Concise Synthesis of Broussonone A
Abstract
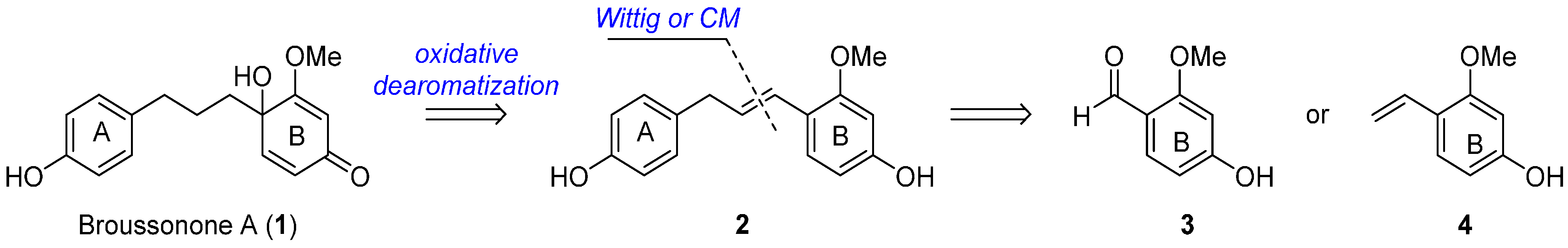
:1. Introduction
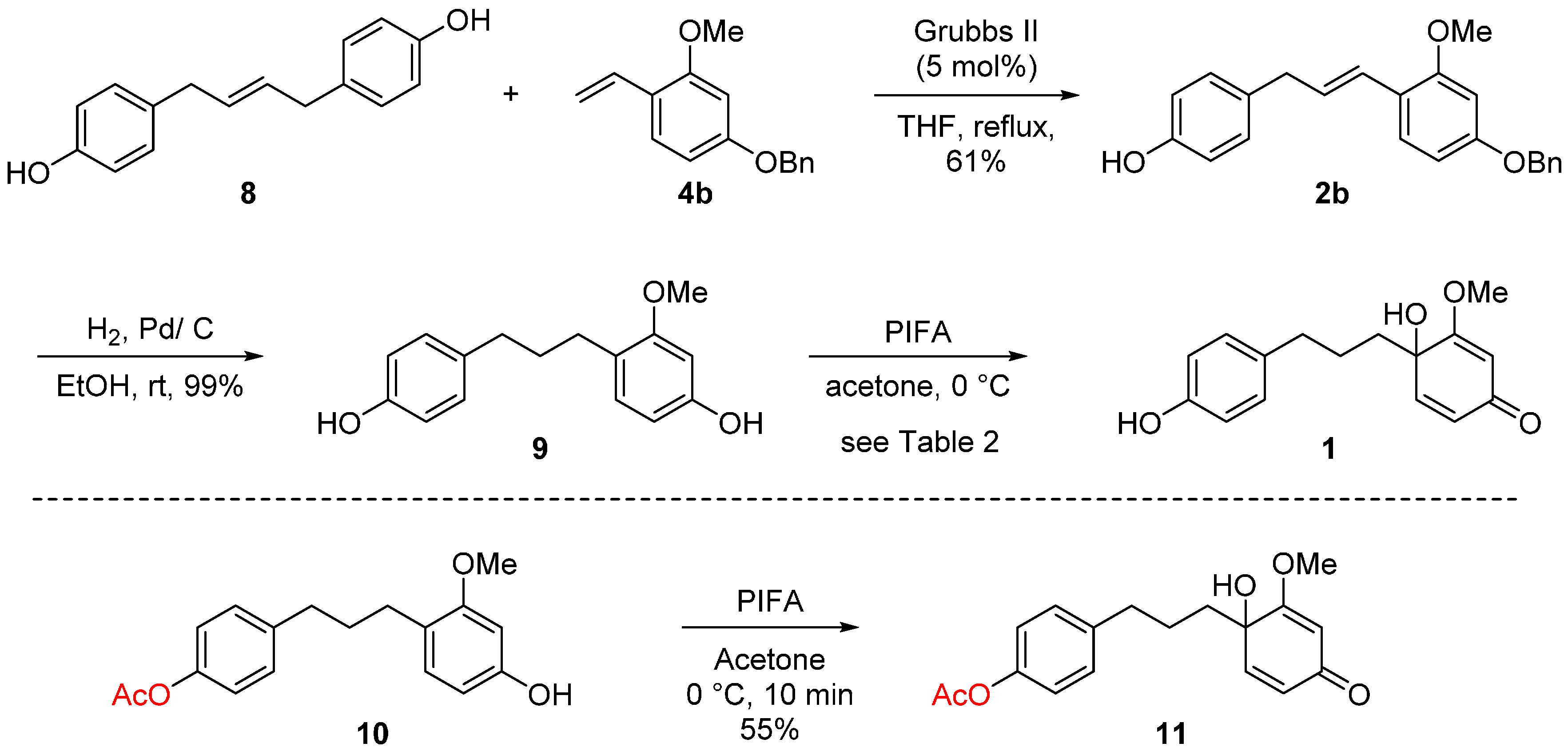
2. Results and Discussion
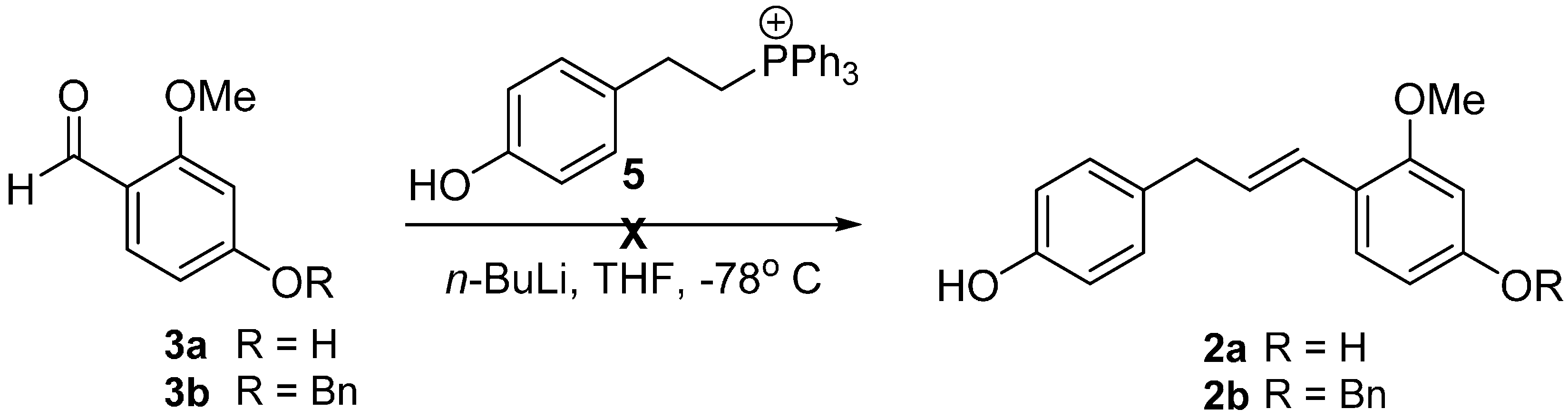
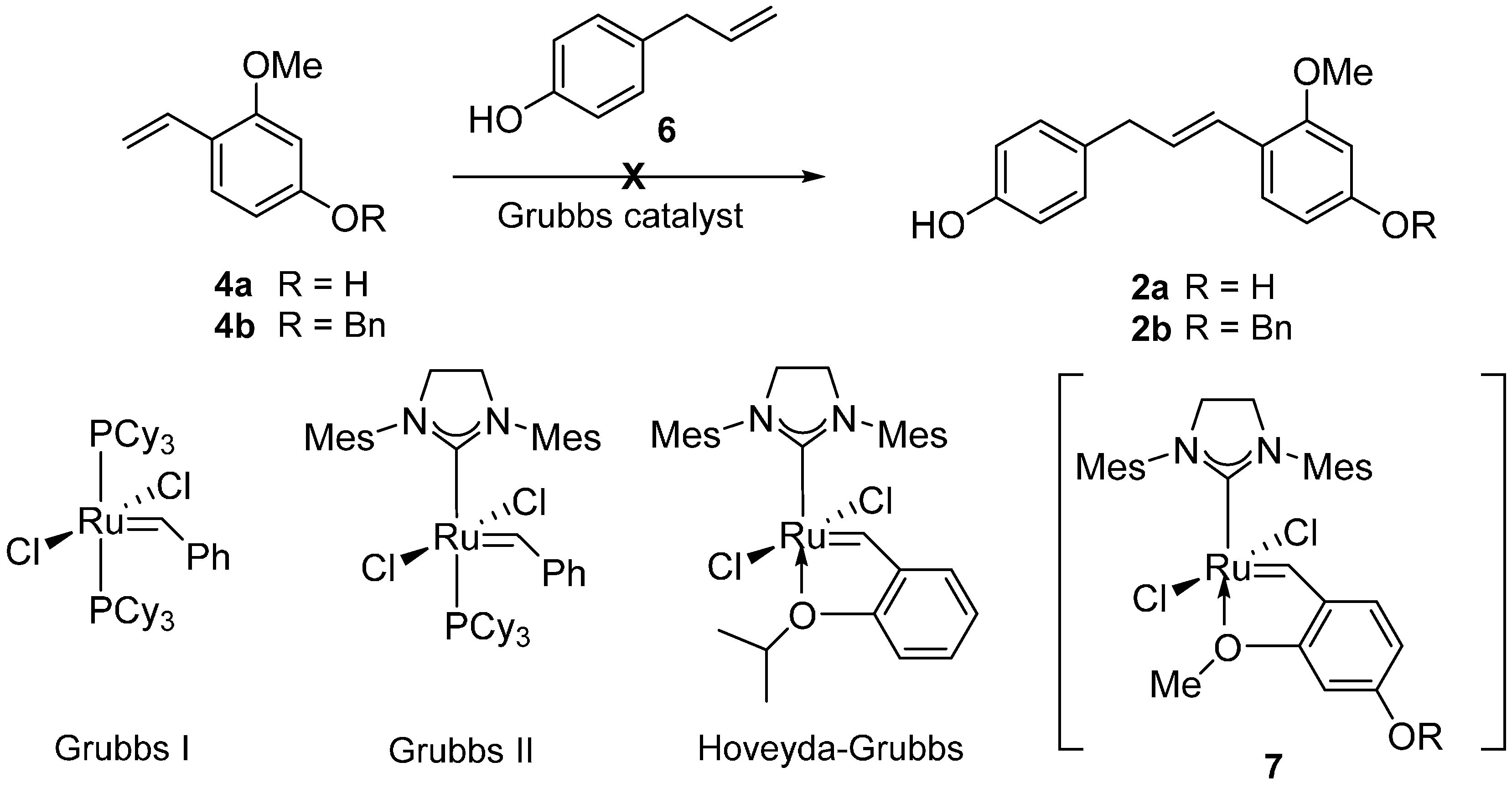
| Entry | 8 (equiv.) | Catalyst (mol %) | Solvent | Yield (%) b |
|---|---|---|---|---|
| 1 | 2.0 | Grubbs I (2) | THF | NR c |
| 2 | 2.0 | Grubbs II (2) | THF | 9 |
| 3 | 2.0 | Hoveyda-Grubbs II (2) | THF | 8 |
| 4 | 2.0 | Grubbs II (5) | THF | 41 |
| 5 | 2.0 | Grubbs II(10) | THF | 37 |
| 6 | 2.0 | Grubbs II (5) | CH2Cl2 | 4 |
| 7 | 2.0 | Grubbs II (5) | DCE | 12 |
| 8 | 2.0 | Grubbs II (5) | toluene | 11 |
| 9 | 1.0 | Grubbs II (5) | THF | 30 |
| 10 | 4.0 | Grubbs II (5) | THF | 36 |
| 11 | 2.0 | Grubbs II (5) | THF | 61 d |
| Entry | Hypervalent Iodine | Solvent | Yield (%) b | |
|---|---|---|---|---|
| 1 | 12 | |||
| 1 | PhI(OAc)2 | CH3CN/H2O (2/1) | 7 | 3 |
| 2 | PhI(OAc)2 | Acetone | 16 | 27 |
| 3 | PhI(OCOCF3)2 | CH3CN/H2O (2/1) | 38 | 31 |
| 4 | PhI(OCOCF3)2 | CH3CN | - | >49 |
| 5 | PhI(OCOCF3)2 | THF | - | >49 |
| 6 | PhI(OCOCF3)2 | Acetone | 40 | 31 |
| 7 | PhI(OCOCF3)2 | Acetone/H2O (2/1) | 22 | 21 |
| 8 | PhI(OCOCF3)2 | CH3OH/H2O (2/1) | - | 31 |
3. Experimental Section
3.1. General Information
3.2. Synthesis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Ahn, J.H.; Liu, Q.; Lee, C.; Ahn, M.J.; Yoo, H.S.; Hwang, B.Y.; Lee, M.K. A new pancreatic lipase inhibitor from Broussonetia kanzinoki. Bioorg. Med. Chem. Lett. 2012, 22, 2760–2763. