3. Experimental Section
3.1. General
All reagents and solvents were of reagent grade or purified according to standard methods before use. Analytical thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC) were performed with silica gel plates using silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao city, China). Melting points were determined in Kofler apparatus and were uncorrected. IR spectra were obtained on NIC-5DX spectra photometer,
1H-NMR spectra were recorded at 400 MHz on a Bruker AM-400 spectrometer using TMS as reference (Bruker Company, Boston, MA, USA). Elemental analyses were determined on a Vario El Gmbh elemental analyzer. The starting camptothecin was isolated from a Chinese medicinal plant
C. acuminata and was purified before being used. A key intermediate for the synthesis of three series of target compounds was camptothecin-7-aldehyde (
3), which was prepared according to Sawada
et al. [
38].
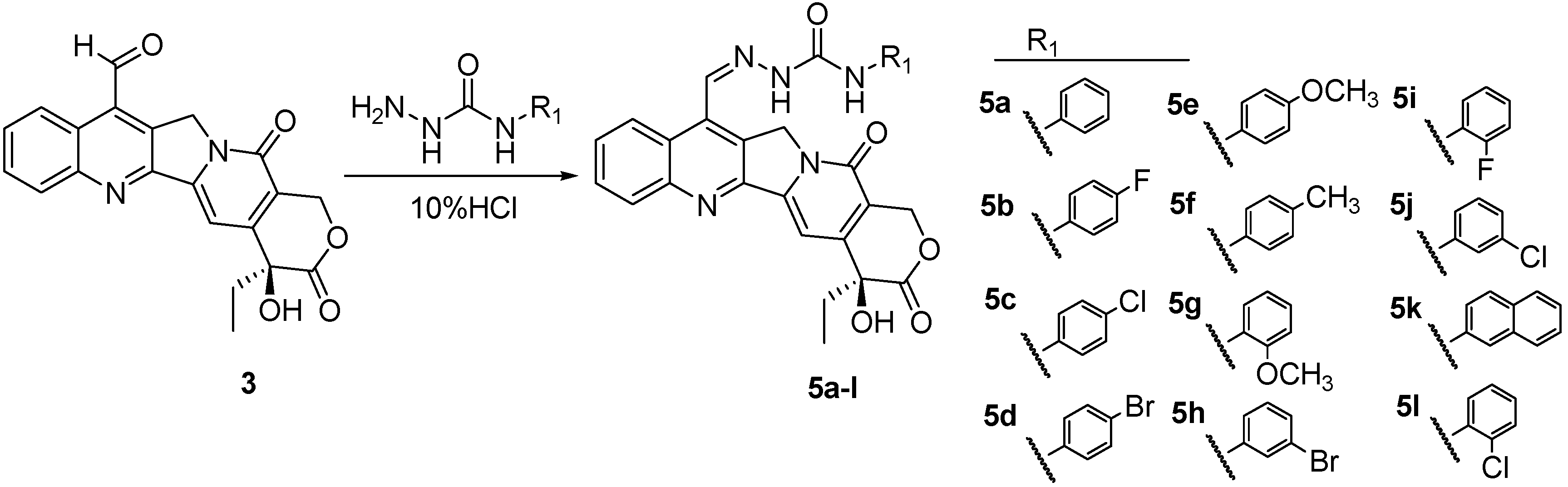
3.2. General Synthetic Procedure for Target Compounds 4a–l
To a solution of 7-formyl camptothecin (0.1 mmol) in chloroform (10 mL) and methanol (10 mL), an appropriate N-substituent-thioureidohydrazine (0.12 mmol) and 10% hydrochloric (0.5 mL) were added. The reaction mixture was stirred for 2 h at room temperature. A large amount of precipitate was producted. After filteration, the precipitate was washed with chloroform to give a yellow solid. Recrystallization from N, N-dimethyl formamine gave compounds 4a–l.
7-(N-phenylthioureidoiminomethyl)-camptothecin (4a). Yield: 85%; mp: 235 °C (decomp.); IR(KBr) cm−1: 3444 (OH), 3324, 3287 (N-H), 1737 (γ-lactone), 1655 (C=N), 1253 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.90 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 5.44 (s, 2H, 5-H), 5.66–5.78 (m, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.22–7.26 (m, 1H, 4'-H), 7.36 (s, 1H, 14-H), 7.40–7.44 (m, 2H, 2', 6'-H), 7.82 (t, 1H, 11-H), 7.85–7.89 (m, 2H, 3', 5'-H), 7.92 (t, 1H, 10-H), 8.24 (d, J = 8.0 Hz, 1H, 12-H), 8.33 (d, J = 8.0 Hz, 1H, 9-H), 9.19 (s, 1H, 7-CH), 10.01 (s, 1H, CSNHC), 12.32 (s, 1H, NNH). Anal. calc. for C28H23N5O4S: C 63.99%, H 4.41%, N 13.33%. Found: C 63.98%, H 4.42%, N 13.34%.
7-(N-benzylthioureidoiminomethyl)-camptothecin (4b). Yield: 89%; mp: 243 °C (decomp.); IR(KBr) cm−1: 3436 (OH), 3387, 3266 (N-H), 1747 (γ-lactone), 1659 (C=N), 1258 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.89 (m, 3H, 19-H), 1.84–1.91 (m, 2H, 18-H), 4.89 (d, J = 5.6 Hz, 2H, benzyl-CH2), 5.45 (s, 2H, 5-H), 5.57–5.73 (m, 2H, 17-H), 6.53 (s, 1H, 20-OH), 7.23–7.27 (m, 1H, 4'-H), 7.35 (s, 1H, 14-H), 7.38 (d, J = 7.2 Hz, 2H, 2', 6'-H), 7.45 (d, J = 7.2 Hz, 2H, 3', 5'-H), 7.79 (t, 1H, 11-H), 7.90 (t, 1H, 10-H), 8.21 (d, J = 8.4 Hz, 1H, 12-H), 8.32 (d, J = 8.4 Hz, 1H, 9-H), 9.07 (s, 1H, 7-CH), 9.08 (s, 1H, CSNHC), 12.05 (s, 1H, NNH). Anal. calc. for C29H25N5O4S: C 64.55%, H 4.67%, N 12.98%. Found: C 64.56%, H 4.66%, N 12.98%.
