Determination of the Molecular Weight of Low-Molecular-Weight Heparins by Using High-Pressure Size Exclusion Chromatography on Line with a Triple Detector Array and Conventional Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of HP-SEC Calibrants
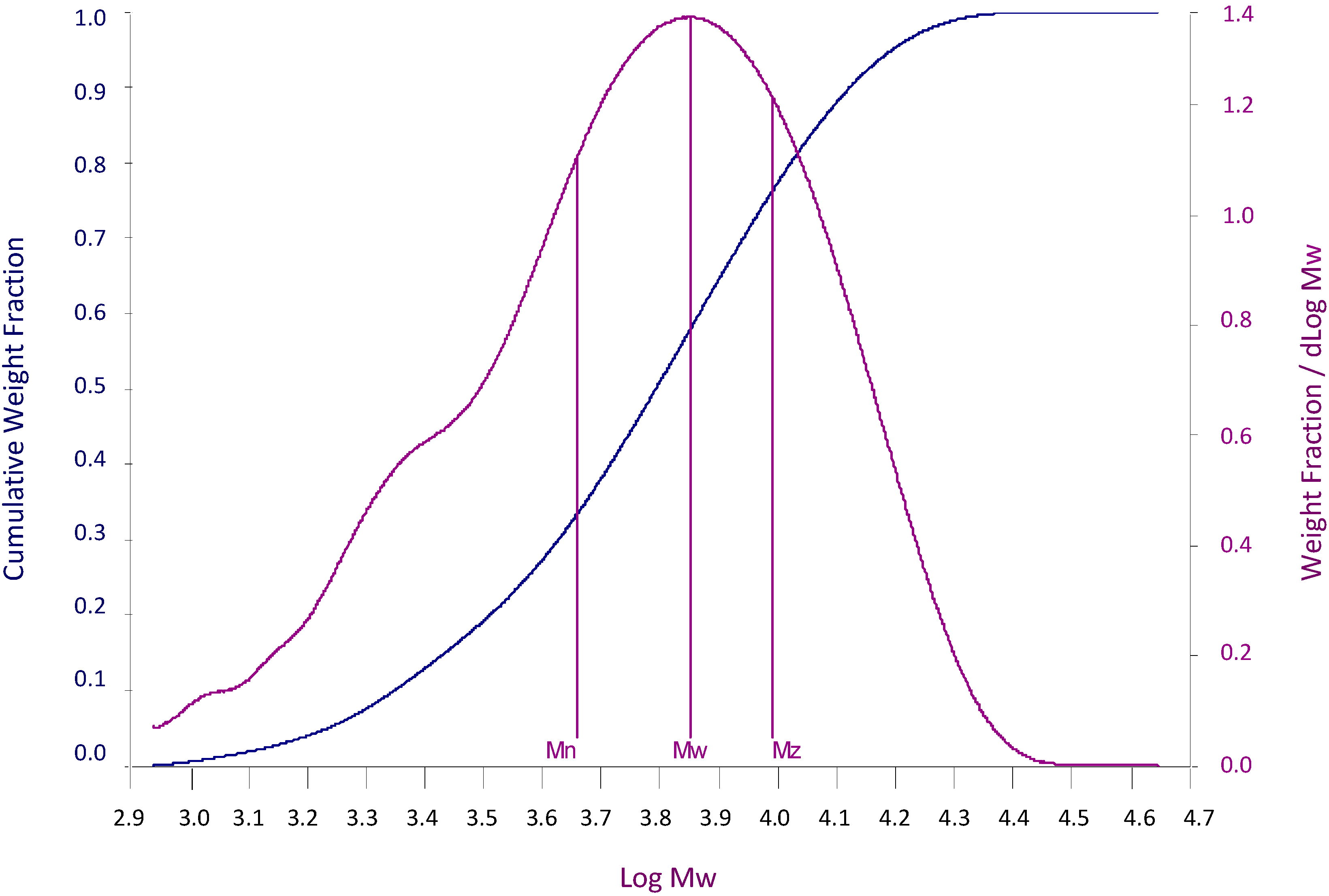
| Point | Cum.% | log (Mr) | Mr |
|---|---|---|---|
| 1 | 2.1 | 4.258 | 18,100 |
| 2 | 3.1 | 4.228 | 16,900 |
| 3 | 5.2 | 4.182 | 15,200 |
| 4 | 6.9 | 4.155 | 14,300 |
| 5 | 9.1 | 4.124 | 13,300 |
| 6 | 12.2 | 4.090 | 12,300 |
| 7 | 17.6 | 4.037 | 10,900 |
| 8 | 33.7 | 3.914 | 8200 |
| 9 | 41.8 | 3.857 | 7200 |
| 10 | 51.0 | 3.792 | 6200 |
| 11 | 61.0 | 3.716 | 5200 |
| 12 | 71.0 | 3.623 | 4200 |
| 13 | 80.4 | 3.505 | 3200 |
| 14 | 88.5 | 3.342 | 2200 |
| 15 | 96.8 | 3.079 | 1200 |
| 16 | 98.1 | 2.991 | 980 |
| Fraction | Vr # | Mp | Mn | Mw | Pd |
|---|---|---|---|---|---|
| 1 | 11.70 | 18,776 | 15,323 | 17,615 | 1.15 |
| 2 | 11.86 | 14,646 | 13,778 | 15,256 | 1.10 |
| 3 | 11.93 | 13,046 | 12,173 | 13,383 | 1.10 |
| 4 | 12.14 | 9815 | 9444 | 10,262 | 1.09 |
| 5 | 12.17 | 9231 | 9183 | 9676 | 1.05 |
| 6 | 12.18 | 8390 | 8332 | 8714 | 1.05 |
| 7 | 12.31 | 6883 | 6862 | 7151 | 1.04 |
| 8 | 12.35 | 6286 | 6023 | 6482 | 1.07 |
| 9 | 12.48 | 5410 | 5044 | 5666 | 1.12 |
| 10 | 12.49 | 4663 | 4500 | 4796 | 1.07 |
| 11 | 12.64 | 3871 | 3686 | 3881 | 1.05 |
| 12 | 12.75 | 3554 | 3476 | 3599 | 1.04 |
| 13 | 12.93 | 2808 | 2713 | 2824 | 1.04 |
| 14 | 13.05 | 2653 | 2501 | 2631 | 1.05 |
| 15 | 13.19 | 2192 | 2059 | 2198 | 1.07 |