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Shin, E.; Liu, Q.; Kim, S.B.; Choi, K.M.; Yoo, H.S.; Hwang, B.Y.; Lee, M.K. Secoiridoids from the stem barks of Fraxinus rhynchophylla with pancreatic lipase inhibitory activity. Nat. Prod. Res. 2013, 27, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Andrae-Marobela, K.; Ghislain, F.W.; Okatch, H.; Majinda, R.R.T. Polyphenols: A Diverse Class of Multi-Target Anti-HIV-1 Agents. Curr. Drug Metab. 2013, 14, 392–413. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Nakagomi, R.; Gunawan-Puteri, M.D.P.T.; Kawabata, J. Identification of hydroxychavicol and its dimers, the lipase inhibitors contained in the Indonesian spice, Eugenia polyantha. Food Chem. 2013, 136, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Yama, M.; Nakagomi, R.; Shibata, T.; Hosokawa, K.; Kawabata, J. Substrate-like water soluble lipase inhibitors from Filipendula kamtschatica. Bioorg. Med. Chem. Lett. 2012, 22, 6410–6412. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, Z.; Tang, R.; Jia, H.; Liu, B. Novel (E)-5-styryl-2,2′-bithiophene derivatives as ligands for β-amyloid plaques. Eur. J. Med. Chem. 2011, 46, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Grice, C.A.; Tays, K.L.; Savall, B.M.; Wei, J.; Butler, C.R.; Axe, F.U.; Bembenek, S.D.; Fourie, A.M.; Dunford, P.J.; Lundeen, K.; et al. Identification of a Potent, Selective, and Orally Active Leukotriene A4 Hydrolase Inhibitor with Anti-Inflammatory Activity. J. Med. Chem. 2008, 51, 4150–4169. [Google Scholar] [CrossRef] [PubMed]