7-[N-(4-chlorinephenyl)thioureidoiminomethyl]-camptothecin (4c). Yield: 76%; mp: 265 °C (decomp.); IR(KBr) cm−1: 3437 (OH), 3321, 3273 (N-H), 1730 (γ-lactone), 1651(C=N), 1249(C=S); 1H-NMR (400 MHz, DMSO-d6): δ 0.86–0.89 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 5.44 (s, 2H, 5-H), 5.69–5.81 (m, 2H, 17-H), 6.55 (s, 1H, 20-OH), 7.37 (s, 1H, 14-H), 7.47 (d, J = 8.8Hz, 2H, 2', 6'-H), 7.83–7.87 (m, 1H, 11-H), 7.91 (d, J = 8.8Hz, 2H, 3', 5'-H), 7.94–7.96 (m, 1H, 10-H), 8.26 (d, J = 8.0 Hz, 1H, 12-H), 8.35 (d, J = 8.0 Hz, 1H, 9-H), 9.20 (s, 1H, 7-CH), 10.11 (s, 1H, CSNHC), 12.42 (s, 1H, NNH). Anal. calc. for C28H22N5O4SCl: C 60.05%, H 3.96%, N 12.51%. Found: C 60.07%, H 3.94%, N 12.51%.
7-[N-(4-bromophenyl)-thioureidoiminomethyl]-camptothecin (4d). Yield: 83%; mp: 253 °C (decomp.); IR(KBr) cm−1: 3436 (OH), 3321, 3266 (N-H), 1729 (γ-lactone), 1650 (C=N), 1248 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.89 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 2.33 (s, 3H, 4'-CH3), 5.44 (s, 2H, 5-H), 5.70–5.81 (m, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.37 (s, 1H, 14-H), 7.60 (d, J = 8.8Hz, 2H, 2', 6'-H), 7.84 (d, J = 8.8Hz, 2H, 3', 5'-H), 7.85 (t, 1H, 11-H), 7.95 (t, 1H, 10-H), 8.26 (d, J = 8.4 Hz, 1H, 12-H), 8.35 (d, J = 8.4 Hz, 1H, 9-H), 9.20 (s, 1H, 7-CH), 10.10 (s, 1H, CSNHC), 12.43 (s, 1H, NNH). Anal. calc. for C28H22N5O4SBr: C 55.64%, H 3.67%, N 11.59%. Found: C 55.64%, H 3.65%, N 11.61%.
7-[N-(4-methoxylphenyl)-thioureidoiminomethyl]-camptothecin (4e). Yield: 82%; mp: 234 °C (decomp.); IR(KBr) cm−1: 3437 (OH), 3325, 3262 (N-H), 1728 (γ-lactone), 1653 (C=N), 1251 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.89 (m, 3H, 19-H), 1.82–1.92 (m, 2H, 18-H), 3.78 (s, 3H, 4'-OCH3), 5.43 (s, 2H, 5-H), 5.64–5.76 (m, 2H, 17-H), 6.54 (s, 1H, 20-OH), 6.97 (d, J = 8.8Hz, 1H, 2', 6'-H), 7.36 (s, 1H, 14-H), 7.67 (d, J = 8.8Hz, 1H, 3', 5'-H), 7.82 (t, 1H, 11-H), 7.92 (t, 1H, 10-H), 8.23 (d, J = 8.4 Hz, 1H, 12-H), 8.32 (d, J = 8.4 Hz, 1H, 9-H), 9.15 (s, 1H, 7-CH), 9.91 (s, 1H, CSNHC), 12.22 (s, 1H, NNH). Anal. calc. for C29H25N5O5S: C 62.69%, H 4.54%, N 12.60%. Found: C 62.67%, H 4.56%, N 12.60%.
7-[N-(4-methylphenyl)-thioureidoiminomethyl]-camptothecin (4f). Yield: 73%; mp: 246 °C (decomp.); IR(KBr) cm−1: 3439 (OH), 3325, 3284 (N-H), 1731 (γ-lactone), 1650 (C=N), 1250 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.89 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 2.33 (s, 3H, 4'-CH3), 5.44 (s, 2H, 5-H), 5.69–5.81 (m, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.22 (d, J = 8.0Hz, 2H, 2', 6'-H), 7.37 (s, 1H, 14-H), 7.73 (d, J = 8.0Hz, 2H, 3', 5'-H), 7.85 (t, 1H, 11-H), 7.94 (t, 1H, 10-H), 8.25 (d, J = 8.4 Hz, 1H, 12-H), 8.34 (d, J = 8.4 Hz, 1H, 9-H), 9.20 (s, 1H, 7-CH), 9.97 (s, 1H, CSNHC), 12.29 (s, 1H, NNH). Anal. calc. for C29H25N5O4S: C 64.55%, H 4.67%, N 12.98%. Found: C 64.56%, H 4.66%, N 12.98%.
7-[N-(2-methoxylphenyl)-thioureidoiminomethyl]-camptothecin (4g). Yield: 81%; mp: 272 °C (decomp.); IR(KBr) cm−1: 3436 (OH), 3324 (N-H), 1741 (γ-lactone), 1657 (C=N), 1233 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.90 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 3.88 (s, 3H, 2'-OCH3), 5.42 (s, 2H, 5-H), 5.53–5.64 (m, 2H, 17-H), 6.53 (s, 1H, 20-OH), 6.98–7.02 (m, 1H, 4'-H), 7.12–7.14 (m, 1H, 3'-H), 7.22–7.24 (m, 1H, 5'-H), 7.37 (s, 1H, 14-H), 7.85 (t, 1H, 11-H), 7.94 (t, 1H, 10-H), 8.25 (d, J = 8.4 Hz, 1H, 12-H), 8.38–8.41 (m, 2H, 9, 6' -H), 9.22 (s, 1H, 7-CH), 9.83 (s, 1H, CSNHC), 12.35 (s, 1H, NNH). Anal. calc. for C29H25N5O5S: C 62.69%, H 4.54%, N 12.60%. Found: C 62.68%, H 4.55%, N 12.61%.
7-[N-(2-methylphenyl)-thioureidoiminomethyl]-camptothecin (4h). Yield: 77%; mp: 265 °C (decomp.); IR(KBr) cm−1: 3471 (OH), 3279, 3126 (N-H), 1741 (γ-lactone), 1664 (C=N), 1257 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.85–0.88 (m, 3H, 19-H), 1.82–1.90 (m, 2H, 18-H), 2.38 (s, 3H, 2'-CH3), 5.41 (s, 2H, 5-H), 5.66–5.82 (m, 2H, 17-H), 6.53 (s, 1H, 20-OH), 7.22–7.42 (m, 4H, 3', 4', 5', 6'-H), 7.35 (s, 1H, 14-H), 7.83 (t, 1H, 11-H), 7.93 (t, 1H, 10-H), 8.24 (d, J = 8.4 Hz, 1H, 12-H), 8.35 (d, J = 8.4 Hz, 1H, 9-H), 9.19 (s, 1H, 7-CH), 9.82 (s, 1H, CSNHC), 12.25 (s, 1H, NNH). Anal. calc. for C29H25N5O4S: C 64.55%, H 4.67%, N 12.98%. Found: C 64.56%, H 4.65%, N 12.99%.
