Anti-Allergic Properties of Curine, a Bisbenzylisoquinoline Alkaloid
Abstract
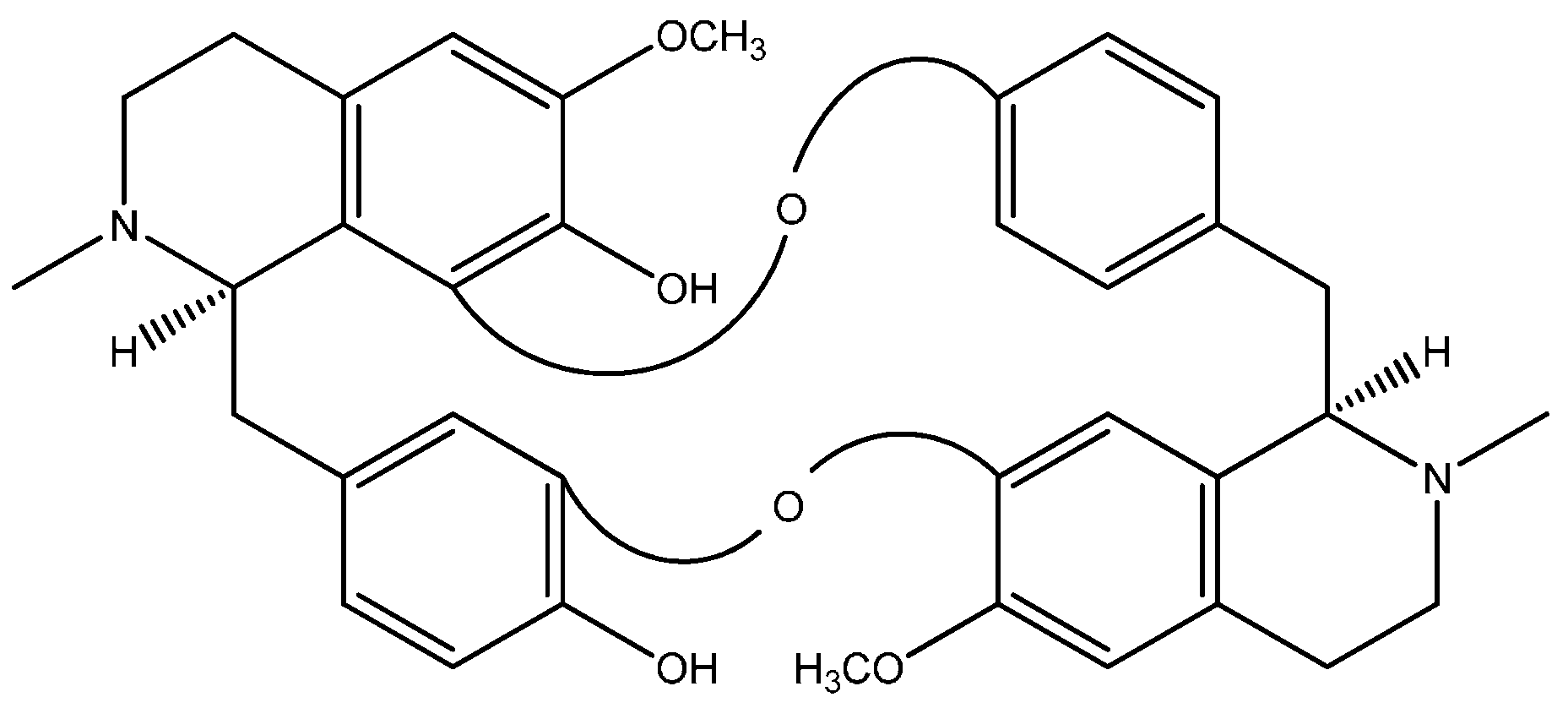
:1. Curine, a Bisbenzylisoquinoline Alkaloid
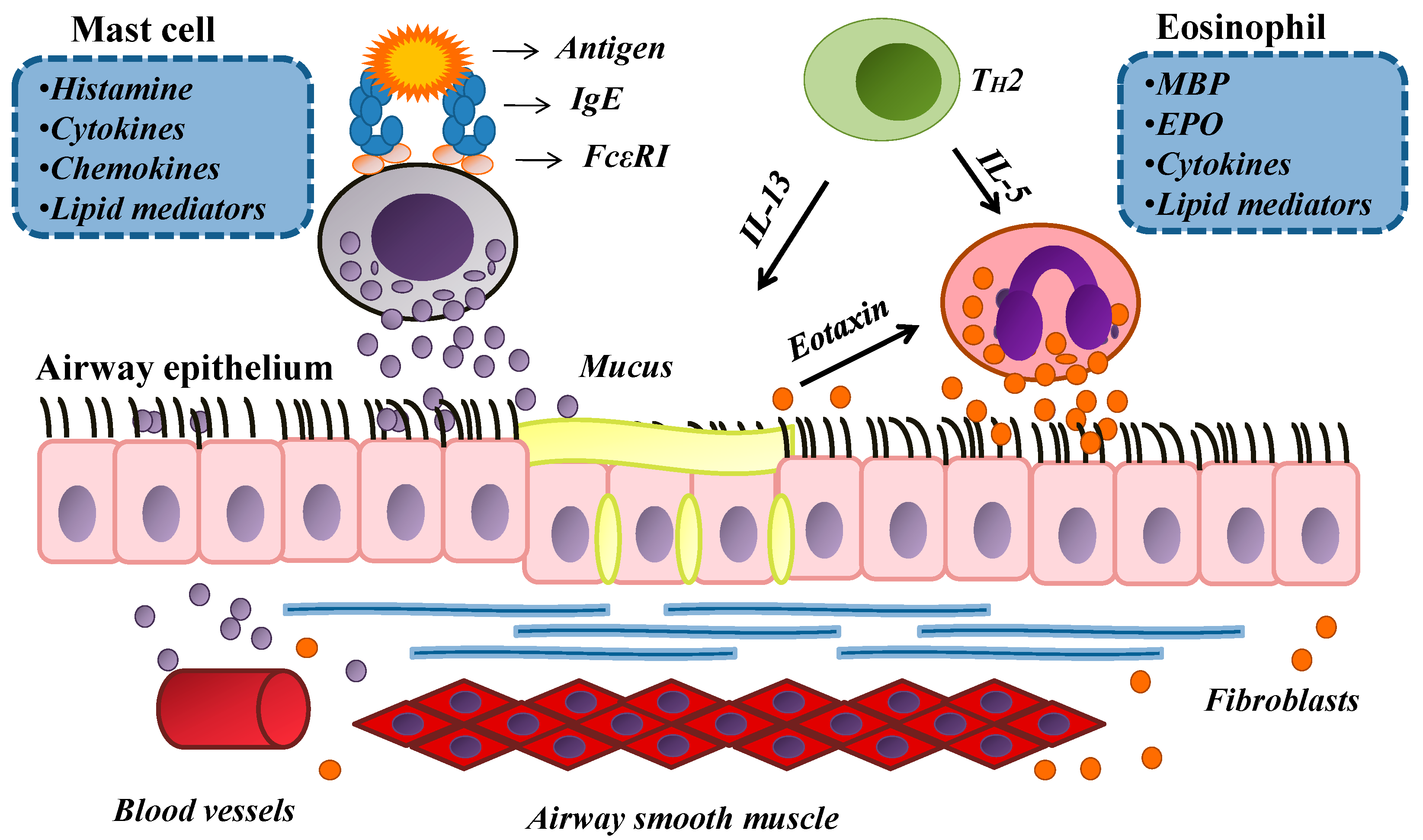
2. An Overview of Allergy
3. The Anti-Allergic Effects of Curine
3.1. The Effects of Curine on Experimental Allergic Asthma

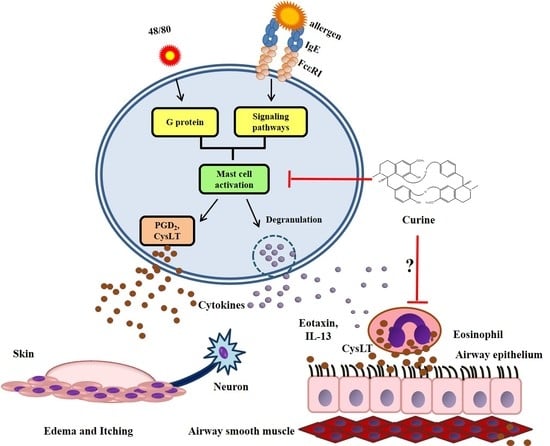
3.2. The Effects of Curine on Mast Cell-Dependent Responses
4. Other Pharmacological Effects of Curine
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 2nd ed.; Chapman and Hall: London, UK, 1984; p. 288. [Google Scholar]
- Barbosa-Filho, J.M.; Piuvezam, M.R.; Moura, M.D.; Silva, M.S.; Batista-Lima, K.V.; Leitão-da-Cunha, E.V.; Fechine, I.M.; Takemura, O.S. Anti-inflammatory activity of alkaloids: A twenty-century review. Braz. J. Pharmacogn. 2006, 16, 109–139. [Google Scholar] [CrossRef]
- Tolkachev, O.N.; Nakova, E.P.; Evstigneeva, R.P. Bisbenzylisoquinoline alkaloids. Chem. Nat. Prod. 1977, 13, 382–405. [Google Scholar]
- Barbosa-Filho, J.M. Quimiodiversidade e Potencialidade Farmacológica da Flora Paraibana. Cad. Farm. 1997, 13, 85–102. [Google Scholar]
