Synthesis and Disinfection Effect of the Pyridine-4-aldoxime Based Salts
Abstract
:1. Introduction

2. Results and Discussion
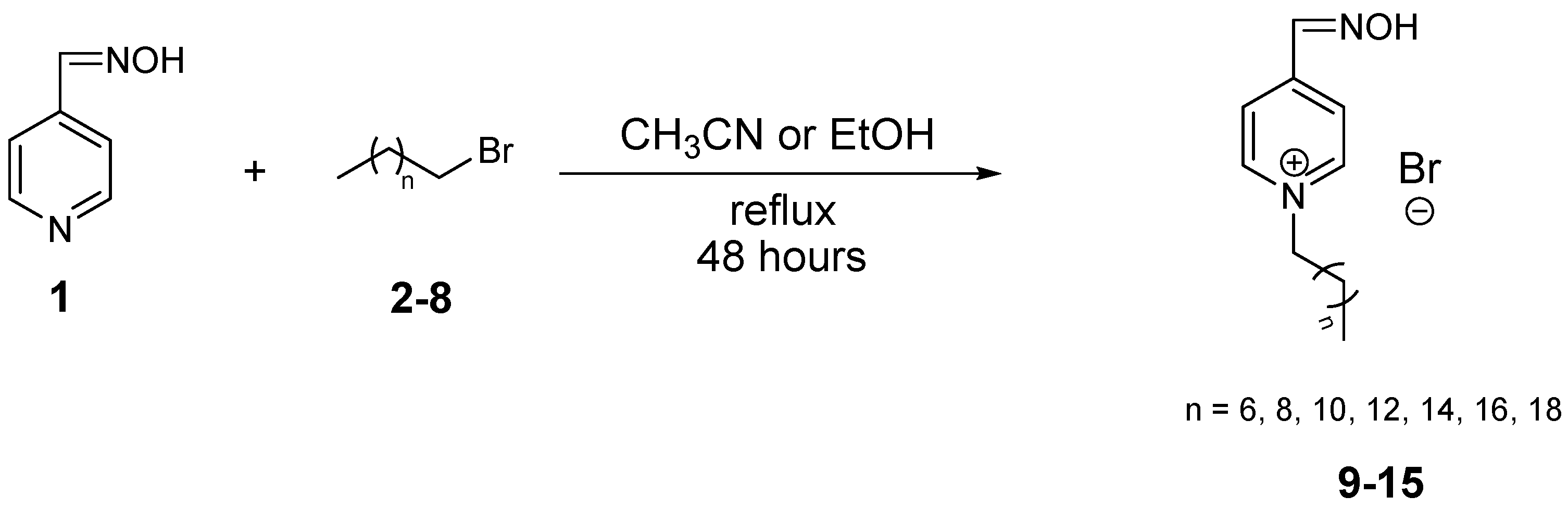
2.1. Synthesis and Analysis
| Comp. | R | Yields (%) EtOH | Yields (%) CH3CN | m.p. (°C) | HPLC Rt (min) | ClogP |
|---|---|---|---|---|---|---|
| 9 | C8 | 15 | X | 92–93 | 4.15 | 0.04 |
| 10 | C10 | 27 | 41 | 130–132 | 4.67 | 1.10 |
| 11 | C12 | 70 | 55 | 136–137 | 5.22 | 2.15 |
| 12 | C14 | 72 | 75 | 144–146 | 5.82 | 3.21 |
| 13 | C16 | 75 | 87 | 144–145 | 6.49 | 4.27 |
| 14 | C18 | 47 | 76 | 128–130 | 7.23 | 5.33 |
| 15 | C20 | 45 | X | 126–128 | 8.06 | 6.39 |

2.2. Antimicrobial Activity
| Microorganisms | MIC (μmol/L); 24 h/48 h Incubation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MFC (μmol/L); 48 h Incubation | ||||||||||
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | B12 a | B14 a | B16 a | |
| Candida albicans ATCC 44859 (CA) | >500/>500 | 125/250 | 15.62/15.62 | 1.95/1.95 | 3.9/3.9 | 7.81/7.81 | >500/>500 | 0.49/0.49 | 7.81/7.81 | 3.91/7.81 |
| >500 | 250 | 15.62 | 1.95 | 3.9 | 15.62 | >500 | 0.98 | 125 | 7.81 | |
| Candida tropicalis 156 (CT) | 125/125 | 31.25/31.25 | 7.81/7.81 | 3.9/3.9 | 3.9/3.9 | 7.81/7.81 | >500/>500 | 0.49/0.49 | 3.91/3.91 | 3.91/7.81 |
| 250 | 125 | 31.25 | 15.62 | 3.9 | 15.62 | >500 | 0.98 | 125 | 7.81 | |
| Candida krusei E28 (CK) | 31.25/62.5 | 3.9/3.9 | 0.98/1.95 | 1.95/1.95 | 3.9/ 3.9 | 3.9/3.9 | >500/>500 | 0.49/0.49 | 3.91/3.91 | 1.95/1.95 |
| 125 | 15.62 | 3.9 | 15.62 | 3.9 | 15.62 | >500 | 0.49 | 125 | 1.95 | |
| Candida glabrata 20/I (CG) | 125/125 | 31.25/31.25 | 3.9/3.9 | 1.95/3.9 | 3.9/3.9 | 3.9/3.9 | >500/>500 | 0.49/0.49 | 7.81/7.81 | 1.95/3.91 |
| 250 | 62.5 | 3.9 | 31.25 | 3.9 | 7.81 | >500 | 0.49 | 125 | 62.5 | |
| Trichosporon asahii 1188 (TA) | >500 >500 | 500/>500 | 125/125 | 15.62/15.62 | 7.81/7.81 | 7.81/7.81 | >500/>500 | 0.49/1.95 | 31.25/31.25 | 7.81/7.81 |
| >500 | >500 | 500 | 31.25 | 7.81 | 7.81 | >500 | 1.95 | 125 | 62.5 | |
| Aspergillus fumigatus 231 (AF) | >500/>500 | >500/>500 | 125/250 | 62.5/62.5 | 15.62/15.62 | >500/>500 | >500/>500 | 0.98/3.91 | 7.81/15.62 | 7.81/7.81 |
| >500 | >500 | >500 | 250 | 62.5 | >500 | >500 | 3.91 | 125 | 62.5 | |
| Absidia corymbifera 272 (AC) | >500/>500 | >500/>500 | 500/500 | 62.5/62.5 | 15.62/31.25 | >500/>500 | >500/>500 | 7.81/7.81 | 31.25/31.25 | 7.81/7.81 |
| >500 | >500 | 500 | 500 | 62.5 | >500 | >500 | 7.81 | 125 | 62.5 | |
| Trichophyton mentagrophytes 445 (TM) | >500>500 | >500/>500 | 62.5/62.5 | 15.62/62.5 | 15.62/15.62 | >500/>500 | >500/>500 | 0.98/0.98 | 15.62/15.62 | 7.81/7.81 |
| >500 | >500 | 125 | 125 | 62.5 | >500 | >500 | 1.95 | 15.62 | 62.5 | |