7-[N-(1-naphthyl)-thioureidoiminomethyl]-camptothecin (4i). Yield: 87%; mp: 278 °C (decomp.); IR(KBr) cm−1: 3436 (broad, OH, N-H), 1745 (γ-lactone), 1656 (C=N), 1227(C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.85–0.88 (m, 3H, 19-H), 1.80–1.91 (m, 2H, 18-H), 5.39 (s, 2H, 5-H), 5.74–5.90 (m, 2H, 17-H), 6.53 (s, 1H, 20-OH), 7.36 (s, 1H, 14-H), 7.55–7.66 (m, 4H, naphthyl-H), 7.85 (t, 1H, 11-H), 7.93 (t, 1H, 10-H), 7.94–8.01 (m, 3H, naphthyl-H), 8.25 (d, J = 8.4 Hz, 1H, 12-H), 8.42 (d, J = 8.4 Hz, 1H, 9-H), 9.23 (s, 1H, 7-CH), 10.37 (s, 1H, CSNHC), 12.37 (s, 1H, NNH). Anal. calc. for C32H25N5O4S: C 66.77%, H 4.38%, N 12.17%. Found: C 66.76%, H 4.37%, N 12.19%.
7-[N-(4-fluorophenyl)-thioureidoiminomethyl]-camptothecin (4j). Yield: 74%; mp: 271 °C (decomp.); IR(KBr) cm−1: 3438 (OH), 3321, 3266 (N-H), 1744 (γ-lactone), 1655 (C=N), 1236 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.85–1.92 (m, 2H, 18-H), 5.45 (s, 2H, 5-H), 5.74–5.81 (m, 2H, 17-H), 6.56 (s, 1H, 20-OH), 7.25–7.29 (m, 2H, 2', 6'-H), 7.38 (s, 1H, 14-H), 7.81–7.85 (m, 2H, 3', 5'-H), 7.87 (s, 1H, 11-H), 7.95 (t, 1H, 10-H), 8.25 (d, J = 8.4 Hz, 1H, 12-H), 8.35 (d, J = 8.4 Hz, 1H, 9-H), 9.19 (s, 1H, 7-CH), 10.07 (s, 1H, CSNHC), 12.37 (s, 1H, NNH). Anal. calc. for C28H22N5O4SF: C 61.87%, H 4.08%, N 12.88%. Found: C 61.88%, H 4.09%, N 12.86%.
7-[N-(3-chlorinephenyl)-thioureidoiminomethyl]-camptothecin (4k). Yield: 69%; mp: 269 °C (decomp.); IR(KBr) cm−1: 3439 (OH), 3267, 3132 (N-H), 1741 (γ-lactone), 1651 (C=N), 1254 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.86–1.90 (m, 2H, 18-H), 5.45 (s, 2H, 5-H), 5.75–5.77 (m, 2H, 17-H), 6.56 (s, 1H, 20-OH), 7.31 (d, J = 7.6 Hz, 1H, 6'-H), 7.38 (s, 1H, 14-H), 7.46 (t, 1H, 5'-H), 7.80 (d, J = 9.6 Hz, 1H, 4'-H), 7.86 (t, 1H, 11-H), 7.95 (t, 1H, 10-H), 8.12 (s, 1H, 2'-H), 8.25 (d, J = 8.4 Hz, 1H, 12-H), 8.35 (d, J = 8.4 Hz, 1H, 9-H), 9.22 (s, 1H, 7-CH), 10.14 (s, 1H, CSNHC), 12.47 (s, 1H, NNH). Anal. calc. for C28H22N5O4SCl: C 60.05%, H 3.96%, N 12.51%. Found: C 60.07%, H 3.93%, N 12.52%.
7-[N-cyclohexyl-thioureidoiminomethyl]-camptothecin (4l). Yield: 58%; mp: 263 °C (decomp.); IR(KBr) cm−1: 3438 (OH), 3279, 3126 (N-H), 1746 (γ-lactone), 1652 (C=N), 1226 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.21–1.27 (m, 1H, cyclohexane-H), 1.32–1.40 (m, 2H, cyclohexane-H), 1.47–1.55 (m, 2H, cyclohexane-H), 1.63–1.65 (m, 1H, cyclohexane-H), 1.77–1.80 (m, 2H, cyclohexane-H), 1.86–1.90 (m, 2H, 18-H), 2.01–2.03 (m, 2H, cyclohexane-H), 4.19 (s, 1H, cyclohexane-H), 5.46 (s, 2H, 5-H), 5.53 (s, 2H, 17-H), 6.56 (s, 1H, 20-OH), 7.36 (s, 1H, 14-H), 7.83 (t, 1H, 11-H), 7.89 (d, J = 8.4 Hz, 1H, CSNHC), 7.94 (t, 1H, 10-H), 8.24 (d, J = 8.8 Hz, 1H, 12-H), 8.28 (d, J = 8.4 Hz, 1H, 9-H), 9.12 (s, 1H, 7-CH), 12.05 (s, 1H, NNH). Anal. calc. for C28H29N5O4S: C 63.26%, H 5.50%, N 13.17%. Found: C 63.25%, H 5.51%, N 13.17%.
3.3. General Synthetic Procedure for Target Compounds 5a–l
To a solution of 7-formyl camptothecin (0.1 mmol) in chloroform (10 mL) and methanol (10 mL), an appropriate N-substituent-carbamidohydrazine (0.12 mmol) and 10% hydrochloric acid (0.5 mL) were added. The reaction mixture was stirred for 2 h at room temperature. A large amount of precipitate was produced. After filtration, the precipitate was washed with chloroform to give a pale yellow solid. Recrystallization from N, N-dimethyl dimethylformamide gave compounds 5a–l.
7-(N-phenylaminocarbonylhydrazonomethyl)camptothecin (5a). Yield: 82%; mp: 232 °C (decomp.); IR(KBr) cm−1: 3397 (broad, OH, N-H), 1751 (γ-lactone), 1689 (C=O) 1658(C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.83–1.93 (m, 2H, 18-H), 5.45 (s, 2H, 5-H), 5.67 (s, 2H, 17-H), 6.53 (s, 1H, 20-OH), 7.05–7.08 (m, 1H, 4'-H), 7.33–7.35 (m, 2H, 2', 6'-H), 7.36 (s, 1H, 14-H), 7.75–7.77 (m, 2H, 3', 5'-H), 7.80 (t, 1H, 11-H), 7.90 (t, 1H, 10-H), 8.22 (d, J = 8.4 Hz, 1H, 12-H), 8.34 (d, J = 8.4 Hz, 1H, 9-H), 8.79 (s, 1H, 7-CH), 8.96 (s, 1H, CONHC), 11.37 (s, 1H, NNH). Anal. alc. for C28H23N5O5: C 66.00%, H 4.55%, N 13.75%. Found: C 66.02%, H 4.53%, N 13.75%.
