1: Synthesis, structural studies and cytotoxic activities. Eur. J. Med. Chem. 2014, 85, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Nauš, P.; Perlíková, P.; Bourderioux, A.; Pohl, R.; Slavětínská, L.; Votruba, I.; Bahador, G.; Birkuš, G.; Cihlář, T.; Hocek, M. Sugar-modified derivatives of cytostatic 7-(het)aryl-7-deazaadenosines: 2′-C-methylribonucleosides, 2′-deoxy-2′-fluoroarabinonucleosides, arabinonucleosides and 2′-deoxyribonucleosides. Bioorg. Med. Chem. 2012, 20, 5202–5214. [Google Scholar] [CrossRef] [PubMed]
- Perlíková, P.; Jornet Martínez, N.; Slavětínská, L.; Hocek, M. Synthesis of 2′-deoxy-2′-fluororibo- and 2′-deoxy-2′,2′-difluororibonucleosides derived from 6-(het)aryl-7-deazapurines. Tetrahedron 2012, 68, 8300–8310. [Google Scholar] [CrossRef]
- Nauš, P.; Caletková, O.; Konečny, P.; Džubak, P.; Bogdanova, K.; Kolář, M.; Vrbková, J.; Slavětinská, L.; Tloušt’ová, E.; Perlíková, P.; et al. Synthesis, Cytostatic, Antimicrobial, and Anti-HCV Activity of 6-Substituted 7-(Het)aryl-7-deazapurine Ribonucleosides. J. Med. Chem. 2014, 57, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, S.; Maćičková-Cahová, H.; Pohl, R.; Šanda, M.; Hocek, M. Synthesis of nucleoside and nucleotide conjugates of bile acids, and polymerase construction of bile acid-functionalized DNA. Org. Biomol. Chem. 2010, 8, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Prickett, C.D.; Shaughnessy, K.H. Efficient Sonogashira Coupling of Unprotected Halonucleosides in Aqueous Solvents Using Water-Soluble Palladium Catalysts. Eur. J. Org. Chem. 2010, 3678–3683. [Google Scholar] [CrossRef]
- Cho, J.H.; Shaughnessy, K.H. Aqueous-phase Sonogashira alkynylation to synthesize 5-substituted pyrimidine and 8-substituted purine nucleosides. Curr. Prot. Nucleic Acid Chem. 2012, 49. [Google Scholar] [CrossRef]
- Cho, J.H.; Shaughnessy, K.H. Aqueous-Phase Heck Coupling of 5-Iodouridine and Alkenes under Phosphine-Free Conditions. Synlett 2011, 20, 2963–2966. [Google Scholar]
- Thoresen, L.H.; Jiao, G.S.; Haaland, W.C.; Metzker, M.L.; Burgess, K. Rigid, conjugated, fluoresceinated thymidine triphosphates: Syntheses and polymerase mediated incorporation into DNA analogs. Chem. Eur. J. 2003, 9, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.; Wagner, G. A facile two-step synthesis of 8-arylated guanosine mono- and triphosphates (8-aryl GXPs). Org. Biomol. Chem. 2006, 4, 4526–4532. [Google Scholar] [CrossRef] [PubMed]
- Čapek, P.; Cahová, H.; Pohl, R.; Hocek, M.; Gloeckner, C.; Marx, A. An efficient method for the construction of functionalized DNA bearing amino acid groups through cross-coupling reactions of nucleoside triphosphates followed by primer extension or PCR. Chem. Eur. J. 2007, 13, 6196–6203. [Google Scholar] [CrossRef] [PubMed]
- Cahová, H.; Pohl, R.; Bednárová, L.; Nováková, K.; Cvačka, J.; Hocek, M. Synthesis of 8-bromo, 8-methyl- and 8-phenyl-dATP and their polymerase incorporation into DNA. Org. Biomol. Chem. 2008, 6, 3657–3660. [Google Scholar] [CrossRef] [PubMed]
- Cahová, H.; Havran, L.; Brázdilová, P.; Pivoňková, H.; Pohl, R.; Fojta, M.; Hocek, M. Aminophenyl- and nitrophenyl-labeled nucleoside triphosphates: Synthesis, enzymatic incorporation, and electrochemical detection. Angew. Chem. Int. Ed. 2008, 47, 2059–2062. [Google Scholar] [CrossRef]
