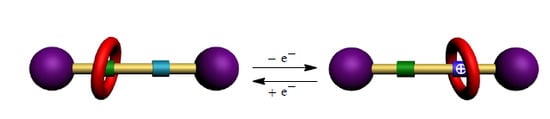
A Redox-Controllable Molecular Switch Based on Weak Recognition of BPX26C6 at a Diphenylurea Station
Abstract
:1. Introduction
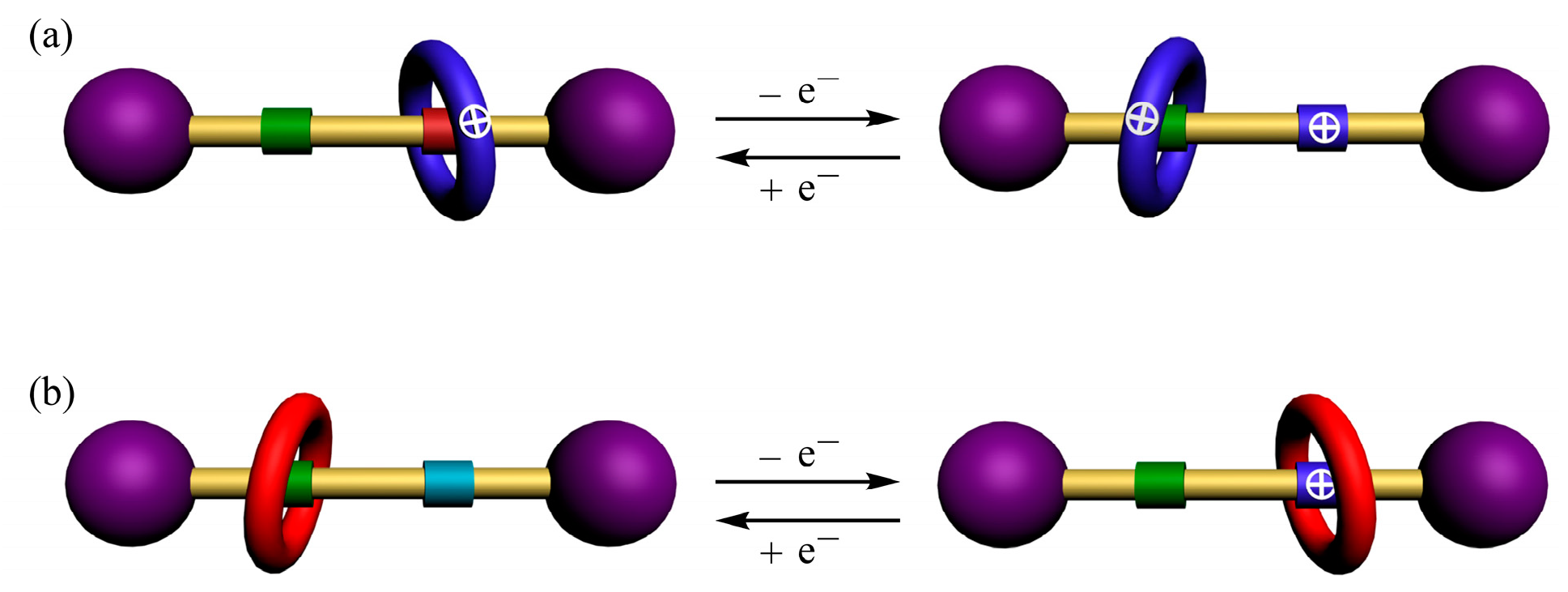
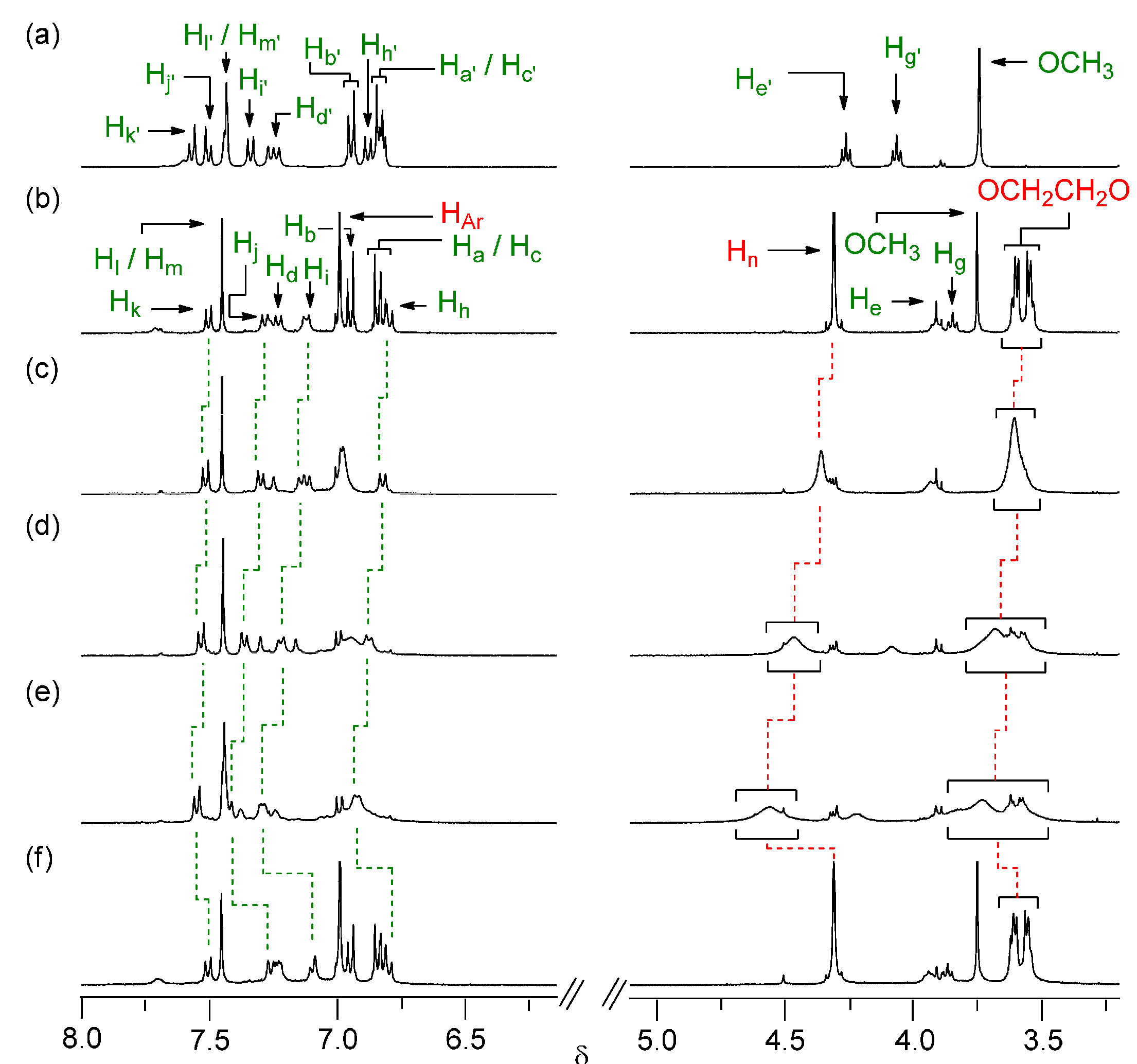
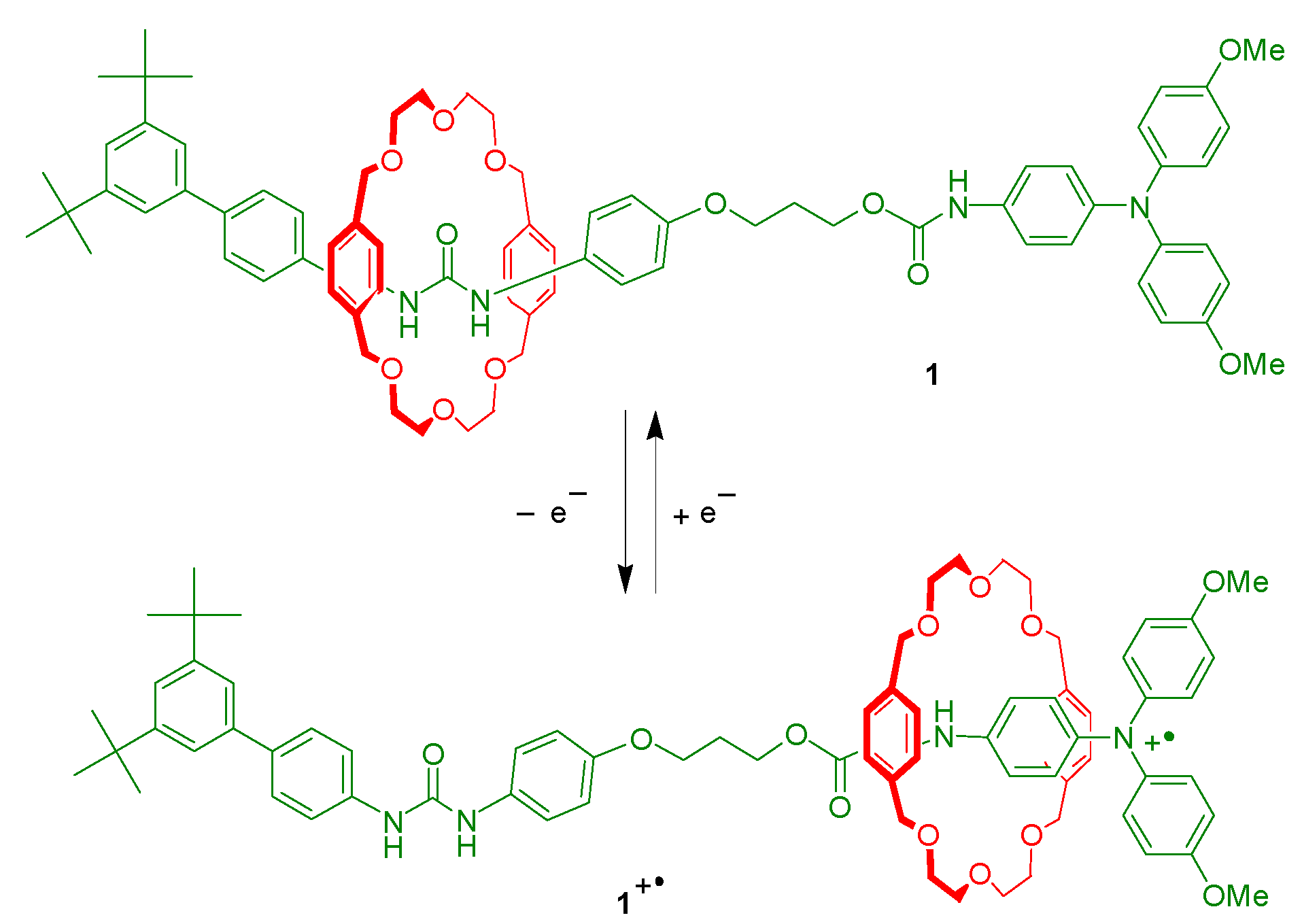
2. Results and Discussion
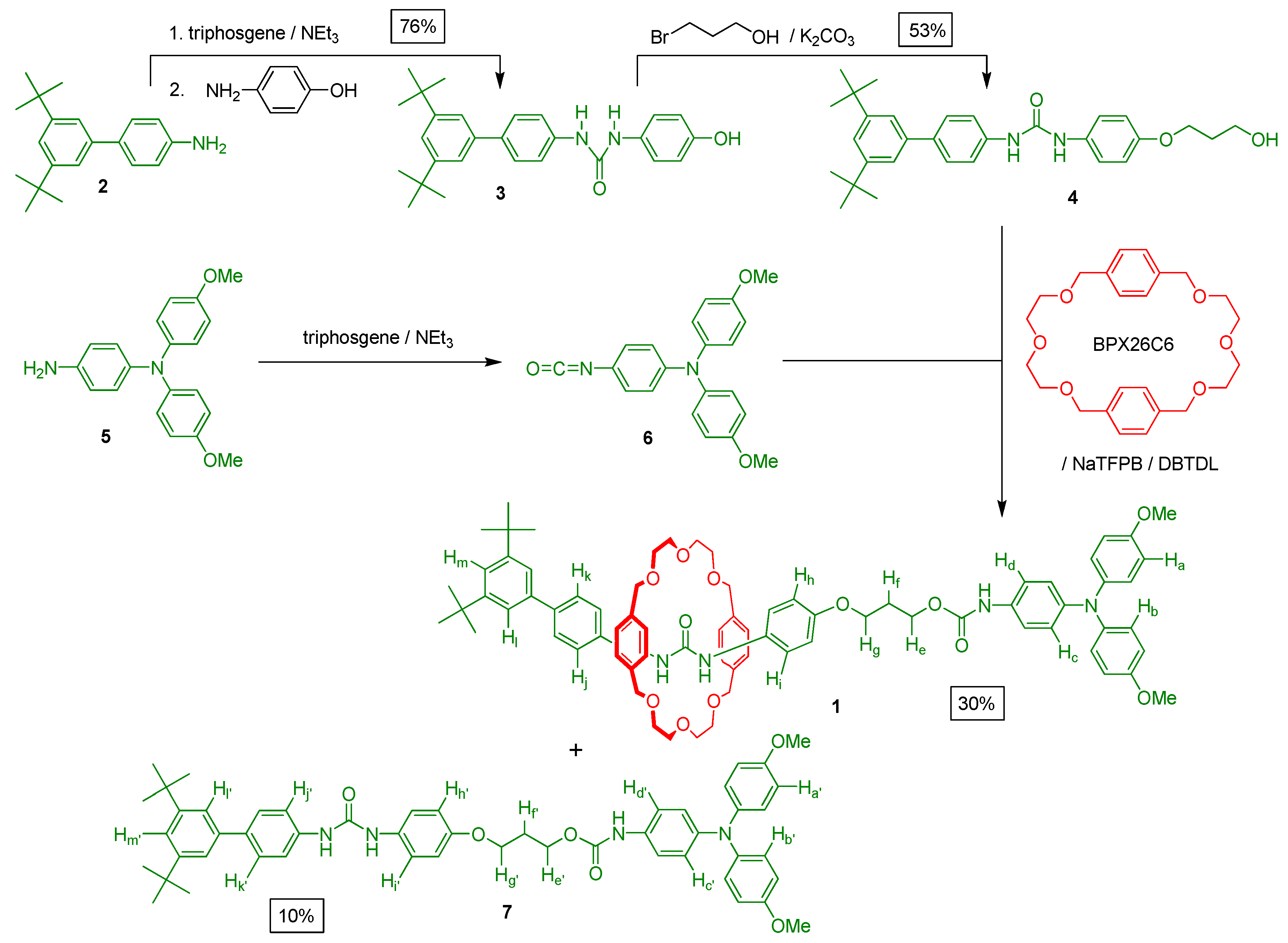
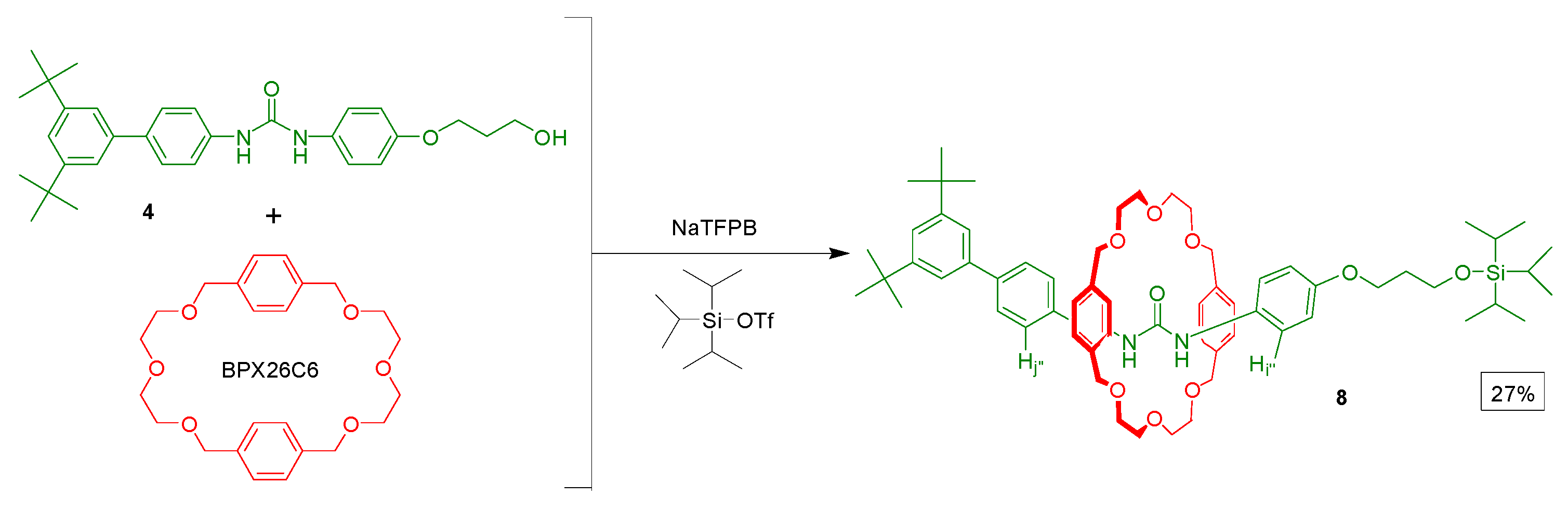

3. Experimental Section
3.1. General Information
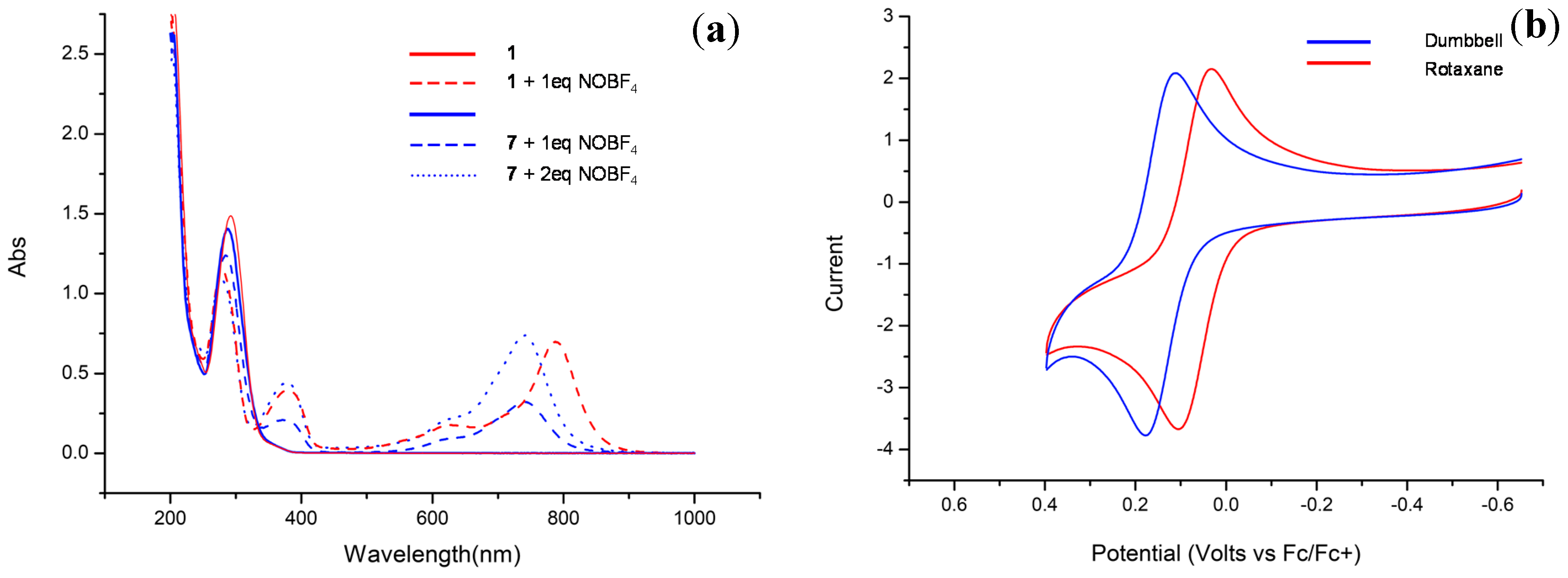
3.2. UV–Vis Spectroscopy
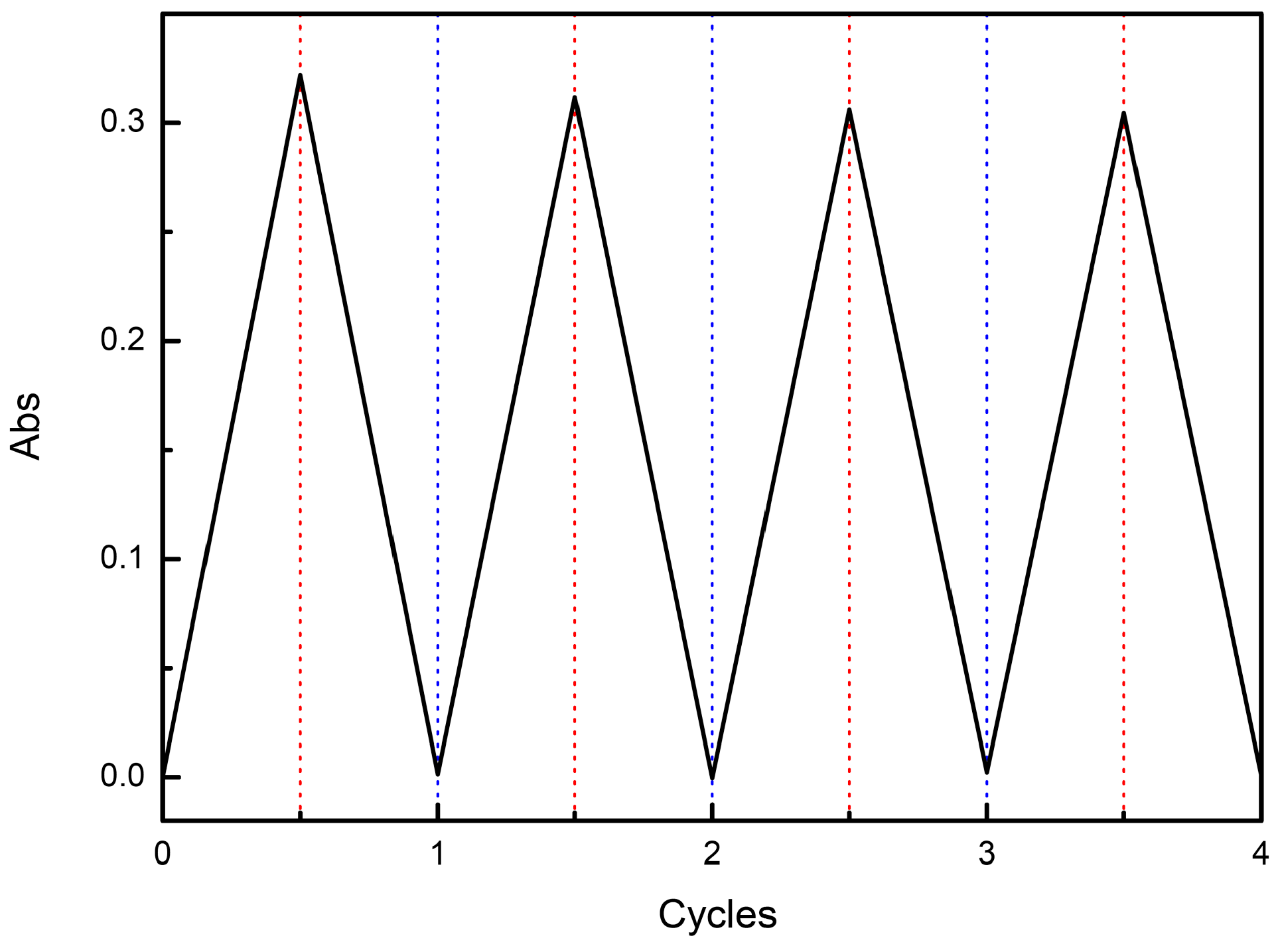
3.3. Electrochemistry
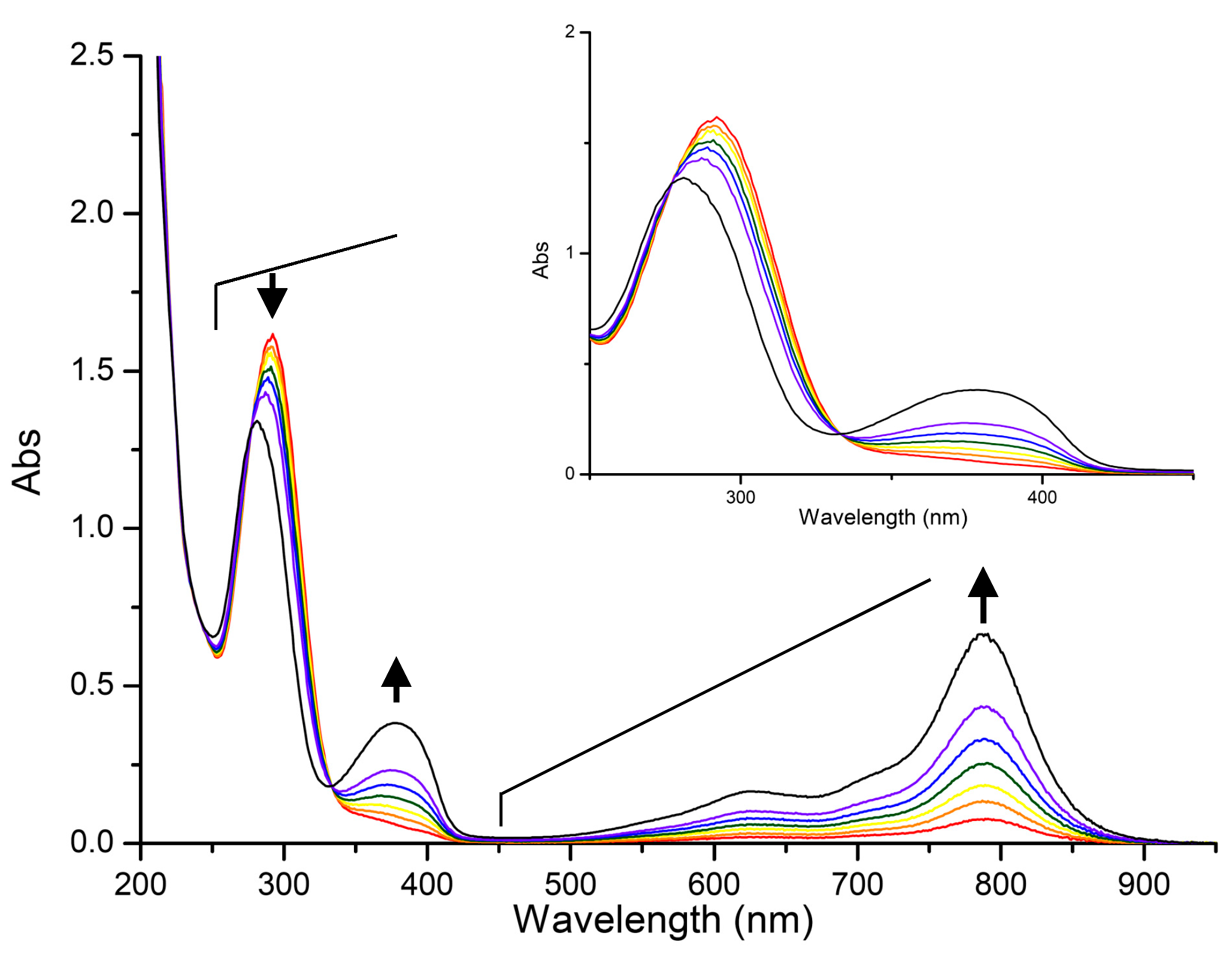
3.4. Spectroelectrochemistry
3.5. Electron Paramagnetic Resonance Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berná, J.; Leigh, D.A.; Lubomska, M.; Mendoza, S.M.; Pérez, E.M.; Rudolf, P.; Teobaldi, G.; Zerbetto, F. Macroscopic transport by synthetic molecular machines. Nat. Mater. 2005, 4, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Baumes, J.M.; Gassensmith, J.J.; Giblin, J.; Lee, J.