| 16 | 13.29 | 2022 | 2006 | 2098 | 1.05 |
| 17 | 13.47 | 1830 | 1746 | 1827 | 1.05 |
| 18 | 13.82 | 1198 | 1369 | 1479 | 1.08 |
| 19 | 566 |
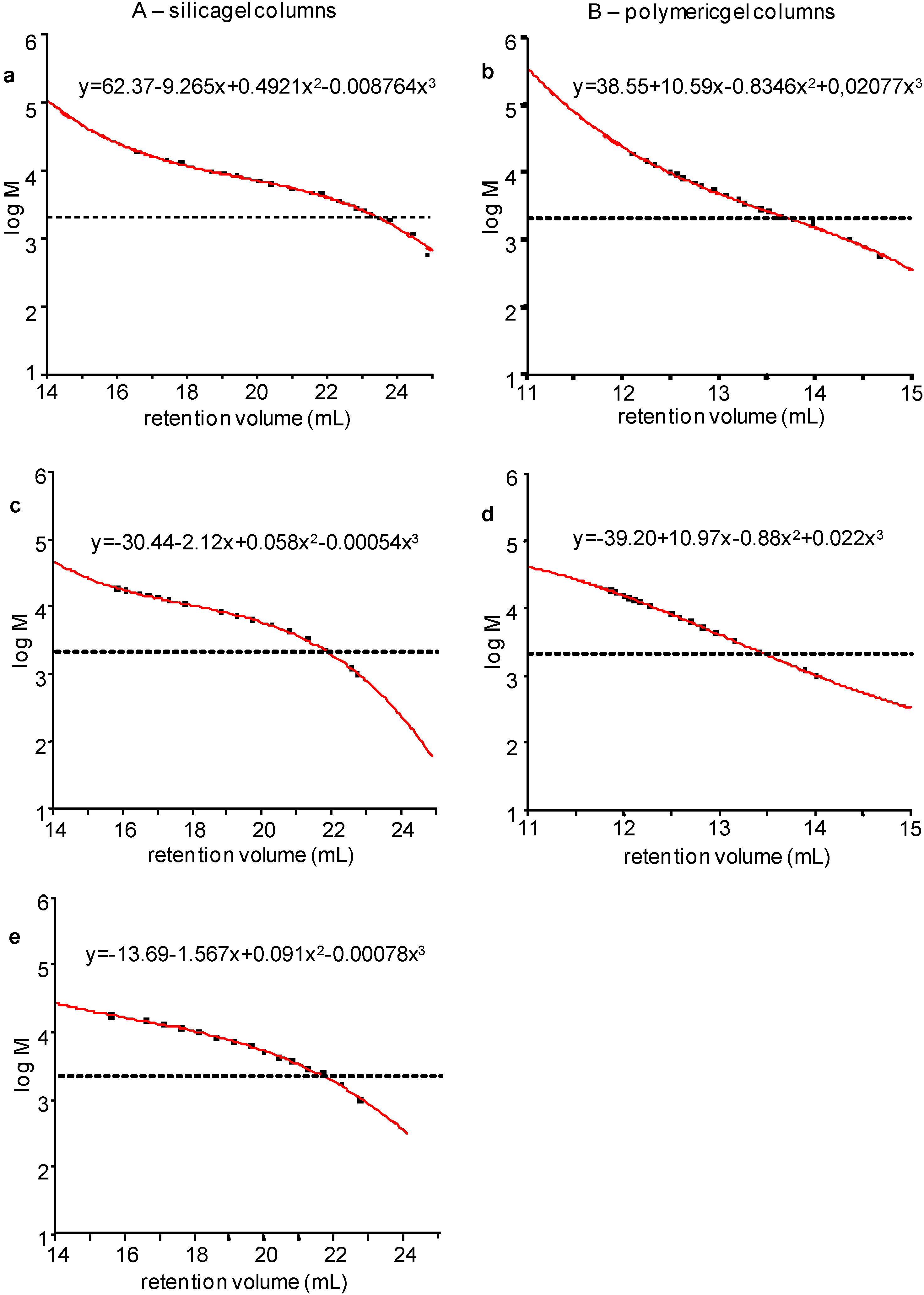
2.2. Calibration of the HP-SEC Systems
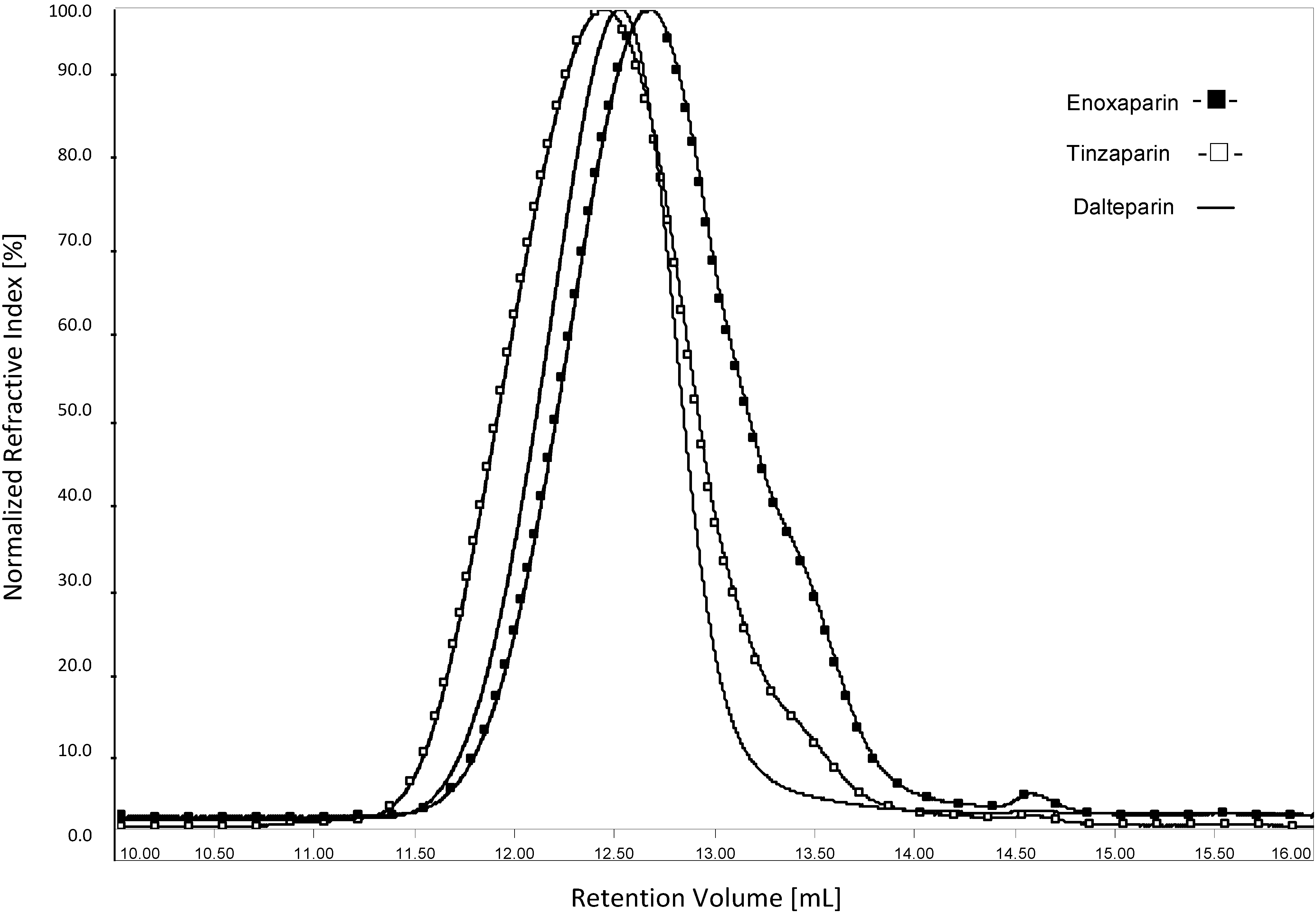
2.3. Measurement of Molecular Weight Parameters of LMWHs
2.3.1. HP-SEC/TDA
| LMWH | Mn | Mw | Pd |
|---|---|---|---|
| Enoxaparin | 4036 ±123 | 5426 ± 101 | 1.34 |
| Tinzaparin | 5920 ± 207 | 8270 ± 235 | 1.40 |
| Dalteparin | 5668 ± 96 | 6910 ± 131 | 1.22 |
2.3.2. HP-SEC/RI and HP-SEC/RI-UV
| LMWH | Detector | Calibration | Stationary Phase | Mw | Pd | % Mw | |||
|---|---|---|---|---|---|---|---|---|---|
| <2000 | <3000 | 2000–8000 | >8000 | ||||||
| Enoxaparin | TDA | - | polymer | 5400 | 1.34 | 7 | nd | 76 | 17 |
| RI | narrow fr. | polymer | 4900 | 1.39 | 12 | nd | 76 | 13 | |
| silica | 5000 | 1.35 | 8 | nd | 82 | 10 | |||
| broad cal. | polymer | 5300 | 1.45 | 11 | nd | 72 | 17 | ||
| silica | 4800 | 1.60 | 19 | nd | 65 | 16 | |||
| RI-UV | EP std | silica | 4600 | 1.59 | 22 | nd | 65 | 14 | |
| Tinzaparin | TDA | - | polymer | 8300 | 1.40 | 2 | nd | 56 | 42 |
| RI | narrow fr. | polymer | 7300 | 1.48 | 5 | nd | 62 | 34 | |
| silica | 7600 | 1.36 | 3 | nd | 68 | 30 | |||
| broad cal. | polymer | 7900 | 1.45 | 4 | nd | 56 | 40 | ||
| silica | 7900 | 1.57 | 6 | nd | 52 | 42 | |||
| RI-UV | EP std | silica | 7400 | 1.59 | 8 | nd | 53 | 39 | |
| Dalteparin | TDA | - | polymer | 6900 | 1.22 | nd | 5 | 68 | 30 |