| Microorganisms | MIC (μmol/L); 24 h/48 h Incubation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MBC (μmol/L); 48 h Incubation | ||||||||||
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | B12 a | B14 a | B16 a | |
| Staphylococcus aureus CCM 451608 (SA) | 125/ 125 | 15.62/15.62 | 15.62/15.62 | 1.95/7.81 | 0.98/0.98 | 7.81/7.81 | 15.62/15.62 | 0.49/1.95 | 0.98/0.98 | 0.98/0.98 |
| 125 | 15.62 | 15.62 | 7.81 | 0.98 | 7.81 | 15.62 | 1.95 | 3.91 | 3.91 | |
| Staphylococcus aureus H 599608 (MRSA) | 3.9/15.62 | 31.25/31.25 | 7.81/15.62 | 0.49/3.9 | 0.98/7.81 | 15.62/125 | 62.5/>250 | 0.49/0.49 | 1.95/1.95 | 1.95/1.95 |
| 15.62 | 31.25 | 15.62 | 7.81 | 7.81 | 125 | >250 | 0.98 | 3.91 | 3.91 | |
| Staphylococcus epidermidis H 696608 (SE) | 62.5/125 | 15.62/15.62 | 0.98/1.95 | 0.98/0.98 | 0.98/0.98 | 1.95/1.95 | 1.95/1.95 | 0.49/0.49 | 0.98/0.98 | 0.49/0.49 |
| 250 | 15.62 | 1.95 | 0.98 | 0.98 | 1.95 | 1.95 | 0.49 | 0.98 | 3.91 | |
| Enterococcus sp. J 1436508 (ES) | 500/>500 | 62.5/125 | 31.25/31.25 | 0.98/7.81 | 7.81/7.81 | 3.9/15.62 | 62.5/62.5 | 0.49/0.98 | 1.95/1.95 | 1.95/1.95 |
| >500 | 500 | 62.5 | 7.81 | 7.81 | 15.62 | 62.5 | 0.98 | 7.81 | 3.91 | |
| Escherichia coli CCM 4517 (EC) | >500/>500 | 500/500 | 62.5/62.5 | 15.62/15.62 | 15.62/15.62 | >500/>500 | >250/>250 | 0.49/1.95 | 7.81/7.81 | 7.81/7.81 |
| >500 | 500 | 62.5 | 15.62 | 15.62 | >500 | >250 | 1.95 | 7.81 | 7.81 | |
| Klebsiella pneumoniae D 1175008 (KP) | >500/>500 | 500/500 | 62.5/62.5 | 15.62/15.62 | 15.62/15.62 | >500/>500 | >250/>250 | 0.49/0.49 | 7.81/7.81 | 7.81/7.81 |
| >500 | 500 | 62.5 | 15.62 | 15.62 | >500 | >250 | 0.49 | 7.81 | 7.81 | |
| Klebsiella pneumoniae J 1436808 (KP-E)) | >500/>500 | >500/>500 | 125/125 | 15.62/15.62 | 31.25/31.25 | >500/>500 | >250/>250 | 0.98/0.98 | 7.81/7.81 | 7.81/7.81 |
| >500 | >500 | 125 | 15.62 | 31.25 | >500 | >250 | 0.98 | 7.81 | 7.81 | |
| Pseudomonas aeruginosa CCM 1961 (PA) c | >500/>500 | 500/500 | 125/125 | 15.62/15.62 | 250/250 | >500/>500 | >250/>250 | 3.91/3.91 | 15.62/31.25 | 15.62/31.25 |
| >500 | 500 | 125 | 15.62 | 250 | >500 | >250 | 7.81 | 62.5 | 125 | |
2.3. Cytotoxicity
| Cell Line | IC50 (μmol/L); 24 h Incubation ± SEM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | B12 a | B14 a | B16 a | |
| CHO-K1 | >1000 | 108 ± 5 | 16 ± 2 | 14 ± 1 | 7 ± 1 | 3.5 ± 0.1 | 2.9 ± 0.2 | 29 ± 3 | 24 ± 4 | 15 ± 1 |
3. Experimental Section
3.1. Synthesis
3.2. HPLC Analysis
3.3. In-Vitro Antimicrobial Testing
3.3.1. Antifungal Activity
3.3.2. Antibacterial Activity
