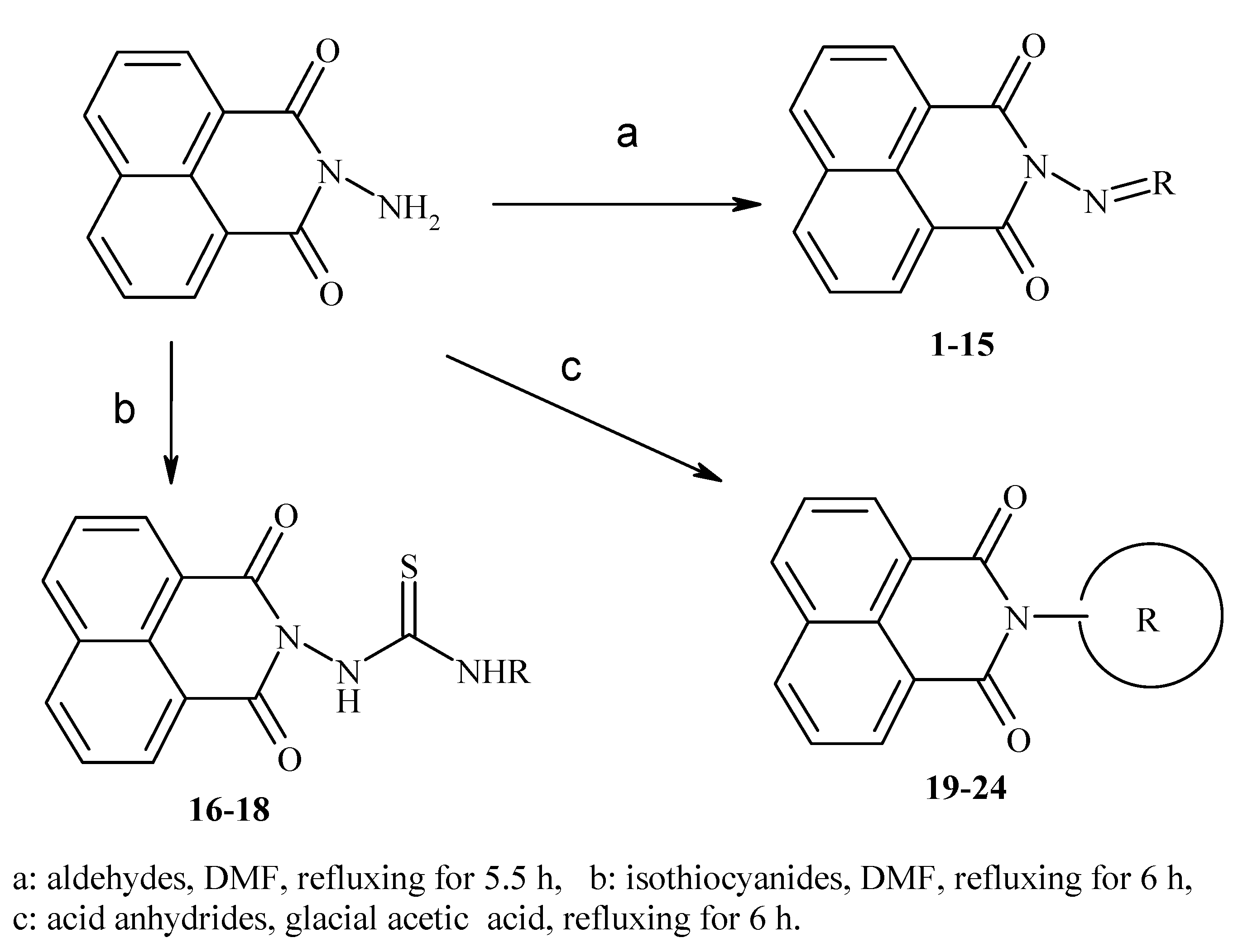
Docking and Antiherpetic Activity of 2-Aminobenzo[de]-isoquinoline-1,3-diones
Abstract
:1. Introduction
2. Results and Discussion
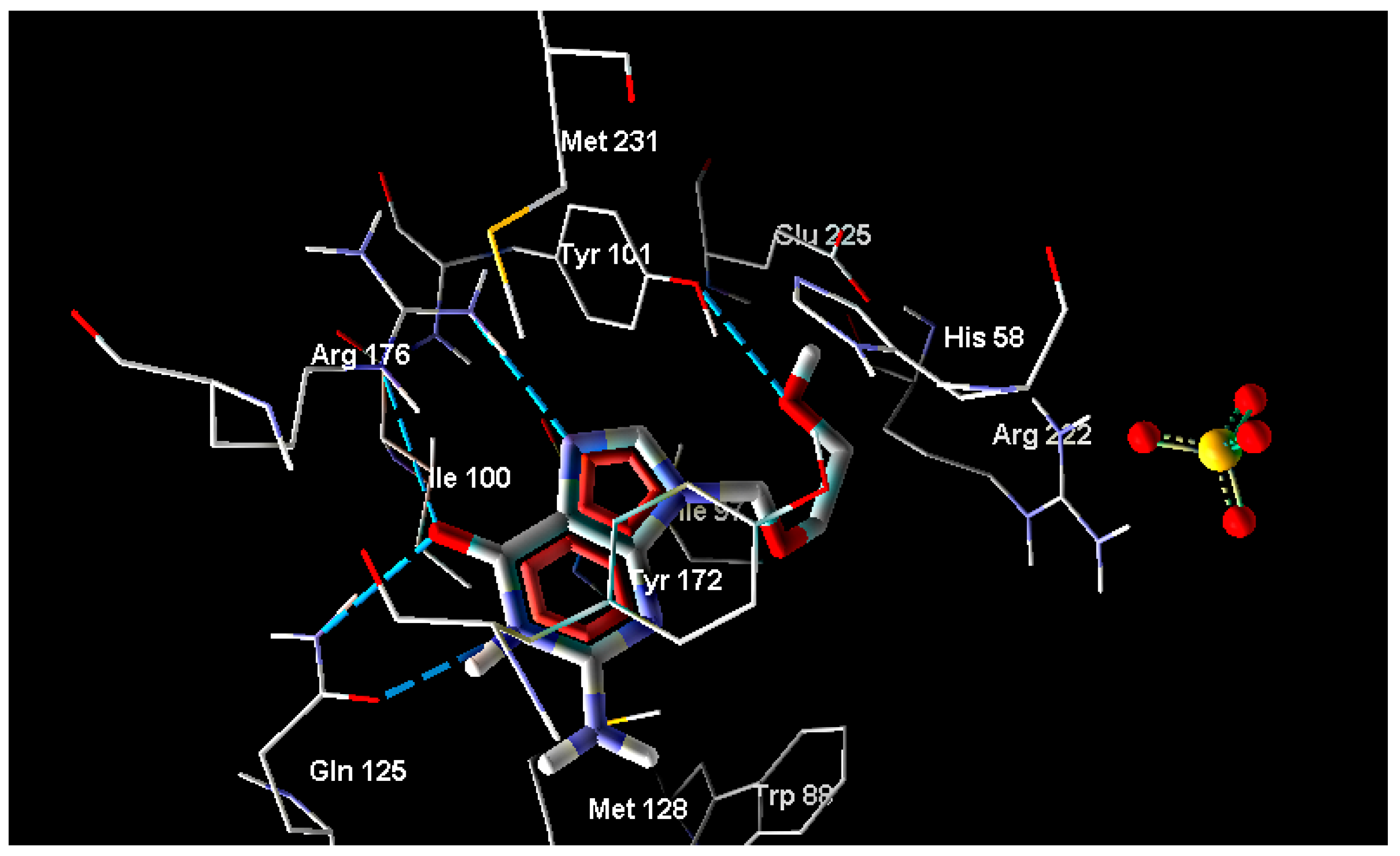
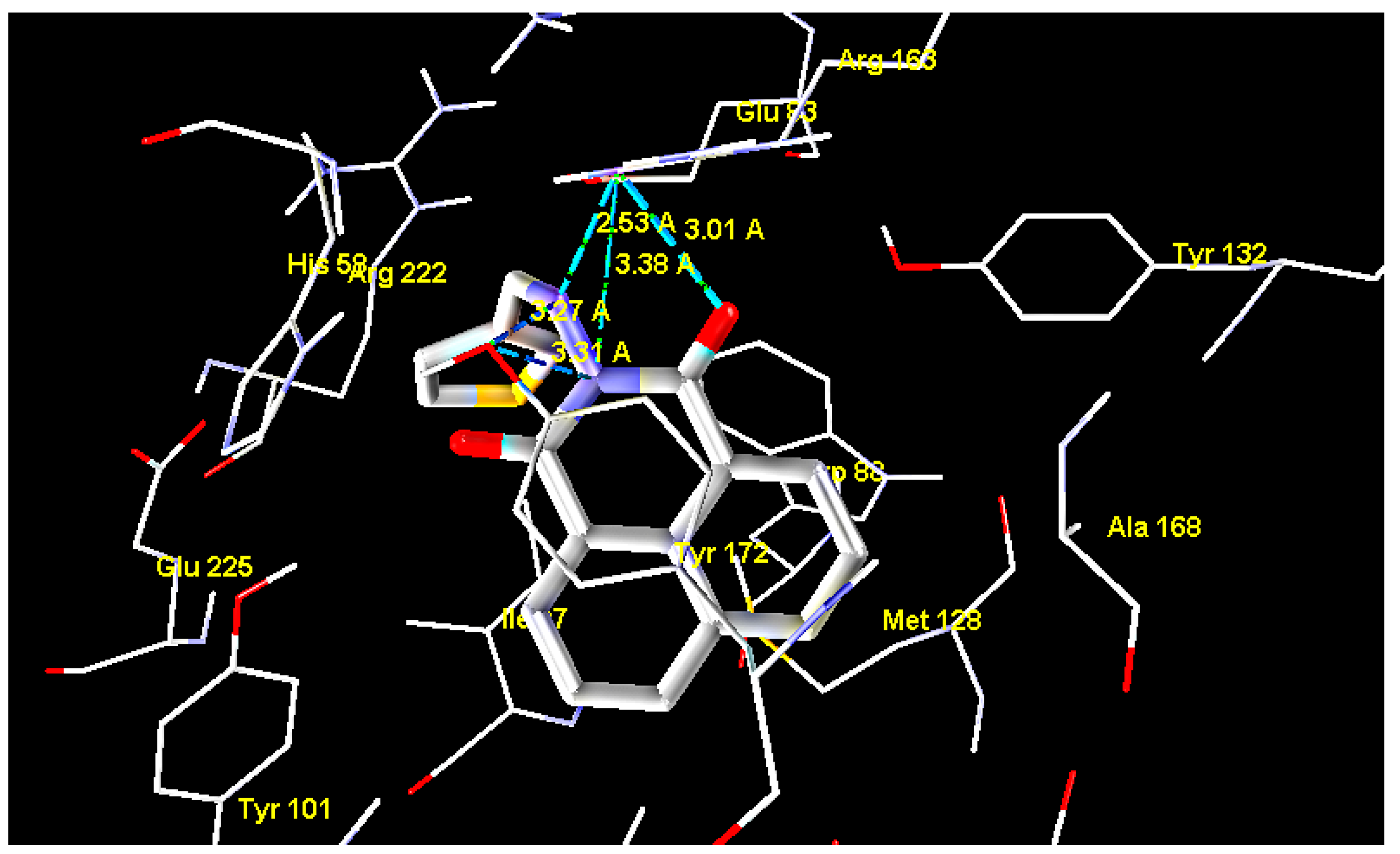
2.2. Molecular Docking

| Ligand | MolDock Score | Ligand | MolDock Score | Ligand | MolDock Score |
|---|---|---|---|---|---|
| Parent | −101.205 | 9 | −112.001 | 18 | −134.765 |
| 18 | −97.8437 | 10 | −108.487 | 19 | −112.904 |
| 2 | −102.893 | 11 | −104.531 | 20 | −118.027 |
| 3 | −102.959 | 12 | −115.317 | 21 | −93.0316 |
| 4 | −95.8232 | 13 | −101.817 | 22 | −91.99 |
| 5 | −113.363 | 14 | −116.126 | 23 | −73.5124 |
| 6 | −127.137 | 15 | −112.552 | 24 | −133.133 |
| 7 | −109.968 | 16 | −129.001 | acyclovir | −111.737 |
| 8 | −121.392 | 17 | −132.879 |
3. Experimental Section
3.1. Mammalian Cell Line
3.3. Cytotoxicity Evaluation Using Viability Assay
3.4. Molecular Docking
3.5. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, J.S.; Robinson, J.N. Age-specific prevalence of infection with herpes simplex virus types 1 and 2: A global review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef] [PubMed]
- Akanitapichata, P.; Wangmaneerata, A.; Wilairatb, P.; Bastowc, K.F. Anti-herpes virus activity of Dunbari abella Prain. J. Ethnopharmacol. 2006, 105, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Cowan, F.M.; French, R.S.; Mayaud, P.; Gopal, R.; Robinson, N.J.; de Oliveira, S.A.; Faillace, T.; Uusküla, A.; Nygård-Kibur, M.; Ramalingam, S.; et al. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex. Transm. Infect. 2003, 79, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Safrin, S.; Crumpacker, C.; Chatis, P.; Davis, R.; Hafner, R.; Rush, J.; Kessler, H.A.; Landry, B.; Mills, J. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant muco cutaneous herpes simplex in the acquired immunodeficiency syndrome: The AIDS clinical trials group. N. Eng. J. Med. 1991, 325, 551–555. [Google Scholar] [CrossRef]
- White, M.K.; Gorrill, T.S.; Khalili, K. Reciprocal transactivation between HIV-1 and other human viruses. Virology 2006, 352, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes 2004, 11, 70–76. [Google Scholar] [PubMed]
- Barooah, N.; Tamuly, C.; Baruah, J.B. Synthesis, characterisation of few N-substituted 1,8-naphthalimide derivatives and their copper(II) complexes. J. Chem. Sci. 2005, 117, 117–122. [Google Scholar]
- Xu, Y.; Qu, B.; Qian, X.; Li, Y. Five-member thio-heterocyclic fused naphthalimides with aminoalkyl side chains: Intercalation and photocleavage to DNA. Bioorg. Med. Chem. Lett. 2005, 15, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Hazra, S.; Dutta, S.; Muthiah, S.; Mondhe, D.M.; Sharma, P.R.; Singh, S.K.; Saxena, A.K.; Qazi, G.N.; Sanyal, U. Antitumor efficacy and apoptotic activity of substituted chloroalkyl 1H-benzo[de]isoquinoline-1,3-diones: A new class of potential antineoplastic agents. Investg. New Drugs 2011, 29, 434–442. [Google Scholar] [CrossRef]
- Brana, M.F.; Castellano, J.M.; Moran, M.; Pérez de Vega, M.J.; Romerdahl, C.R.; Qian, X.D.; Bousquet, P.; Emling, F.; Schlick, E.; Keilhauer, G. Bis-naphthalimides: A new class of antitumor agents. Anti-Cancer Drug Des. 1993, 8, 257–268. [Google Scholar]