| RI | narrow fr. | polymer | 6400 | 1.26 | nd | 9 | 75 | 23 | |
| silica | 6600 | 1.14 | nd | 2 | 81 | 18 | |||
| broad cal. | polymer | 7200 | 1.23 | nd | 4 | 66 | 33 | ||
| silica | 6900 | 1.28 | nd | 5 | 66 | 32 | |||
| RI-UV | EP std | silica | 6600 | 1.32 | nd | nd | 69 | 29 | |
| Fondaparinux | TDA | - | polymer | 1890 | 1.01 | 97 | |||
| RI | narrow fr. | polymer | 2030 | 1.04 | 46 | ||||
| silica | 2080 | 1.01 | 28 | ||||||
| broad cal. | polymer | 1920 | 1.08 | 55 | |||||
| silica | 1410 | 1.02 | 100 | ||||||
| RI-UV | EP std | silica | 1420 | 1.02 | 100 | ||||
| LMWH | Mass-Average Mw Range | Molecular Weight Distribution | |||
|---|---|---|---|---|---|
| <2000 Da | <3000 Da | 2000–8000 Da | >8000 Da | ||
| Enoxaparin | 3800–5000 | 12%–20% | - | 68%–82% | - |
| Tinzaparin | 5500–7500 | ≤10% | - | 60%–72% | 22%–36% |
| Dalteparin | 5600–6400 | - | ≤13% | - | 15%–25% |
2.3.3. Measurement of Molecular Weight Parameters of a Synthetic Pentasaccharide
3. Materials and Methods
3.1. Materials
3.2. Fractionation of Depolymerized Heparin
3.3. HP-SEC/TDA Analysis
3.4. HP-SEC/RI-UV Analysis
3.5. Sample Preparation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bertini, S.; Bisio, A.; Torri, G.; Bensi, D.; Terbojevich, M. Molecular weight determination of heparin and dermatan sulfate by size exclusion chromatography with a triple detector array. Biomacromolecules 2005, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B. Structure and physicochemical characterization of heparin. In Heparin—A Century of Progress; Lever, R., Mulloy, B., Page, C.P., Eds.; Springer: London, UK, 2012; pp. 77–98. [Google Scholar]