- With electron-deficient aldehyde such as nitrobenzaldehyde, the reaction occurred efficiently with 5.
- Grubbs, R.H. Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2003; Volumes 1–3. [Google Scholar]
- Alcaide, B.; Almendros, P.; Luna, A. Grubbs’ Ruthenium-Carbenes Beyond the Metathesis Reaction: Less Conventional Non-Metathetic Utility. Chem. Rev. 2009, 109, 3817–3858. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Vila, A.M.; Monsaert, S.; Bajek, A.; Verpoort, F. Ruthenium-Based Olefin Metathesis Catalysts Derived from Alkynes. Chem. Rev. 2010, 110, 4865–4909. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J. S.; Grubbs, R.H. Alkene Chemoselectivity in Ruthenium-Catalyzed Z-Selective Olefin Metathesis. Angew. Chem. Int. Ed. 2013, 52, 9001–9004. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, J.S.; Harrity, J.P.A.; Bonitatebus, P.J.; Hoveyda, A.H. A Recyclable Ru-Based Metathesis Catalyst. J. Am. Chem. Soc. 1999, 121, 791–799. [Google Scholar] [CrossRef]
- Garber, S.B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. [Google Scholar] [CrossRef]
- Tzeng, S.-C.; Liu, Y.-C. Peroxidase-catalyzed synthesis of neolignan and itsanti-inflammatory activity. J. Mol. Catal. B: Enzym. 2004, 32, 7–13. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.L.; Sanders, D.P.; Grubbs, R.H. A General Model for Selectivity in Olefin Cross Metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed]