7-[N-(4-fluorophenyl)-carbonylhydrazonomethyl]-camptothecin (5b). Yield: 74%; mp: 236 °C (decomp.); IR(KBr) cm−1: 3406 (broad, OH, N-H), 1742 (γ-lactone), 1706 (C=O), 1656 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.90 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 5.46 (s, 2H, 5-H), 5.71 (s, 2H, 17-H), 6.55 (s, 1H, 20-OH), 7.17–7.21 (m, 2H, 2', 6'-H), 7.37 (s, 1H, 14-H), 7.75–7.78 (m, 2H, 3', 5'-H), 7.83 (t, 1H, 11-H), 7.93 (t, 1H, 10-H), 8.24 (d, J = 8.0 Hz, 1H, 12-H), 8.36 (d, J = 8.0 Hz, 1H, 9-H), 8.92 (s, 1H, 7-CH), 8.98 (s, 1H, CONHC), 11.40 (s, 1H, NNH). Anal. calc. for C28H22N5O5F: C 63.75%, H 4.20%, N 13.28%. Found: C 63.75%, H 4.21%, N 13.27%.
7-[N-(4-chlorophenyl)-carbonylhydrazonomethyl]-camptothecin (5c). Yield: 85%; mp: 225 °C (decomp.); IR(KBr) cm−1: 3294–3401 (broad, OH, N-H), 1751 (γ-lactone), 1706 (C=O), 1668 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 5.46 (s, 2H, 5-H), 5.65 (s, 2H, 17-H), 6.53 (s, 1H, 20-OH), 7.36 (s, 1H, 14-H), 7.39 (d, J = 8.8Hz, 2H, 2', 6'-H), 7.79 (d, J = 8.8Hz, 2H, 3', 5'-H), 7.80 (t, 1H, 11-H), 7.91 (t, 1H, 10-H), 8.22 (d, J = 8.4 Hz, 1H, 12-H), 8.34 (d, J = 8.4 Hz, 1H, 9-H), 8.96 (s, 1H, 7-CH), 8.97 (s, 1H, CONHC), 11.43 (s, 1H, NNH). Anal. calc. for C28H22N5O5Cl: C 61.82%, H 4.08%, N 12.87%. Found: C 61.81%, H 4.09%, N 12.87%.
7-[N-(4-bromophenyl)-carbonylhydrazonomethyl]-camptothecin (5d). Yield: 72%; mp: 237 °C (decomp.); IR(KBr) cm−1: 3443 (OH), 3393 (N-H), 1733 (γ-lactone), 1709 (C=O), 1650 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.83–1.93 (m, 2H, 18-H), 5.45 (s, 2H, 5-H), 5.66 (s, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.36 (s, 1H, 14-H), 7.51 (d, J = 8.8Hz, 2H, 2', 6'-H), 7.74 (d, J = 8.8Hz, 2H, 3', 5'-H), 7.80 (t, 1H, 11-H), 7.91 (t, 1H, 10-H), 8.22 (d, J = 8.4 Hz, 1H, 12-H), 8.34 (d, J = 8.4 Hz, 1H, 9-H), 8.96 (s, 1H, 7-CH), 8.97 (s, 1H, CONHC), 11.45 (s, 1H, NNH). Anal. calc. for C28H22N5O5Br: C 57.15%, H 3.77%, N 11.90%. Found: C 57.15%, H 3.76%, N 11.91%.
7-[N-(4-methoxyphenyl)-carbonylhydrazonomethyl]-camptothecin (5e). Yield: 76%; mp: 254 °C (decomp.); IR(KBr) cm−1: 3259–3409 (broad, OH, N-H), 1739 (γ-lactone), 1687 (C=O), 1661 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.90 (m, 3H, 19-H), 1.83–1.93 (m, 2H, 18-H), 3.75(s, 3H, 4'-OCH3), 5.46 (s, 2H, 5-H), 5.67–5.70 (m, 2H, 17-H), 6.54 (s, 1H, 20-OH), 6.92 (d, J = 8.8Hz, 2H, 2', 6'-H), 7.37 (s, 1H, 14-H), 7.65 (d, J = 8.8Hz, 2H, 3', 5'-H), 7.82 (t, 1H, 11-H), 7.92 (t, 1H, 10-H), 8.23 (d, J = 8.4 Hz, 1H, 12-H), 8.35 (d, J = 8.4 Hz, 1H, 9-H), 8.70 (s, 1H, 7-CH), 8.96 (s, 1H, CONHC), 11.31 (s, 1H, NNH). Anal. calc. for C29H25N5O6: C 64.56%, H 4.67%, N 12.98%. Found: C 64.56%, H 4.68%, N 12.97%.
7-[N-(4-methylphenyl)-carbonylhydrazonomethyl]-camptothecin (5f). Yield: 87%; mp: 239 °C (decomp.); IR(KBr) cm−1: 3502 (OH), 3401, 3211(N-H), 1749 (γ-lactone), 1688 (C=O), 1659 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.83–1.93 (m, 2H, 18-H), 2.28(s, 3H, 4'-CH3), 5.46 (s, 2H, 5-H), 5.66 (s, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.15 (d, J = 8.4Hz, 2H, 2', 6'-H), 7.36 (s, 1H, 14-H), 7.64 (d, J = 8.4Hz, 2H, 3', 5'-H), 7.79–7.83 (m, 1H, 11-H), 7.89–7.93 (m, 1H, 10-H), 8.22 (d, J = 8.0 Hz, 1H, 12-H), 8.34 (d, J = 8.0 Hz, 1H, 9-H), 8.71 (s, 1H, 7-CH), 8.96 (s, 1H, CONHC), 11.35 (s, 1H, NNH). Anal. calc. for C29H25N5O5: C 66.53%, H 4.81%, N 13.38%. Found: C 66.52%, H 4.81%, N 13.37%.
7-[N-(2-methoxyphenyl)-carbonylhydrazonomethyl]-camptothecin (5g). Yield: 78%; mp: 242 °C (decomp.); IR(KBr) cm−1: 3469 (OH), 3384, 3298 (N-H), 1746 (γ-lactone), 1686 (C=O), 1660 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.86–1.90 (m, 2H, 18-H), 3.91(s, 3H, 2'-OCH3), 5.44 (s, 2H, 5-H), 5.51 (s, 2H, 17-H), 6.54 (s, 1H, 20-OH), 6.93–7.09 (m, 3H, 3', 4', 5'-H), 7.37 (s, 1H, 14-H), 7.83 (t, 1H, 11-H), 7.93 (t, 1H, 10-H), 8.17(dd, J = 8.0, 1.2Hz, 6'-H), 8.25 (d, J = 8.4 Hz, 1H, 12-H), 8.42 (d, J = 8.4 Hz, 1H, 9-H), 8.69 (s, 1H, 7-CH), 9.00 (s, 1H, CONHC), 11.47 (s, 1H, NNH). Anal. calc. for C29H25N5O6: C 64.56%, H 4.67%, N 12.98%. Found: C 64.54%, H 4.68%, N 12.98%.