- Fareed, J.; Ma, Q.; Florian, M.; Maddineni, J.; Iqbal, O.; Hoppensteadt, D.A.; Bick, R.L. Differentiation of low molecular-weight heparins: Impact on the future of the management of thrombosis. Semin. Thromb. Hemost. 2004, 30, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Linhardt, R.J.; Gunay, N.S. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 1999, 25, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Vecchietti, D.; Citterio, L.; Guerrini, M.; Raman, R.; Bertini, S.; Eisele, G.; Naggi, A.; Sasisekharan, R.; Torri, G. Structural features of low-molecular-weight heparins affecting their affinity to antithrombin. Thromb. Haemost. 2009, 102, 865–873. [Google Scholar] [PubMed]
- Fareed, J.; Walenga, J.M.; Hoppensteadt, D.; Huan, X.; Racanelli, A. Comparative study on the in vitro and in vivo activities of seven low-molecular-weight heparins. Haemostasis 1988, 18, 3–15. [Google Scholar] [PubMed]
- Malsch, R.; Harenberg, J.; Piazolo, L.; Hule, G.; Heene, D.L. Chromatographic and electrophoretic applications for the analysis of heparin and dermatan sulfate. J. Chromatogr. B 1996, 685, 223–231. [Google Scholar] [CrossRef]
- Mulloy, B.; Gee, C.; Wheeler, S.F.; Walt, R.; Gray, E.; Barrowcliffe, T.W. Molecular weight measurements of low molecular weight heparins by gel permeation chromatography. Thromb. Haemost. 1997, 77, 668–674. [Google Scholar] [PubMed]