-J.; White, A.G.; Culligan, W.J.; Leevy, W.M.; Kuno, M.; Smith, B.D. Storable, thermally activated, near-infrared chemiluminescent dyes and dye-stained microparticles for optical imaging. Nat. Chem. 2010, 2, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Green, J.E.; Choi, J.W.; Boukai, A.; Bunimovich, Y.; Johnston-Halperin, E.; DeIonno, E.; Luo, Y.; Sheriff, B.A.; Xu, K.; Shin, Y.S.; et al. A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature 2007, 445, 414–417. [Google Scholar] [CrossRef]
- Collins, C.G.; Peck, E.M.; Kramer, P.J.; Smith, B.D. Squaraine rotaxane shuttle as a ratiometric deep-red optical chloride sensor. Chem. Sci. 2013, 4, 2557–2563. [Google Scholar] [CrossRef]
- Caballero, A.; Zapata, F.; Beer, P.D. Interlocked host molecules for anion recognition and sensing. Coord. Chem. Rev. 2013, 257, 2434–2455. [Google Scholar] [CrossRef]
- Hsueh, S.-Y.; Kuo, C.-T.; Lu, T.-W.; Lai, C.-C.; Liu, Y.-H.; Hsu, H.-F.; Peng, S.-M.; Chen, C.-H.; Chiu, S.-H. Acid/Base- and Anion-Controllable Organogels Formed From a Urea-Based Molecular Switch. Angew. Chem. Int. Ed. 2010, 49, 9170–9173. [Google Scholar] [CrossRef]
- Kohsaka, Y.; Nakazono, K.; Koyama, Y.; Asai, S.; Takata, T. Size-complementary rotaxane cross-linking for the stabilization and degradation of a supramolecular network. Angew. Chem. Int. Ed. 2011, 50, 4872–4875. [Google Scholar] [CrossRef]
- Lee, S.; Chen, C.-H.; Flood, A.H. A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes. Nat. Chem. 2013, 5, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Ko, J.-L.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.-H. Using “threading followed by shrinking” to synthesize highly stable dialkylammonium-ion-based rotaxanes. Chem. Eur. J. 2013, 19, 8850–8860. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.B.; Zhu, K.; Vukotic, V.N.; Loeb, S.J.; Stephan, D.W. Heterolytic activation of H2 using a mechanically interlocked molecule as a frustrated Lewis base. Angew. Chem. Int. Ed. 2013, 52, 960–963. [Google Scholar] [CrossRef]