7-[N-(3-bromophenyl)-carbonylhydrazonomethyl]-camptothecin (5h). Yield: 72%; mp: 253 °C (decomp.); IR(KBr) cm−1: 3282–3396 (broad, OH, N-H), 1746 (γ-lactone), 1699 (C=O), 1665 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.90 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 5.46 (s, 2H, 5-H), 5.70 (s, 2H, 17-H), 6.55 (s, 1H, 20-OH), 7.11–7.33 (m, 3H, 4', 5', 6'-H), 7.36 (s, 1H, 14-H), 7.70 (d, J = 8.4Hz, 1H, 2'-H), 7.81–7.86 (m, 1H, 11-H), 7.93 (t, 1H, 10-H), 8.24 (d, J = 8.4 Hz, 1H, 12-H), 8.36 (d, J = 8.4 Hz, 1H, 9-H), 8.99 (s, 1H, 7-CH), 9.04 (s, 1H, CONHC), 11.49 (s, 1H, NNH). Anal. calc. for C28H22N5O5Br: C 57.15%, H 3.77%, N 11.90%. Found: C 57.16%, H 3.76%, N 11.92%.
7-[N-(2-fluorophenyl)-carbonylhydrazonomethyl]-camptothecin (5i). Yield: 67%; mp: 232 °C (decomp.); IR(KBr) cm−1: 3276–3398 (broad, OH, N-H), 1732 (γ-lactone), 1702 (C=O), 1655 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.86–0.90 (m, 3H, 19-H), 1.85–1.89 (m, 2H, 18-H), 5.43 (s, 2H, 5-H), 5.54 (s, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.15–7.34 (m, 3H, 3', 4', 5'-H), 7.35 (s, 1H, 14-H), 7.80–7.84 (m, 1H, 6'-H), 7.90–7.92 (m, 1H, 11-H), 7.94–8.00 (m, 1H, 10-H), 8.23 (d, J = 8.0 Hz, 1H, 12-H), 8.43 (d, J = 8.0 Hz, 1H, 9-H), 8.68 (s, 1H, 7-CH), 8.97 (s, 1H, CONHC), 11.45 (s, 1H, NNH). Anal. calc. for C28H22N5O5F: C 63.75%, H 4.20%, N 13.28%. Found: C 63.74%, H 4.23%, N 13.26%.
7-[N-(3-chlorophenyl)-carbonylhydrazonomethyl]-camptothecin (5j). Yield: 83%; mp: 248 °C (decomp.); IR(KBr) cm−1: 3631 (OH), 3398, 3292 (N-H), 1751 (γ-lactone), 1691(C=O), 1659 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.90 (m, 3H, 19-H), 1.82–1.93 (m, 2H, 18-H), 5.45 (s, 2H, 5-H), 5.69 (s, 2H, 17-H), 6.55 (s, 1H, 20-OH), 7.11(dd, J = 8.0, 1.2Hz, 1H, 6'-H), 7.34–7.38 (m, 1H, 5'-H), 7.37 (s, 1H, 14-H), 7.65–7.67 (m, 1H, 4'-H), 7.82 (t, 1H, 11-H), 7.89 (t, 1H, 10-H), 7.94–7.96 (m, 1H, 2'-H), 8.23 (d, J = 8.4 Hz, 1H, 12-H), 8.35 (d, J = 8.4 Hz, 1H, 9-H), 8.98 (s, 1H, 7-CH), 9.03 (s, 1H, CONHC), 11.49 (s, 1H, NNH). Anal. calc. for C28H22N5O5Cl: C 61.82%, H 4.08%, N 12.87%. Found: C 61.84%, H 4.07%, N 12.85%.
7-[N-(1-naphthyl)-carbonylhydrazonomethyl]-camptothecin (5k). Yield: 84%; mp: 275 °C (decomp.); IR(KBr) cm−1: 3402 (broad, OH, N-H), 1741 (γ-lactone), 1694 (C=O), 1658 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.88–0.91 (m, 3H, 19-H), 1.84–1.98 (m, 2H, 18-H), 5.50 (s, 2H, 5-H), 5.70–5.80 (m, 2H, 17-H), 6.53 (s, 1H, 20-OH), 7.38 (s, 1H, 14-H), 7.38–7.50 (m, 2H, naphthyl-H), 7.83 (t, 1H, 11-H), 7.85–7.90 (m, 4H, naphthyl-H), 7.93 (t, 1H, 10-H), 8.24 (d, J = 8.0 Hz, 1H, 12-H), 8.36 (d, J = 8.4 Hz, 1H, 9-H), 9.01 (s, 1H, 7-CH), 9.03 (s, 1H, CONHC), 11.54 (s, 1H, NNH). Anal. calc. for C32H25N5O5: C 68.69%, H 4.50%, N 12.52%. Found: C 68.69%, H 4.51%, N 12.54%.
7-[N-(2-chlorophenyl)-carbonylhydrazonomethyl]-camptothecin (5l). Yield: 69%; mp: 251 °C (decomp.); IR(KBr) cm−1: 3281–3397 (broad, OH, N-H), 1748 (γ-lactone), 1689 (C=O), 1664 (C=N); 1H-NMR (400 MHz, DMSO-d6) δ: 0.88–0.92 (m, 3H, 19-H), 1.87–1.90 (m, 2H, 18-H), 5.45 (s, 2H, 5-H), 5.56 (s, 2H, 17-H), 6.55 (s, 1H, 20-OH), 7.13 (t, 1H, 4'-H), 7.37 (s, 1H, 14-H), 7.38 (t, 1H, 5'-H), 7.56 (d, J = 8.0 Hz, 1H, 3'-H), 7.83 (t, 1H, 11-H), 7.93 (t, 1H, 10-H), 8.27(dd, J = 8.0, 18.0 Hz, 6'-H), 8.32 (d, J = 8.4 Hz, 1H, 12-H), 8.40 (d, J = 8.4 Hz, 1H, 9-H), 8.73 (s, 1H, 7-CH), 9.08 (s, 1H, CONHC), 11.58 (s, 1H, NNH). Anal. calc. for C28H22N5O5Cl: C 61.82%, H 4.08%, N 12.87%. Found: C 61.83%, H 4.07%, N 12.86%.