- Seow, W.K.; Li, S.T.Y.; Thong, Y.H. Inhibitory effect of tetrandrine on human neutrophil and monocyte adherence. Immunol. Lett. 1986, 13, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Teh, B.S.; Seow, W.K.; Li, S.Y.; Thong, Y.H. Inhibition of prostaglandin and leukotriene generation by the plant alkaloids tetrandrine and berbamine. Int. J. Immunopharmacol. 1990, 12, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Piuvezam, M.R.; Peçanha, L.M.; Alexander, J.; Thomas, G. Cissampelos sympodialis Eichl. leaf extract increases the production of IL-10 by concanavalin-A treated BALB/c spleen cells. J. Ethnopharmacol. 1999, 67, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, C.R.; Balestieri, F.M.; Rossi-Bergmann, B.; Peçanha, L.M.; Piuvezam, M.R. Cissampelos sympodialis Eichl. (Menispermaceae): Oral treatment decreases IgE levels and induces a Th1-skewed cytokine production in ovalbumin-sensitized mice. J. Ethnopharmacol. 2004, 95, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, C.R.; Peçanha, L.M.T.; Piuvezam, M.R. Cissampelos sympodialis Eichl. (Menispermaceae) inhibits anaphylactic shock reaction in murine allergic model. Braz. J. Pharmacog. 2005, 15, 287–291. [Google Scholar]