3.4. Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, S.; Bhadani, A.; Kataria, H.; Kaur, G.; Kamboj, R. Synthesis of glycerol-based pyridinium surfactants and appraisal of their properties. Ind. Eng. Chem. Res. 2009, 48, 1673–1677. [Google Scholar] [CrossRef]
- Earle, M.J.; Katdare, S.P.; Seddon, K.R. Paradigm confirmed: The first use of ionic liquids to dramatically influence the outcome of chemical reactions. Org. Lett. 2004, 6, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Price, P.B. Benzalkonium chloride (zephiran chloride) as a skin disinfectant. Arch. Surg. 1950, 61, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Kohler, H.H. Surface properties of alkylpyridinium chlorides and the applicability of the pendant drop technique. J. Colloid Interface Sci. 1999, 218, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Akbas, H.; Kartal, C.C.I. Reactive orange 16-dodecylpyridinium chloride interactions in electrolytic solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, S.; Compadre, R.L.; Castillo, R.; Breen, P.J.; Compadre, C.M. 3D-QSAR, synthesis, and antimicrobial activity of 1-alkylpyridinium compounds as potential agents to improve food safety. Eur. J. Med. Chem. 2005, 40, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Sivak, A.; Goyer, M.; Perwak, J.; Thayer, P. Environmental and Human Health Aspects of Commercially Important Surfactants. In Solution Behaviour of Surfactants (Theoretical and Applied Aspects); Mittal, K.L., Fendler, E.J., Eds.; Plenum Press: New York, NY, USA, 1982; Volume 1, pp. 161–188. [Google Scholar]
- Kuca, K.; Bielavska, M.; Cabal, J.; Dohnal, V. Determination of benzalkonium bromide homologues in disinfection products using high-performance liquid chromatography. Anal. Lett. 2005, 38, 673–682. [Google Scholar] [CrossRef]
- Kuca, K.; Marek, J.; Stodulka, P.; Musilek, K.; Hanusova, P.; Hrabinova, M.; Jun, D. Preparation of benzalkonium salts differing in the length of a side alkyl chain. Molecules 2007, 12, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.L.; Hubieki, M.P.; Curfman, C.L.; Doncel, G.F.; Dudding, T.C.; Savle, P.S.; Gandour, R.D. A structure-activity study of spermicidal and anti-hiv properties of hydroxylated cationic surfactants. Bioorg. Med. Chem. 2002, 10, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Qiu, S.Y.; Lewis, K.; Klibanov, A.M. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol. Prog. 2002, 18, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Faraj, J.A.; Dorati, R.; Schoubben, A.; Worthen, D.; Selmin, F.; Capan, Y.; Leung, K.; DeLuca, P.P. Development of a peptide-containing chewing gum as a sustained release antiplaque antimicrobial delivery system. AAPS PharmSciTech 2007, 8, E177–E185. [Google Scholar] [CrossRef]