- Guo, X.; Condra, M.; Kimura, K.; Berth, G.; Dautzenberg, H.; Dubin, P.L. Determination of molecular weight of heparin by size exclusion chromatography with universal calibration. Anal. Biochem. 2003, 312, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.E.; Shaklee, P.N. Absolute molecular weight distribution of low-molecular-weight heparins by size-exclusion chromatography with multiangle laser light scattering detection. Anal. Biochem. 1997, 245, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.D.; Ye, H.; Kolinski, R.E.; Nasr, M.; Buhse, L.F.; Hakin, A.; Keire, D.A. Characterization of currently marketed heparin products: Analysis of molecular weight and heparinase-I digest patterns. Anal. Bioanal. Chem. 2011, 401, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Takahata, T.; Ishimitsu, S.; Okada, S. Molecular mass determination of low-molecular-mass heparins. Application of wide collection angle measurements of light scattering using a high-performance gel permeation chromatographic system equipped with a low-angle laser light-scattering photometer. J. Chromatogr. A 1993, 644, 17–24. [Google Scholar] [CrossRef]
- Bisio, A.; Guglieri, S.; Frigerio, M.; Torri, G.; Vismara, E.; Cornelli, U.; Bensi, D.; Gonella, S.; De Ambrosi, L. Controlled γ-ray irradiation of heparin generates oligosaccharides enriched in highly sulfated sequences. Carbohydr. Polym. 2004, 455, 101–112. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2014; Volume 2.