- Bezerra-Santos, C.R.; Vieira-de-Abreu, A.; Barbosa-Filho, J.M.; Bandeira-Melo, C.; Piuvezam, M.R.; Bozza, P.T. Anti-allergic properties of Cissampelos sympodialis and its isolated alkaloid warifteine. Int. Immunopharmacol. 2006, 6, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.F.; Bezerra-Santos, C.R.; Barbosa-Filho, J.M.; Martins, M.A.; Piuvezam, M.R. Warifteine, a bisbenzylisoquinoline alkaloid, decreases immediate allergic and thermal hyperalgesic reactions in sensitized animals. Int. Immunopharmacol. 2008, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, C.R.; Vieira-de-Abreu, A.; Vieira, G.C.; Ribeiro-Filho, J.; Barbosa-Filho, J.M.; Pires, A.L.; Martins, M.A.; Souza, H.S.; Bandeira-Melo, C.; Bozza, P.T.; et al. Effectiveness of Cissampelos sympodialis and its isolated alkaloid warifteine in airway hyperreactivity and lung remodeling in a mouse model of asthma. Int. Immunopharmacol. 2012, 13, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.M. Dicionário das plantas úteis do Brasil e das exóticas cultivadas; Ministério da Agricultura; Instituto Brasileiro de Desenvolvimento Florestal: Brasília, Brazil, 1984. [Google Scholar]
- Gotfredsen, E. Liber Herbarum II: Chondrodendron platyphyllum (A.St.Hil.) Miers. Available online: http://www.liberherbarum.com (accessed on 29 September 2013).