- Gresser, M.J.; Wales, S.M.; Keller, P.A. The attempted stereoselective synthesis of chiral 2,2ʹ-biindoline. Tetrahedron 2010, 66, 6965–6976. [Google Scholar] [CrossRef]
- Previously, the ortho-alkoxy-β-methyl styrenes instead of the simple ortho-alkoxystyrenes have been used for this type CM reactions, see: Strych, S.; Trauner, D. Biomimetic Synthesis of Santalin A,B and Santarubin A,B, the Major Colorants of Red Sandalwood. Angew. Chem. Int. Ed. 2013, 52, 9509–9512. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Toste, D.; Choi, T.-L.; Grubbs, R.H. Ruthenium-Catalyzed Olefin Cross Metathesis of Styrenes as an Alternative to the Heck and Cross-Coupling Reactions. Adv. Synth. Catal. 2002, 344, 634–637. [Google Scholar] [CrossRef]
- Compound 9 is also known as broussonin B of which the only synthetic study was reported, see: de Almeida, P.A.; Fraiz, S.V.; Braz-Filho, R. Synthesis and Structural Confirmation of Natural 1,3-Diarylpropanes. J. Braz. Chem. Soc. 1999, 10, 347–353. [Google Scholar] [CrossRef]
- Rappoport, Z. The Chemistry of Phenols; Wiley: Chichester, UK, 2003; Volumes 1–2. [Google Scholar]
- Hata, K.; Hamamoto, H.; Shiozaki, Y.; Cammerer, S.B.; Kita, Y. Nucleophilic attack of intramolecular hydroxyl groups onelectron-rich aromatics using hypervalent iodine(III) oxidation. Tetrahedron 2007, 63, 4052–4060. [Google Scholar] [CrossRef]
- Plourde, G.L. Studies towards the diastereoselective spiroannulation of phenolic derivatives. Tetrahedron Lett. 2002, 43, 3597–3599. [Google Scholar] [CrossRef]