- Aibin, W.; Jide, L.L.; Shaoxiong, Q.; Ping, M. Derivatives of 5-nitro-1H-benzo[de]isoquinoline-1,3(2H)-dione: Design, synthesis, and biological activity. Monatsh. Chem. 2010, 141, 95–99. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dutta, S.; Shanmugavel, M.; Mondhe, D.M.; Sharma, P.R.; Singh, S.K.; Saxena, A.K.; Sanyal, U. 6-Nitro-2-(3 hydroxypropyl)-1H-benz[de]isoquinoline-1,3-dione, a potent antitumor agent, induces cell cycle arrest and apoptosis. J. Exp. Clin. Cancer Res. 2010, 29, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Qazi, G.N.; Saxena, A.K.; Muthiah, S.; Mondhe, D.M.; Sharma, P.R.; Singh, S.K.; Sanyal, U.; Mukherjee, A.; Hazra, S.; Dutta, S. Preparation of benzisoquinolinedione derivatives for us as antitumor agents. WO 2008084496 A1, 17 July 2008. [Google Scholar]
- Cholody, W.M.; Kosakowska-Cholody, T.; Michejda, C.J. Preparation of 1,8-naphthalimido-linked imidazo[4,5,1-de]acridones as bis-intercalating antitumor agents. WO 2001066545 A2, 13 September 2001. [Google Scholar]
- Vaisburg, A.; Bernstein, N.; Frechette, S.; Allan, M.; Abou-Khalil, E.; Leit, S.; Moradei, O.; Bouchain, G.; Wang, J.; Woo, S.H.; et al. (2-Aminophenyl)-amides of omega-substituted alkanoic acids as new histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Leopoldo, M.; Masotti, A.C.; Inglese, C.; Berardi, F.; Perrone, R.; Ganguly, S.; Jafurulla, M.; Chattopadhyay, A. Synthesis and characterization of environment-sensitive fluorescent ligands for human 5-HT1A receptors with 1-arylpiperazine structure. J. Med. Chem. 2009, 52, 7892–7896. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Marzouk, M. Some 2-amino-benzo[de]isoquinolin-1,3-diones as antimicrobial agents. Asian J. Chem. 2014, 26, 8163–8165. [Google Scholar]
- Al-Salahi, R.; Alswaidan, I.; Marzouk, M. Cytotoxicity evaluation for a new set of 2-aminobenzo[de]iso-quinolin-1,3-diones. Int. J. Mol. Sci. 2014, 15, 22483–22491. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Marzouk, M. Synthesis of novel 2-aminobenzo[de]isoquinolin-1,3-dione derivatives. Asian J. Chem. 2014, 26, 2166–2172. [Google Scholar]