- Dias, C.S.; Barbosa-Filho, J.M.; Lemos, V.S.; Cortes, S.F. Mechanisms involved in the vasodilator effect of curine in rat resistance arteries. Planta Med. 2002, 68, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.A.; Pinho, J.F.; de-Lira, D.P.; Barbosa-Filho, J.M.; Araújo, D.A.; Cortes, S.F.; Lemos, V.S.; Cruz, J.S. Curine, a bisbenzylisoquinoline alkaloid, blocks l-type Ca2+ channels and decreases intracellular Ca2+transients in A7r5 cells. Eur. J. Pharmacol. 2011, 669, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.A.; Nunes, X.P.; Barbosa-Filho, J.M.; Lemos, V.S.; Pinho, J.F.; Roman-Campos, D.; de Medeiros, I.A.; Araújo, D.A.; Cruz, J.S. (S)-reticuline induces vasorelaxation through the blockade of l-type Ca2+ channels. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Jones, R.D.; Jones, T.H.; Channer, K.S.; Peers, C. Selective inhibition of l-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 2006, 147, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, J.; Calheiros, A.S.; Vieira-de-Abreu, A.; Carvalho, K.I.M.; Mendes, D.S.; Bandeira-Melo, C.; Martins, M.A.; Dias, C.S.; Piuvezam, M.R.; Bozza, P.T. Curineinibits eosinophil activation and airway hyper-responsiveness in a mouse model of allergic asthma. Toxicol. Appl. Pharmacol. 2013, 273, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008, 8, 116–124. [Google Scholar] [CrossRef]
- Allen, D.B. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv. Pediatr. 2006, 53, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Peanut allergy: Emerging concepts and approaches for an apparent epidemic. J. Allergy Clin. Immunol. 2007, 120, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.R.; Lloyd, C.R. Chronic inflammation and asthma. Mutat. Res. 2010, 690, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Willart, M.A.M.; Hammad, H. Alarming Dendritic Cells for Allergic Sensitization. Allergol. Int. 2010, 59, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Salomon, B.; Klatzmann, D.; Pauwels, R.A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J. Immunol. 1998, 160, 4090–4097. [Google Scholar] [PubMed]
- Chomarat, P.; Dantin, C.; Bennett, L.; Banchereau, J.; Palucka, A.K. TNF skews monocyte differentiation from macrophages to dendritic cells. J. Immunol. 2003, 171, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Zammit, D.J.; Lefrancois, L.; Vella, A.T. Effects of LPS-mediated bystander activation in the innate immune system. J. Leukoc. Biol. 2006, 80, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palmero, M.; Hara, T.; Thumbs, A.; Hunig, T. Triggering of T cell proliferation through CD28 induces Gata-3 and promotes T helper type 2 differentiation in vitro and in vivo. Eur. J. Immunol. 1999, 29, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Zhu, J. How are TH2-type immune responses initiated and amplified. Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Maurer, M.; Lantz, C.S. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 1999, 11, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Hughes, M.R.; Krystal, G. Thapsigargin-induced degranulation of mast cells is dependent on transient activation of phosphatidylinositol-3 kinase. J. Immunol. 2000, 165, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Kruhlak, M.J.; Whiteheart, S.W.; Roche, P.A. Mast cell degranulation requires Nethylmaleimide-sensitive factor-mediated SNARE disassembly. J. Immunol. 2003, 17, 5345–5352. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.F.; Lockey, R.F. Anaphylaxis: A review of causes and mechanisms. J. Allergy Clin. Immunol. 2002, 110, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.P.; Liang, M.H.; Sheffer, A.L. Exercise-induced anaphylaxis: Epidemiologic observations. Prog. Clin. Biol. Res. 1989, 297, 175–182. [Google Scholar] [PubMed]