- Tanzer, J.M.; Slee, A.M.; Kamay, B.; Scheer, E.R. In vitro evaluation of seven cationic detergents as antiplaque agents. Antimicrob. Agents Chemother. 1979, 15, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.S.; Vancampen, M.G.; Tilford, C.H.; Lang, H.C.; Nisonger, L.; Bandelin, F.J.; Rubenkoenig, H.L. Quaternary ammonium salts as germicides 1. Non-acylated quaternary ammonium salts derived from aliphatic amines. J. Am. Chem. Soc. 1946, 68, 753–755. [Google Scholar]
- Li, Y.B.; Slavik, M.F.; Walker, J.T.; Xiong, H. Pre-chill spray of chicken carcasses to reduce salmonella typhimurium. J. Food Sci. 1997, 62, 605–607. [Google Scholar] [CrossRef]
- Brill, F.; Goroncy-Bermes, P.; Sand, W. Influence of growth media on the sensitivity of staphylococcus aureus and pseudomonas aeruginosa to cationic biocides. Int. J. Hyg. Environ. Health 2006, 209, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dwars, T.; Paetzold, E.; Oehme, G. Reactions in micellar systems. Angewa. Chem. Int. Ed. 2005, 44, 7174–7199. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Kolay, S.; Bal, S.; Satnami, M.L.; Quagliotto, P.; Dafonte, P.R. Effect of cationic gemini surfactants on the hydrolysis of carboxylate and phosphate esters using hydroxamate ions. Coll. Polym. Sci. 2008, 286, 293–303. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Satnami, M.L.; Sinha, D. Dephosphorylation of paraoxon by hydroxamate ions in micellar media. Tetrahedron. Lett. 2004, 45, 9103–9105. [Google Scholar] [CrossRef]
- Singh, N.; Ghosh, K.K.; Marek, J.; Kuca, K. Hydrolysis of carboxylate and phosphate esters using monopyridinium oximes in cationic micellar media. Int. J. Chem. Kinet. 2011, 43, 569–578. [Google Scholar] [CrossRef]
- Cabal, J.; Kuca, K.; Sevelova-Bartosova, L.; Dohnal, V. Cyclodextrines as functional agents for decontamination of the skin contaminated by nerve agents. Acta Medica (Hradec Kral.) 2004, 47, 115–118. [Google Scholar]
- Tiwari, S.; Ghosh, K.K.; Marek, J.; Kuca, K. Comparative study of nucleophilic efficacy of pralidoxime towards phosphorus, sulfur and thiophosphorus based esters. React. Kinet. Catal. Lett. 2009, 98, 91–97. [Google Scholar] [CrossRef]
- Tiwari, S.; Ghosh, K.K.; Marek, J.; Kuca, K. Functionalized surfactant mediated reactions of carboxylate, phosphate and sulphonate esters. J. Phys. Org. Chem. 2010, 23, 519–525. [Google Scholar] [CrossRef]
- Tiwari, S.; Ghosh, K.K.; Marek, J.; Kuca, K. Cationic micellar-catalyzed hydrolysis of pesticide fenitrothion using alpha-nucleophiles. Lett. Drug Des. Discov. 2010, 7, 194–199. [Google Scholar] [CrossRef]