- Garett, R.; Romanos, M.T.V.; Borges, R.M.; Santos, M.G.; Rocha, L.; da Silva, A.J.R. Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Braz. J. Pharmacogn. 2012, 22, 306–313. [Google Scholar] [CrossRef]
- Bag, P.; Ojha, D.; Mukherjee, H.; Halder, U.C.; Mondal, S.; Chandra, N.S.; Nandi, S.; Sharon, A.; Sarkar, M.C.; Chakrabarti, S.; et al. An indole alkaloid from a tribal folklore inhibits immediate early event in HSV-2 infected cells with therapeutic efficacy in vaginally infected mice. PLoS One 2013, 8, e77937. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Vogel, J.L.; Narayanan, A.; Peng, H.; Kristie, T.M. Inhibition of the histone demethylase LSD1 blocks α-herpesvirus lytic replication and reactivation from latency. Nat. Med. 2009, 15, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Champness, J.N.; Bennett, M.S.; Wien, F.; Viss, R.; Summers, W.C.; Herdewijn, P.; Ostrowski, E.; Jarvest, R.L.; Sanderson, M.R. Exploring the active site of herpes simplex virus type-1 thymidine kinase by X-ray crystallography of complexes with Aciclovir and other ligands. Proteins Struct. Funct. Genet. 1998, 32, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.S.; Wien, F.; Champness, J.N.; Batuwangala, T.; Rutherford, T.; Summers, W.C.; Sun, H.; Wright, G.; Sanderson, M.R. Structure to 1.9 Å resolution of a complex with herpes simplex virus type-1 thymidine kinase of a novel, non-substrate inhibitor: X-ray crystallographic comparison with binding of acyclovir. FEBS Lett. 1999, 443, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Luyten, I.; de Winter, H.; Busson, R.; Lescrinier, T.; Creuven, I.; Durant, F.; Baizarini, J.; de Clercq, E.; Herdwijn, P. Synthesis of 2'-deoxy-5-(isothiazol-5-yl)uridine and its interaction with the HSV-1 thymidine kinase. Helv. Chim. Acta 1996, 79, 1462–1474. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, M.; Alswaidan, I.; Al-Omar, M. Antiviral activity of 2-phenoxy-4H-[1,2,4]triazolo[1,5-a]quinazoline derivatives. Life Sci. J. 2013, 10, 2164–2169. [Google Scholar]
- Hu, J.M.; Hsiung, G.D. Evaluation of new antiviral agents I: In vitro prospective. Antivir. Res. 1989, 11, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, P.; Raghu, C.; Ashok, G.; Dhanaraj, S.A.; Suresh, B. Antiviral activity of medicinal plants of Nilgiris. Indian J. Med. Res. 2004, 120, 24–29. [Google Scholar] [PubMed]
- Dargan, D.J. Investigation of the anti-HSV activity of candidate antiviral agents. In Methods in Molecular Medicine, Herpes Simplex Virus Protocols; Brown, S.M., MacLean, A.R., Eds.; Humana Press Inc.: Totowa, NJ, USA, 1998; Volume 10, pp. 387–405. [Google Scholar]
- Zandi, K.; Zadeh, M.A.; Sartavi, K.; Rastian, Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Biotechnol. 2007, 6, 1770–1773. [Google Scholar]
- Vega-Avila1, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assesssurvival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar] [PubMed]
- Bajbouj, K.; Schulze-Luehrmann, J.; Diermeier, S.; Amin, A.; Schneider-Stock, R. The anticancer effect of saffron in two p53 isogenic colorectal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Ušaj, M.; Trontelj, K.; Hudej, R.; Kandušer, M.; Miklavčič, D. Cell size dynamics and viability of cells exposed to hypotonic treatment and electroporation for electrofusion optimization. Radiol. Oncol. 2009, 43, 108–119. [Google Scholar] [CrossRef]