- Buddenkotte, J.; Steinhoff, M. Pathophysiology and therapy of pruritus in allergic and atopicdiseases. Allergy 2010, 65, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, L.; Rheingold, C.G.; Lerner, E.A. Understanding the pathophysiology of itch. Dermatol. Ther. 2013, 26, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.W. Pathogenesis and Treatment of Pruritus. Curr. Allergy Asthma Rep. 2010, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.F. Itch and atopic dermatitis: Clinical and experimental studies. Acta Derm. Venereol. 1991, 165, 1–53. [Google Scholar]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, A.-M.; Bates, J.H.T.; Donovan, G.; Tawhai, M.; Sneid, J.; Sanderson, M.J. A multi-scale approach to airway hyperresponsiveness: From molecule to organ. Front. Physiol. 2012, 3, 191. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Capra, V.; Thompson, M.D.; Sala, A.; Cole, D.E.; Folco, G.; Rovati, G.E. Cysteinyl leukotrienes and their receptors in asthma and other inflammatory diseases: Critical update and emerging trends. Med. Res. Rev. 2007, 27, 469–527. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Adolphson, C.R.; Leiferman, K.M. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993, 44, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Akuthota, P.; Xenakis, J.J.; Weller, P.F. Eosinophils: Offenders or General Bystanders in Allergic Airway Disease and Pulmonary Immunity? J. Innate Immun. 2011, 3, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Vieira-de-Abreu, A.; Assis, E.F.; Gomes, G.S.; Castro-Faria-Neto, H.C.; Weller, P.F.; Bozza, P.T.; Bandeira-Melo, C. Allergic Challenge-Elicited Lipid Bodies Compartmentalize in Vivo Leukotriene C4 Synthesis within Eosinophils. Am. J. Respir. Cell Mol. Biol. 2005, 33, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Austen, K.F.; Soberman, R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 1990, 323, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, L.A.; Laitinen, A.; Haahtela, T.; Vilkka, V.; Spur, B.W.; Lee, T.H. Leukotriene E4and granulocytic infiltration into asthmatic airways. Lancet 1993, 341, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.B.; Stahl, J.L.; Barney, N.P.; Graziano, F.M. Mechanisms of antihistamines and mast cell stabilizers in ocular allergic inflammation. Curr. Drug Targets Inflamm. Allergy 2002, 1, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Neuroimmunological Mechanism of Pruritus in Atopic Dermatitis Focused on the Role of Serotonin. Biomol. Ther. 2012, 20, 506–512. [Google Scholar] [CrossRef]
- Szefler, S.J. Advancing asthma care: The glass is only half full. J. Allergy Clin. Immunol. 2011, 128, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Howell, J.B. The chromones: History, chemistry and clinical development. A tribute to the work of Dr REC. Altounyan. Clin. Exp. Allergy 2000, 30, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Gonzalo, J.A.; Nguyen, T.; Delaney, T.; Tian, J.; Oettgen, H.; Gutierrez-Ramos, J.C.; Coyle, A.J. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J. Immunol. 