- Yuki, T.; Koyama, Y.; Matsumura, T.; Takata, T. Click annulation of pseudo[2]rotaxane to [2]catenane exploiting homoditopic nitrile N-oxide. Org. Lett. 2013, 15, 4438–4441. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Yan, X.; Huang, F. Reversible formation of a poly[3]rotaxane based on photo dimerization of an anthracene-capped [3]rotaxane. Chem. Commun. 2014, 50, 14105–14108. [Google Scholar] [CrossRef]
- Caballero, A.; Swan, L.; Zapata, F.; Beer, P.D. Iodide-induced shuttling of a halogen- and hydrogen-bonding two-station rotaxane. Angew. Chem. Int. Ed. 2014, 53, 11854–11858. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-based molecular muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- De Bo, G.; Kuschel, S.; Leigh, D.A.; Lewandowski, B.; Papmeyer, M.; Ward, J.W. Efficient assembly of threaded molecular machines for sequence-specific synthesis. J. Am. Chem. Soc. 2014, 136, 5811–5814. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.-H. Sodium ions template the formation of rotaxanes from BPX26C6 and nonconjugated amide and urea functionalities. Angew. Chem. Int. Ed. 2013, 52, 10231–10236. [Google Scholar] [CrossRef]
- Lu, T.-W.; Chang, C.-F.; Lai, C.-C.; Chiu, S.-H. Molecular switch based on very weak association between BPX26C6 and two recognition units. Org. Lett. 2013, 15, 5742–5745. [Google Scholar]
- Lin, Y.-H.; Lai, C.-C.; Chiu, S.-H. Five additional macrocycles that allow the Na+ ion–templated threading of guest units featuring a single urea or amide functionality. Org. Biomol. Chem. 2014, 12, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Fahrenbach, A.C.; Bruns, C.J.; Cao, D.; Stoddart, J.F. Ground-state thermodynamics of redox-active donor-acceptor mechanically interlocked molecules. Acc. Chem. Res. 2012, 45, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Kihara, N.; Hashimoto, M.; Takata, T. Redox behavior of ferrocene-containing rotaxane: Transposition of the rotaxane wheel by redox reaction of a ferrocene moiety tethered at the