3.4. General Synthetic Procedure for Target Compounds 6a–d
To a solution of 7-formyl camptothecin (0.1 mmol) in chloroform (10 mL) and methanol (10 mL), an appropriate N-acylsubstituent-thioureidohydrazine (0.12 mmol) and 10% hydrochloric acid (0.5 mL) were added. The reaction mixture was stirred for 2 h at room temperature. A large amount of precipitate was produced. After filtration, the precipitate was washed with chloroform to give a deep yellow solid. Recrystallization from N, N-dimethyl dimethylformamide gave compounds 6a–d.
7-(N-benzoylthioureidoiminomethyl)camptothecin (6a). Yield: 91%; mp: 277 °C (decomp.); IR(KBr) cm−1: 3435 (broad, OH, N-H), 1745 (γ-lactone), 1660 (C=O), 1602 (C=N), 1229 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.84–1.91 (m, 2H, 18-H), 4.89 (d, J = 5.6 Hz, 2H, benzyl-CH2), 5.44 (s, 2H, 5-H), 5.54 (s, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.36 (s, 1H, 14-H), 7.55–7.59 (m, 2H, 2', 6'-H), 7.67–7.71 (m, 1H, 4'-H), 7.83 (t, 1H, 11-H), 7.92 (t, 1H, 10-H), 8.03–8.05 (m, 2H, 3', 5'-H), 8.26 (d, J = 8.4 Hz, 1H, 12-H), 8.76 (d, J = 8.4 Hz, 1H, 9-H), 9.81 (s, 1H, 7-CH), 11.94 (s, 1H, CSNHCO), 13.99(s, 1H, NNH). Anal. calc. cor C29H23N5O5S: C 62.92%, H 4.19%, N 12.65%. Found: C 62.91%, H 4.18%, N 12.67%.
7-[N-(4-methylbenzoyl)-thioureidoiminomethyl]-camptothecin (6b). Yield: 84%; mp: 282 °C (decomp.); IR(KBr) cm−1: 3437 (broad, OH, N-H), 1744 (γ-lactone), 1658 (C=O), 1598 (C=N), 1229 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.88–0.92 (m, 3H, 19-H), 1.84–1.95 (m, 2H, 18-H), 2.35(s, 3H, 4'-CH3), 5.46 (s, 2H, 5-H), 5.69–5.83 (m, 2H, 17-H), 6.56 (s, 1H, 20-OH), 7.24 (d, J = 8.0Hz, 2H, 2', 6'-H), 7.39 (s, 1H, 14-H), 7.75 (d, J = 8.0Hz, 2H, 3', 5'-H), 7.87 (t, 1H, 11-H), 7.96 (t, 1H, 10-H), 8.27 (d, J = 8.4 Hz, 1H, 12-H), 8.36 (d, J = 8.4 Hz, 1H, 9-H), 9.22 (s, 1H, 7-CH), 9.99 (s, 1H, CSNHCO), 12.31 (s, 1H, NNH). Anal. calc. for C30H25N5O5S: C 63.48%, H 4.44%, N 12.34%. Found: C 63.47%, H 4.43%, N 12.35%.
7-[N-(4chlorobenzoyl)-thioureidoiminomethyl]-camptothecin (6c). Yield: 76%; mp: 269 °C (decomp.); IR(KBr) cm−1: 3438 (broad, OH, N-H), 1746 (γ-lactone), 1658 (C=O), 1599 (C=N), 1248 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.87–0.91 (m, 3H, 19-H), 1.82–1.94 (m, 2H, 18-H), 5.51 (s, 2H, 5-H), 5.76–5.77 (m, 2H, 17-H), 6.56 (s, 1H, 20-OH), 7.38 (s, 1H, 14-H), 7.48 (d, J = 8.8Hz, 2H, 2', 6'-H), 7.84–7.88 (m, 1H, 11-H), 7.92 (d, J = 8.8Hz, 2H, 3', 5'-H), 7.95–7.97 (m, 1H, 10-H), 8.27 (d, J = 8.0 Hz, 1H, 12-H), 8.36 (d, J = 8.0 Hz, 1H, 9-H), 9.21 (s, 1H, 7-CH), 10.12 (s, 1H, CSNHCO), 12.43 (s, 1H, NNH). Anal. calc. for C29H22N5O5SCl: C 59.23%, H 3.77%, N 11.91%. Found: C 59.25%, H 3.76%, N 11.93%.
7-[N-(2-naphthoyl)-thioureidoiminomethyl] -camptothecin (6d). Yield: 77%; mp: 275 °C (decomp.); IR(KBr) cm−1: 3439 (broad, OH, N-H), 1747 (γ-lactone), 1658 (C=O), 1598 (C=N), 1228 (C=S); 1H-NMR (400 MHz, DMSO-d6) δ: 0.85–0.89 (m, 3H, 19-H), 1.81–1.92 (m, 2H, 18-H), 5.40 (s, 2H, 5-H), 5.75–5.91 (m, 2H, 17-H), 6.54 (s, 1H, 20-OH), 7.37 (s, 1H, 14-H), 7.56–7.67 (m, 4H, naphthyl-H), 7.86 (t, 1H, 11-H), 7.94 (t, 1H, 10-H), 7.95–8.02 (m, 3H, naphthyl-H), 8.26 (d, J = 8.4 Hz, 1H, 12-H), 8.43 (d, J = 8.4 Hz, 1H, 9-H), 9.24 (s, 1H, 7-CH), 10.38 (s, 1H, CSNHCO), 12.38 (s, 1H, NNH). Anal. calc. for C33H25N5O5S: C 65.66%, H 4.17%, N 11.60%. Found: C 65.67%, H 4.16%, N 11.61%.
3.5. Biological Assay
All bioassays were performed on representative test organisms reared in the laboratory. The bioassay was repeated at 25 ± 2 °C according to statistical requirements. Assessments were made on a dead/alive basis, and mortality rates were corrected using Abbott’s formula [
39]. Evaluations are based on a percentage scale of 0~100, in which 0 = no activity and 100 = total kill. The deviation of values was ±5%. Probit analysis was used to determine lethal concentrations of 50% (LC
50) by using the SPSS program, version 13.0. For comparative purposes, CPT was tested as a reference. All bioassay results are summarized in
Table 1,
Table 2 and
Table 3.
3.5.1. Lethal Activity against Tetranychus cinnabarinus
The acaricidal activity of compounds
4a–
l,
5a–
l,
6a–
d, and CPT (positive control) was evaluated using the slide immersion method recommended by FAO [
40]. Thirty adult spider mites were fixed dorsally to a strip of double-sided tape attached to the slide by using a small brush. The slide was immersed and shaken for 3 s in the diluted solution of the test compound. After the excess solution was removed, the treated slides with the mites were kept at 25 ± 2 °C in a Petri dish with moist filter paper. Mortality rates were calculated 24 h after treatment. Each treatment was replicated with triplicate experiments and each replicate involved 30 adult mites. Control groups were tested with acetone only.