- Balintová, J.; Plucnara, M.; Vidláková, P.; Pohl, R.; Havran, L.; Fojta, M.; Hocek, M. Benzofurazane as a New Redox Label for Electrochemical Detection of DNA: Towards Multipotential Redox Coding of DNA Bases. Chem. Eur. J. 2013, 19, 12720–12731. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.; Pohl, R.; Rulíšek, L.; Hocek, M. Synthesis and Photophysical Properties of Biaryl-Substituted Nucleosides and Nucleotides. Polymerase Synthesis of DNA Probes Bearing Solvatochromic and pH-Sensitive Dual Fluorescent and 19F-NMR Labels. J. Org. Chem. 2012, 77, 1026–1044. [Google Scholar] [CrossRef] [PubMed]
- Raindlová, V.; Pohl, R.; Šanda, M.; Hocek, M. Direct Polymerase Synthesis of Reactive Aldehyde-Functionalized DNA and Its Conjugation and Staining with Hydrazines. Angew. Chem. Int. Ed. 2010, 49, 1064–1066. [Google Scholar] [CrossRef]
- Pesnot, T.; Wagner, G.K. Novel derivatives of UDP-glucose: Concise synthesis and fluorescent properties. Org. Biomol. Chem. 2008, 6, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.; Wagner, G.K. A fast synthetic route to GDP-sugars modified at the nucleobase. Chem. Commun. 2008, 2, 178–180. [Google Scholar] [CrossRef]
- Pergolizzi, G.; Butt, J.N.; Bowater, R.P.; Wagner, G.K. A novel fluorescent probe for NAD-consuming enzymes. Chem. Commun. 2011, 47, 12655–12657. [Google Scholar] [CrossRef] [Green Version]
- Brázdilová, P.; Vrábel, M.; Pohl, R.; Pivoňková, H.; Havran, L.; Hocek, M.; Fojta, M. Ferrocenylethynyl derivatives of nucleoside triphosphates: Synthesis, incorporation, electrochemistry, and bioanalytical applications. Chem. Eur. J. 2007, 13, 9527–9533. [Google Scholar] [CrossRef] [PubMed]
- Dadová, J.; Vidlákova, P.; Pohl, R.; Havran, L.; Fojta, M.; Hocek, M. Aqueous Heck Cross-Coupling Preparation of Acrylate-Modified Nucleotides and Nucleoside Triphosphates for Polymerase Synthesis of Acrylate-Labeled DNA. J. Org. Chem. 2013, 78, 9627–9637. [Google Scholar] [CrossRef] [PubMed]
- Omumi, A.; Beach, D.G.; Baker, M.; Gabryelski, W.; Manderville, R.A. Postsynthetic Guanine Arylation of DNA by Suzuki-Miyaura Cross-Coupling. J. Am. Chem. Soc. 2011, 133, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Hertl, A.; Muttach, F.; Jäschke, A. Phosphine-Free Stille-Migita Chemistry for the Mild and Orthogonal Modification of DNA and RNA. Chem. Eur. J. 2014, 20, 16613–16619. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Uozumi, Y. C-N and C-S bond forming cross coupling in water with amphiphilic resin-supported palladium complexes. Chem. Lett. 2011, 40, 934–935. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Abela, A.R.; Moser, R.; Nishikata, T.; Duplais, C.; Krasovskiy, A.; Gaston, R.D.; Gadwood, R.C. TPGS-750-M: A Second-Generation Amphiphile for Metal-Catalyzed Cross-Couplings in Water at Room Temperature. J. Org. Chem. 2011, 76, 4379–4391. [Google Scholar] [CrossRef] [PubMed]
- Tardiff, B.J.; Stradiotto, M. Buchwald-Hartwig Amination of (Hetero)aryl Chlorides by Employing Mor-DalPhos under Aqueous and Solvent-Free Conditions. Eur. J. Org. Chem. 2012, 3972–3977. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaughnessy, K.H. Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides. Molecules 2015, 20, 9419-9454. https://doi.org/10.3390/molecules20059419
Shaughnessy KH. Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides. Molecules. 2015; 20(5):9419-9454. https://doi.org/10.3390/molecules20059419
Chicago/Turabian StyleShaughnessy, Kevin H. 2015. "Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides" Molecules 20, no. 5: 9419-9454. https://doi.org/10.3390/molecules20059419