end of the axle. Org. Lett. 2004, 6, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Dichtel, W.R.; Trabolsi, A.; Saha, S.; Aprahamian, I.; Stoddart, J.F. A redox-switchable α-cyclodextrin-based [2]rotaxane. J. Am. Chem. Soc. 2008, 130, 11294–11296. [Google Scholar] [CrossRef] [PubMed]
- Altieri, A.; Gatti, F.G.; Kay, E.R.; Leigh, D.A.; Martel, D.; Paolucci, F.; Slawin, A.M.Z.; Wong, J.K.Y. Electrochemically switchable hydrogen-bonded molecular shuttles. J. Am. Chem. Soc. 2003, 125, 8644–8654. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Alonso, A.; Fioravanti, G.; Marcaccio, M.; Paolucci, F.; Rahman, G.M.A.; Ehli, C.; Guldi, D.M.; Prato, M. An electrochemically driven molecular shuttle controlled and monitored by C60. Chem. Commun. 2007, 1945–1947. [Google Scholar]
- Jacquot de Rouville, H.-P.; Iehl, J.; Bruns, C.J.; McGrier, P.L.; Frasconi, M.; Sarjeant, A.A.; Stoddart, J.F. A neutral naphthalene diimide [2]rotaxane. Org. Lett. 2012, 14, 5188–5191. [Google Scholar] [CrossRef] [PubMed]