- Tiwari, S.; Kolay, S.; Ghosh, K.K.; Kuca, K.; Marek, J. Kinetic study of the reactions of p-nitrophenyl acetate and p-nitrophenyl benzoate with oximate nucleophiles. Int. J. Chem. Kinet. 2009, 41, 57–64. [Google Scholar] [CrossRef]
- Fisicaro, E.; Pelizzetti, E.; Viscardi, G.; Quagliotto, P.L.; Trossarelli, L. Thermodynamic properties of aqueous micellar solutions of N-(1H,1H,2H,2H perfluorooctyl)pyridinium chloride and N-(1H,1H,2H,2H perfluorodecyl)pyridinium chloride. In Colloids and Surfaces A: Physicochemical and Engineering Aspects, Proceedings of the ACS Symposium on Colloid and Surface Chemistry of Fluorocarbons and Highly Fluorinated Amphiphiles at 205th Annual ACS Meeting, Denver, CO, USA, 28 March–2 April 1993; Franses, E.I., Weers, J.G., Eds.; Elsevier: London, UK, 1994; Volume 84, pp. 59–70. [Google Scholar]
- Fisicaro, E.; Ghiozzi, A.; Pelizzetti, E.; Viscardi, G.; Quagliotto, P.L. Effect of the counterion on thermodynamic properties of aqueous micellar solutions of 1-(3,3,4,4,5,5,6,6,6-nonafluorohexyl) pyridinium halides: Ii. Apparent and partial molar enthalpies and osmotic coefficients at 313 k. J. Colloid Interface Sci. 1996, 184, 147–154. [Google Scholar]
- Fisicaro, E.; Biemmi, M.; Compari, C.; Duce, E.; Peroni, M. Thermodynamics of aqueous solutions of dodecyldimethylethylammonium bromide. J. Colloid Interface Sci. 2007, 305, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Kivala, M.; Dohnal, V. A general method for the quaternization of N,N-dimethyl benzylamines with long chain N-alkylbromides. J. Appl. Biomed. 2004, 2, 195–198. [Google Scholar]
- Spilovska, K.; Korabecny, J.; Kral, J.; Horova, A.; Musilek, K.; Soukup, O.; Drtinova, L.; Gazova, Z.; Siposova, K.; Kuca, K. 7-Methoxytacrine-adamantylamine heterodimers as cholinesterase inhibitors in Alzheimer’s disease treatment—synthesis, biological evaluation and molecular modeling studies. Molecules 2013, 18, 2397–2418. [Google Scholar] [CrossRef] [PubMed]
- Korabecny, J.; Soukup, O.; Dolezal, R.; Spilovska, K.; Nepovimova, E.; Andrs, M.; Nquyen, T.D.; Jun, D.; Musilek, K.; Kucerova-Chlupacova, M.; Kuca, K. From pyridinium-based to central active acetylcholinesterase reactivators. Mini Rev. Med. Chem. 2014, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Malinak, D.; Dolezal, R.; Marek, J.; Salajkova, S.; Soukup, O.; Vejsova, M.; Korabecny, J.; Honegr, J.; Penhaker, M.; Musilek, K.; et al. 6-Hydroxyquinolinium salts differing in the length of alkyl side-chain: Synthesis and antimicrobial activity. Bioorg. Med. Chem. Lett. 2014, 24, 5238–5241. [Google Scholar]
- Jordan, D.; Tan, E.; Hegh, D. Synthesis, characterization and conductivity of quaternary nitrogen surfactants modified by the addition of a hydroxymethyl substructure on the head group. J. Surfactants Deterg. 2012, 15, 587–592. [Google Scholar] [CrossRef]