- Alekseeva, A.; Casu, B.; Cassinelli, G.; Guerrini, M.; Torri, G.; Naggi, A. Structural features of glycol-split low molecular weight heparins and their heparin lyases generated fragments. Anal. Bioanal. Chem. 2014, 406, 249–265, electronic supplementary material. [Google Scholar] [CrossRef] [PubMed]
- Popovici, S.T.; Kok, W.T.; Schoenmakers, P.J. Band broadening in size-exclusion chromatography of polydisperse samples. J. Chromatogr. A 2004, 1060, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisio, A.; Mantegazza, A.; Vecchietti, D.; Bensi, D.; Coppa, A.; Torri, G.; Bertini, S. Determination of the Molecular Weight of Low-Molecular-Weight Heparins by Using High-Pressure Size Exclusion Chromatography on Line with a Triple Detector Array and Conventional Methods. Molecules 2015, 20, 5085-5098. https://doi.org/10.3390/molecules20035085
Bisio A, Mantegazza A, Vecchietti D, Bensi D, Coppa A, Torri G, Bertini S. Determination of the Molecular Weight of Low-Molecular-Weight Heparins by Using High-Pressure Size Exclusion Chromatography on Line with a Triple Detector Array and Conventional Methods. Molecules. 2015; 20(3):5085-5098. https://doi.org/10.3390/molecules20035085
Chicago/Turabian StyleBisio, Antonella, Alessandra Mantegazza, Davide Vecchietti, Donata Bensi, Alessia Coppa, Giangiacomo Torri, and Sabrina Bertini. 2015. "Determination of the Molecular Weight of Low-Molecular-Weight Heparins by Using High-Pressure Size Exclusion Chromatography on Line with a Triple Detector Array and Conventional Methods" Molecules 20, no. 3: 5085-5098. https://doi.org/10.3390/molecules20035085