- Elizarov, A.M.; Chiu, S.-H.; Stoddart, J.F. An acid-base switchable [2]rotaxane. J. Org. Chem. 2002, 67, 9175–9181. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Diaz, M.-V.; Spencer, N.; Stoddart, J.F. The self-assembly of a switchable [2]rotaxane. Angew. Chem. Int. Ed. Engl. 1997, 36, 1904–1907. [Google Scholar] [CrossRef]
- Sauvage, J.-P. Transition metal-containing rotaxanes and catenanes in motion: Toward molecular machines and motors. Acc. Chem. Res. 1998, 31, 611–619. [Google Scholar] [CrossRef]
- Padilla-Tosta, N.E.; Fox, O.D.; Drew, M.G.B.; Beer, P.D. Self-assembly of a mixed-valence copper(II)/copper(III) dithiocarbamate catenane. Angew. Chem. Int. Ed. 2001, 40, 4235–4239. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Mladenova, B.; Grampp, G.; Kaiser, C.; Heckmann, A.; Lambert, C. Electron paramagnetic resonance spectroscopy of bis(triarylamine) paracyclophanes as model compounds for the intermolecular charge-transfer in solid state materials for optoelectronic applications. J. Phys. Chem. C 2009, 113, 2983–2995. [Google Scholar] [CrossRef]
- Sreenath, K.; Thomas, T.G.; Gopidas, K.R. Cu(II) mediated generation and spectroscopic study of the tris(4-anisyl)amine radical cation and dication: Unusually shielded chemical shifts in the dication. Org. Lett. 2011, 13, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-C.; Lai, C.-C.; Chiu, S.-H. A Redox-Controllable Molecular Switch Based on Weak Recognition of BPX26C6 at a Diphenylurea Station. Molecules 2015, 20, 1775-1787. https://doi.org/10.3390/molecules20021775
Chang J-C, Lai C-C, Chiu S-H. A Redox-Controllable Molecular Switch Based on Weak Recognition of BPX26C6 at a Diphenylurea Station. Molecules. 2015; 20(2):1775-1787. https://doi.org/10.3390/molecules20021775
Chicago/Turabian StyleChang, Jia-Cheng, Chien-Chen Lai, and Sheng-Hsien Chiu. 2015. "A Redox-Controllable Molecular Switch Based on Weak Recognition of BPX26C6 at a Diphenylurea Station" Molecules 20, no. 2: 1775-1787. https://doi.org/10.3390/molecules20021775