- Felpin, F.X. Oxidation of 4-arylphenol trimethylsilyl ethers to p-arylquinols using hypervalent iodine(III) reagents. Tetrahedron Lett. 2007, 48, 409–412. [Google Scholar] [CrossRef]
- The Oxone-mediated oxidative dearomitization of the corresponding ketone-contaning phenol has been reported, see: Barrada, S.; Hernandez-Torres, G.; Urbano, A.; Carreno, M.C. Total Synthesis of Natural p-Quinol. Org. Lett. 2012, 14, 5952–5955. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound 1 is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Choi, M.; Viji, M.; Lee, Y.H.; Kwak, Y.-S.; Lee, K.; Choi, N.S.; Lee, Y.-J.; Lee, H.; Hong, J.T.; et al. Concise Synthesis of Broussonone A. Molecules 2015, 20, 15966-15975. https://doi.org/10.3390/molecules200915966
Jo H, Choi M, Viji M, Lee YH, Kwak Y-S, Lee K, Choi NS, Lee Y-J, Lee H, Hong JT, et al. Concise Synthesis of Broussonone A. Molecules. 2015; 20(9):15966-15975. https://doi.org/10.3390/molecules200915966
Chicago/Turabian StyleJo, Hyeju, Minho Choi, Mayavan Viji, Young Hee Lee, Young-Shin Kwak, Kiho Lee, Nam Song Choi, Yeon-Ju Lee, Heesoon Lee, Jin Tae Hong, and et al. 2015. "Concise Synthesis of Broussonone A" Molecules 20, no. 9: 15966-15975. https://doi.org/10.3390/molecules200915966
APA StyleJo, H., Choi, M., Viji, M., Lee, Y. H., Kwak, Y.-S., Lee, K., Choi, N. S., Lee, Y.-J., Lee, H., Hong, J. T., Lee, M. K., & Jung, J.-K. (2015). Concise Synthesis of Broussonone A. Molecules, 20(9), 15966-15975. https://doi.org/10.3390/molecules200915966