2001, 166, 2033–2204. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.A.; Lewis, C.C.; Woodruff, P.G.; Rodriguez, M.W.; Yang, Y.H.; Dolganov, G.M.; Fahy, J.V.; Erle, D.J. Dissecting asthma using focused transgenic modeling and functional genomics. J. Allergy Clin. Immunol. 2005, 116, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, K.; Verstraelen, S.; van-Den-Heuvel, S.; Witters, H.; Nelissen, I.; Schoeters, G. The allergic cascade: Review of the most important molecules in the asthmatic lung. Immunol. Lett. 2007, 113, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Khakzad, M.R.; Mirsadraee, M.; Mohammadpour, A.; Ghafarzadegan, K.; Hadi, R.; Saghari, M.; Meshkat, M. Effect of verapamil on bronchial goblet cells of asthma: An experimental study on sensitized animals. Pulm. Pharmacol. Ther. 2012, 25, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.W.; Okpalugo, B.I.; Small, R.C. Antagonism of Ca2+ and other actions of verapamil in guinea-pig isolated trachealis. Br. J. Pharmacol. 1984, 81, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, J.; Leite, F.C.; Costa, H.F.; Calheiros, A.S.; Torres, R.C.; de Azevedo, C.T.; Martins, M.A.; Dias, C.S.; Bozza, P.T.; Piuvezam, M.R. Curine inhibits mast cell-dependent responses in mice. J. Ethnopharmacol. 2014, 155, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Hargreave, F.E.; O’byrne, P.M.; Boulet, L.P. Understanding allergic asthma from allergen inhalation tests. Canad. Resp. J. 2007, 14, 414–418. [Google Scholar]
- Hossen, M.A.; Inoue, T.; Shinmei, Y.; Minami, K.; Fujii, Y.; Kamei, C. Caffeic Acid Inhibits Compound 48/80-Induced Allergic Symptoms in Mice. Biol. Pharm. Bull. 2006, 29, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Furuno, T. Molecular Basis of Neuroimmune Interaction in an in Vitro Coculture Approach. Cell. Mol. Immunol. 2008, 5, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ash, A.S.; Schild, H.O. Receptors mediating some actions of histamine. Br. J. Pharmacol. 1997, 120, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A. The role of mast cells in asthma. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.C.; Ribeiro-Filho, J.; Costa, H.F.; Salgado, P.R.; Calheiros, A.S.; Carneiro, A.B.; de Almeida, R.N.; Dias Cda, S.; Bozza, P.T.; Piuvezam, M.R. Curine, an Alkaloid Isolated from Chondrodendron platyphyllum Inhibits Prostaglandin E2 in Experimental Models of Inflammation and Pain. Planta Med. 2014, 80, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: an evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Mechanism of action of anti-inflammatory drugs. Scand. J. Rheumatol. 1996, 25, 9–21. [Google Scholar] [CrossRef]
- Van Rossum, D.B.; Patterson, R.L. PKC and PLA2: Probing the complexities of the calcium network. Cell Calcium 2009, 45, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro-Filho, J.; Piuvezam, M.R.; Bozza, P.T. Anti-Allergic Properties of Curine, a Bisbenzylisoquinoline Alkaloid. Molecules 2015, 20, 4695-4707. https://doi.org/10.3390/molecules20034695
Ribeiro-Filho J, Piuvezam MR, Bozza PT. Anti-Allergic Properties of Curine, a Bisbenzylisoquinoline Alkaloid. Molecules. 2015; 20(3):4695-4707. https://doi.org/10.3390/molecules20034695
Chicago/Turabian StyleRibeiro-Filho, Jaime, Márcia Regina Piuvezam, and Patrícia T. Bozza. 2015. "Anti-Allergic Properties of Curine, a Bisbenzylisoquinoline Alkaloid" Molecules 20, no. 3: 4695-4707. https://doi.org/10.3390/molecules20034695