3.5.2. Lethal Activity against Brevicoryne brassicae
The insecticidal activity of compounds
4a–
l,
5a–
l,
6a–
d, and CPT (positive control) against
B. brassicae was evaluated according to the reported procedure [
41]. Thirty healthy adult aphids were dipped into the diluted solutions of tested compound for 5 s, superfluous fluid was removed, and aphids were placed in an air-conditioned room. Mortality rates were calculated 24 h after treatment. Each treatment was performed in triplicate. Control groups were tested with acetone only.
3.5.3. Lethal Activity against Bursaphelenchus xylophilus
Acetone solutions of compounds
4a–
l,
5a–
l,
6a–
d, and CPT (positive control) were first prepared at different concentrations. Then 10 μL of the above solutions was added to the aqueous suspension (90 μL) containing approximately 2500 living nematodes (third-instar and fourth-instar larvae of B. xylophilus) per milliliter. The blank control group was prepared in the same way but lacked the tested compound. Three replicates in each trial were made and kept at 25 °C for 24 h. Finally, the activities of five concentrations of the tested compounds were monitored under a microscope by recording the death rate of the tested nematodes. Nematodes that did not move when prodded with a needle were considered to be dead. The LC
50 values of tested compounds were calculated using the probit method [
42].
3.6. CoMFA Analysis
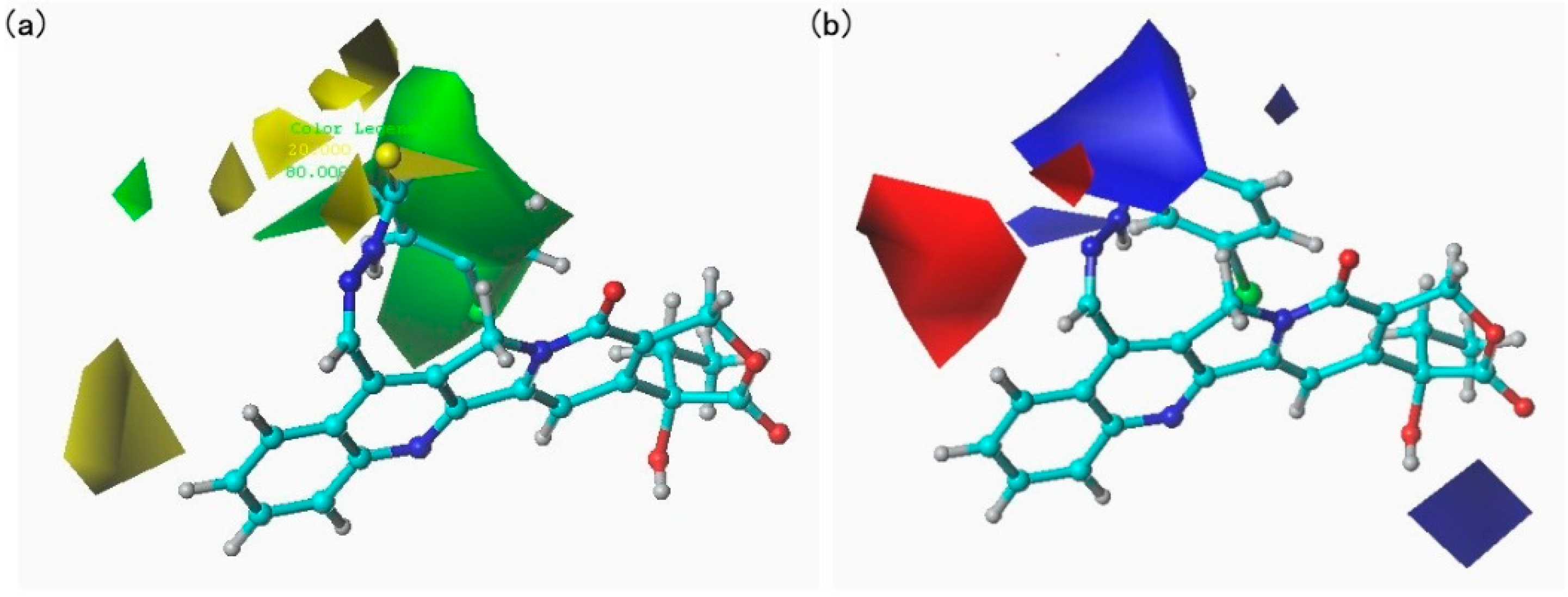
To gain insights into the key structural factors affecting bioactivity, a 3D-QSAR model using comparative molecular field analysis (CoMFA) was performed using the SYBYL 6.9 software package [
43]. Firstly, all compounds were sketched. Then, these compounds were minimized using the Tripos force field with a distance-dependent dielectric and Powell conjugate gradient algorithm with a convergence criterion of 0.05 kcal/mol. Partial atomic charges were calculated using the Gasteiger–Hückel charge. Further conformational search was performed with the multisearch routine.