- Kamboj, R.; Singh, S.; Chauhan, V. Synthesis, characterization and surface properties of N-(2-hydroxyalkyl)-N'-(2-hydroxyethyl)imidazolium surfactants. Coll. Surf. A Physicochem. Eng. Asp. 2014, 441, 233–241. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, Q.X.; Zhi, L.F.; Zhang, M.H. Synthesis, characterization and surface activity of trioctyl hydroxyethyl ammonium chloride. Tenside Surfactants Deterg. 2011, 48, 305–307. [Google Scholar] [CrossRef]
- Marek, J.; Stodulka, P.; Cabal, J.; Soukup, O.; Pohanka, M.; Korabecny, J.; Musilek, K.; Kuca, K. Preparation of the pyridinium salts differing in the length of the N-alkyl substituent. Molecules 2010, 15, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Marek, J.; Buchta, V.; Soukup, O.; Stodulka, P.; Cabal, J.; Ghosh, K.K.; Musilek, K.; Kuca, K. Preparation of quinolinium salts differing in the length of the alkyl side chain. Molecules 2012, 17, 6386–6394. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, R.T.; Tonsager, S.; McGroarty, E.J. Quantitation of metal-cations bound to membranes and extracted lipopolysaccharide of escherichia-coli. Biochemistry 1983, 22, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 2001, 14, 227–228. [Google Scholar]
- Zhong, X.; Guo, J.W.; Fu, S.Q.; Zhu, D.Y.; Peng, J.P. Synthesis, surface property and antimicrobial activity of cationic gemini surfactants containing adamantane and amide groups. J. Surfactants Deterg. 2014, 17, 943–950. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In Approved Standard-Third Edition; CLSI document M27-A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. In Approved Standard-Second Edition; CLSI document M38-A2; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. In Approved Standard-Seventh Edition; CLSI document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marek, J.; Malinak, D.; Dolezal, R.; Soukup, O.; Pasdiorova, M.; Dolezal, M.; Kuca, K. Synthesis and Disinfection Effect of the Pyridine-4-aldoxime Based Salts. Molecules 2015, 20, 3681-3696. https://doi.org/10.3390/molecules20033681
Marek J, Malinak D, Dolezal R, Soukup O, Pasdiorova M, Dolezal M, Kuca K. Synthesis and Disinfection Effect of the Pyridine-4-aldoxime Based Salts. Molecules. 2015; 20(3):3681-3696. https://doi.org/10.3390/molecules20033681
Chicago/Turabian StyleMarek, Jan, David Malinak, Rafael Dolezal, Ondrej Soukup, Marketa Pasdiorova, Martin Dolezal, and Kamil Kuca. 2015. "Synthesis and Disinfection Effect of the Pyridine-4-aldoxime Based Salts" Molecules 20, no. 3: 3681-3696. https://doi.org/10.3390/molecules20033681