- Bernhardt, G.; Reile, H.; Birnboeck, H.; Spruss, T.; Schoenenberger, H. Standardized kinetic microassay to quantify differential chemosensitivity on the basis of proliferative activity. J. Cancer Res. Clin. Oncol. 1992, 118, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ait Mbarek, L.; ait Mouse, H.; Elabbadi, N.; Bensalah, M.; Gamouh, A.; Aboufatima, R.; Benharref, A.; Chait, A.; Kamal, M.; Dalal, A.; et al. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Braz. J. Med. Biol. Res. 2007, 40, 839–847. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, S.M. ChemBioOffice Ultra 2010 suite. J. Am. Chem. Soc. 2010, 132, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Serna, L.; Cravioto, J. Simply Statistic for Health Investigation, 1st ed.; Trillas: Mexico, Mixico, 1999. [Google Scholar]
- Sample Availability: Samples of the compounds (1–24) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Salahi, R.; Alswaidan, I.; Ghabbour, H.A.; Ezzeldin, E.; Elaasser, M.; Marzouk, M. Docking and Antiherpetic Activity of 2-Aminobenzo[de]-isoquinoline-1,3-diones. Molecules 2015, 20, 5099-5111. https://doi.org/10.3390/molecules20035099
Al-Salahi R, Alswaidan I, Ghabbour HA, Ezzeldin E, Elaasser M, Marzouk M. Docking and Antiherpetic Activity of 2-Aminobenzo[de]-isoquinoline-1,3-diones. Molecules. 2015; 20(3):5099-5111. https://doi.org/10.3390/molecules20035099
Chicago/Turabian StyleAl-Salahi, Rashad, Ibrahim Alswaidan, Hazem A. Ghabbour, Essam Ezzeldin, Mahmoud Elaasser, and Mohamed Marzouk. 2015. "Docking and Antiherpetic Activity of 2-Aminobenzo[de]-isoquinoline-1,3-diones" Molecules 20, no. 3: 5099-5111. https://doi.org/10.3390/molecules20035099