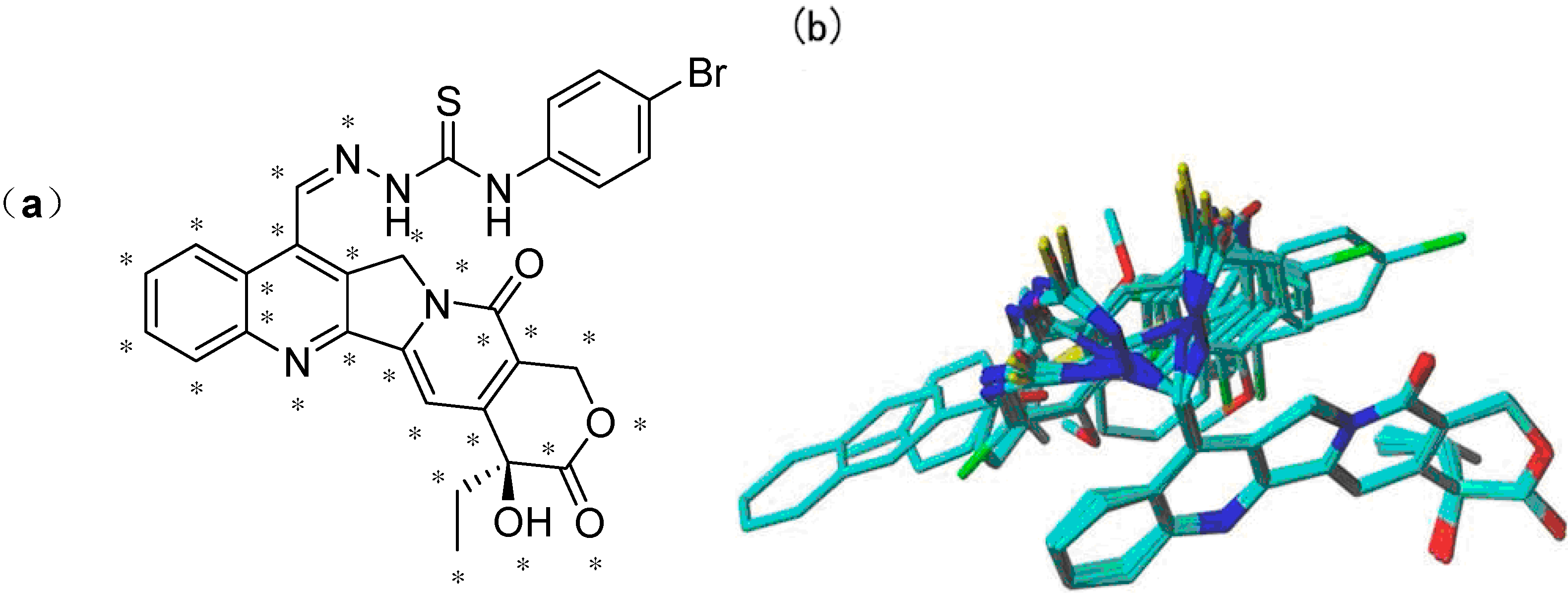
All the molecules were aligned using compound
4d (the most active compound) as the template. The DATABASE ALIGNMENT method was used to align these molecules to the template with common atoms (in total, 28 atoms) in compound
4d (
Figure 4a). The resulting alignment is shown in
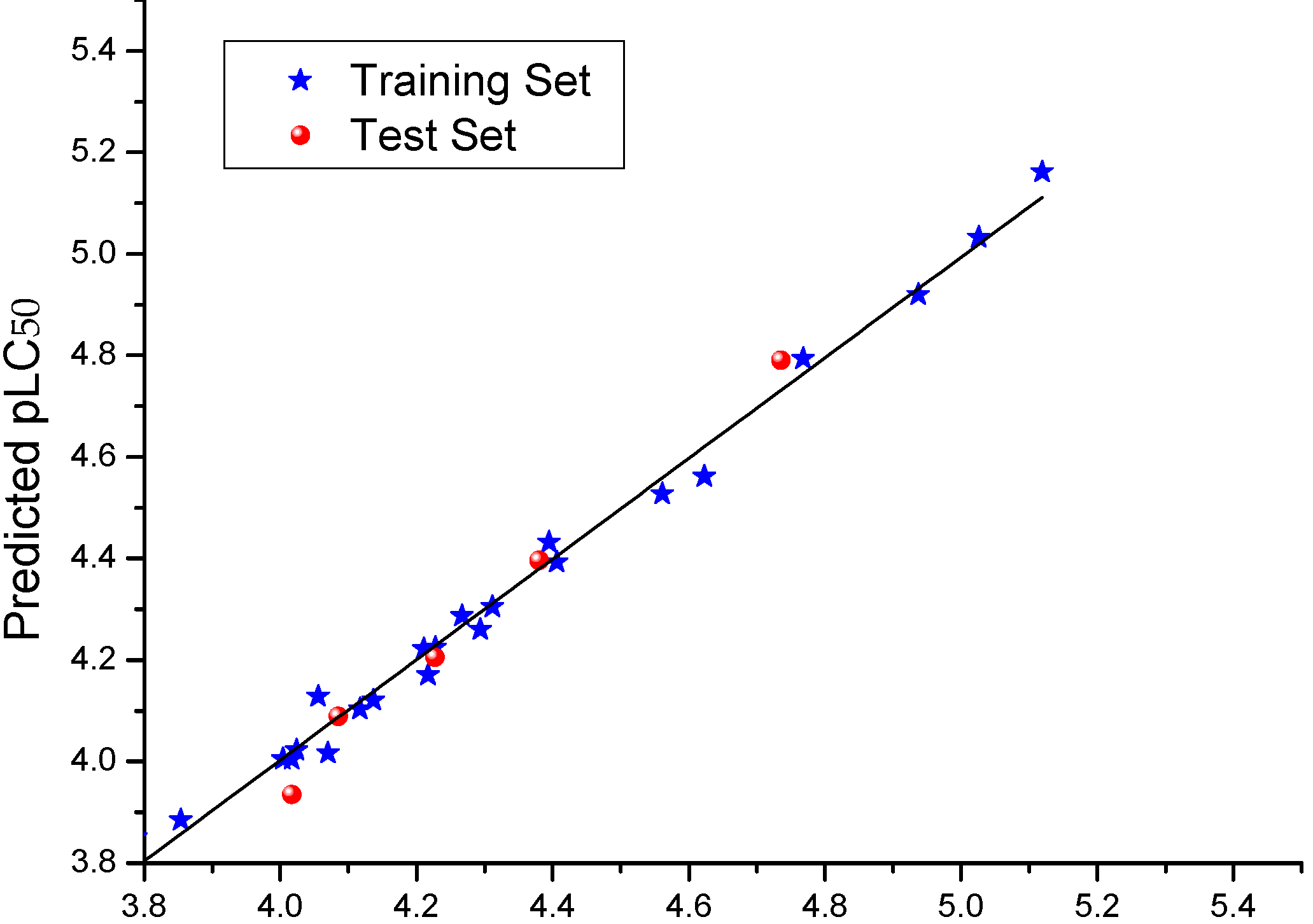
Figure 4b. To build and validate the CoMFA model, the dataset was split into training and test sets by considering the distribution of activity of compounds. The training set contained 23 compounds. The test set was composed of five compounds (labeled in
Table 3).
Figure 4.
(a) The common scaffold (marked atoms with star) of camptothecin derivatives in the alignment shown in compound 4d; (b) the alignment of camptothecin derivatives using the low energy conformers by atom fit.
Figure 4.
(a) The common scaffold (marked atoms with star) of camptothecin derivatives in the alignment shown in compound 4d; (b) the alignment of camptothecin derivatives using the low energy conformers by atom fit.
After the alignment of compounds, the molecular fields including steric (Lennard–Jones potentials) and electrostatic (Coulomb potentials) field energies were calculated using sp3 carbon as a probe atom with a grid step size of 2 Å. The cutoff value of 30 kcal/mol was adopted.
To build the relationship between the molecular fields and the biological activities (Tetranychus cinnabarinus), partial least squares (PLS) regression analyses were used to develop CoMFA models using the standard implementation in the SYBYL package. The CoMFA descriptors were used as independent variables, and pLC50 values were used as the target variables. Cross-validation in PLS was carried out using the leave-one-out method to obtain the optimal number of components. The final model was constructed with the optimum number of components equal to that yielding the highest q2. In addition, a test set of molecules with known biological activities that were not included in the model generation was used to further validate the obtained models.