Lectins: Getting Familiar with Translators of the Sugar Code
Abstract
:1. Why Glycans Are the Most Versatile Platform for Coding
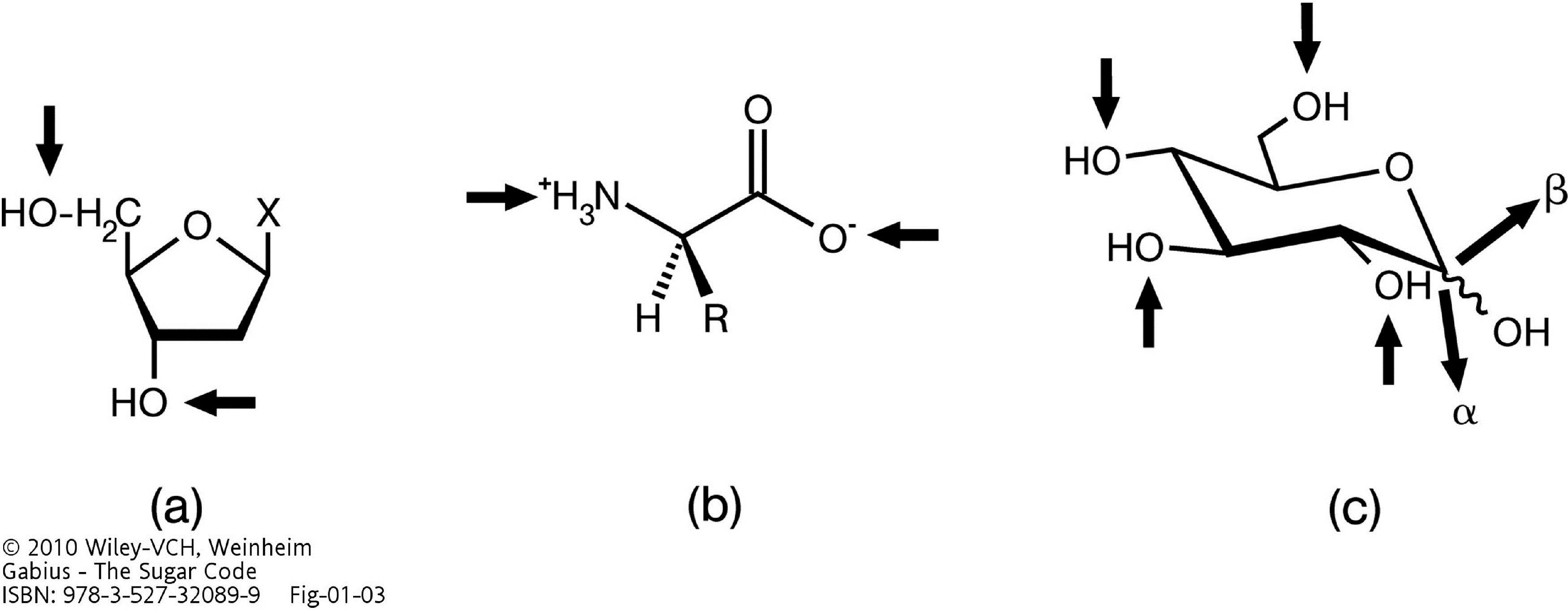
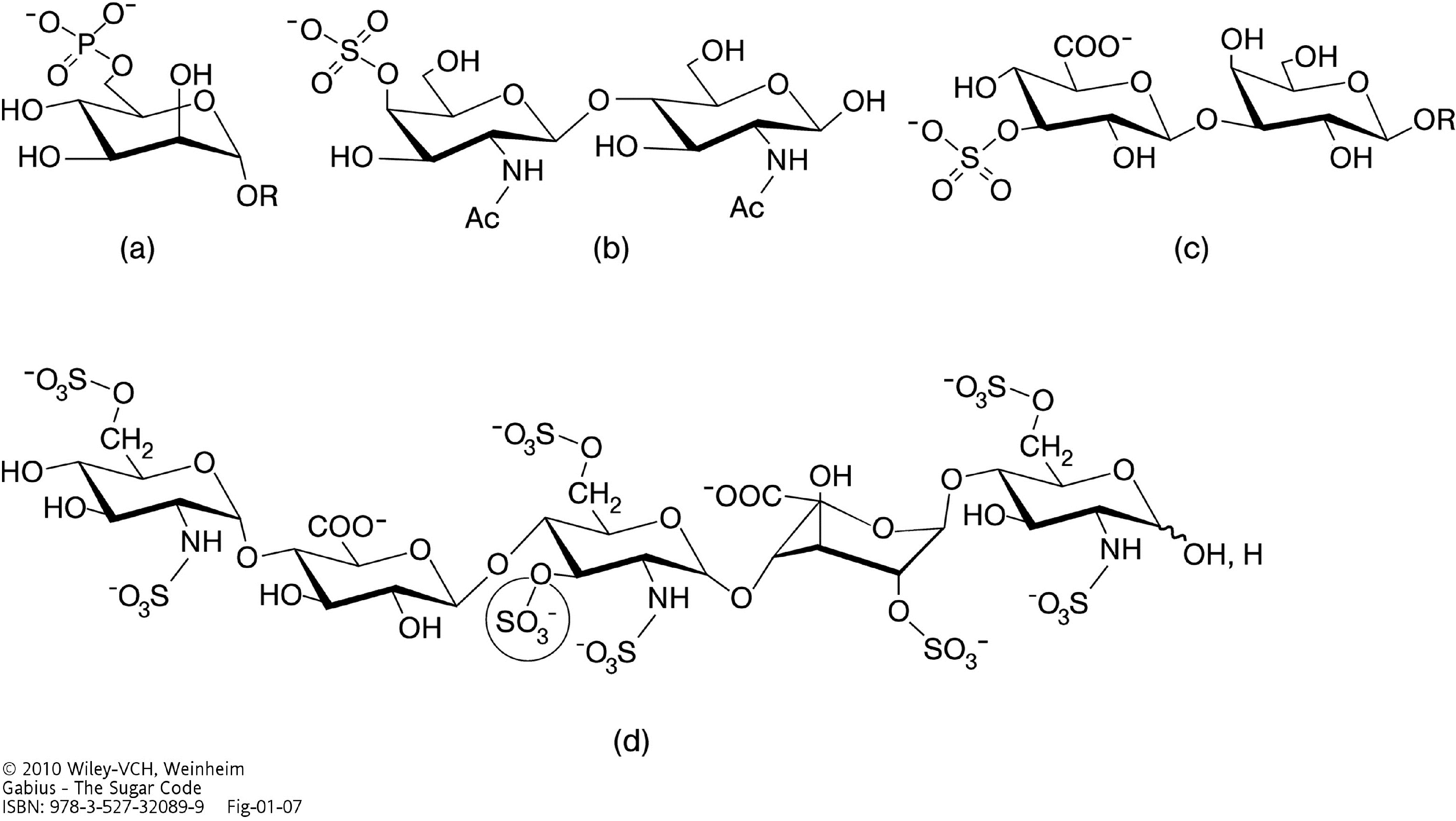
2. Why Glycans are Suited for Biorecognition
3. Lectins: Definition and Overview
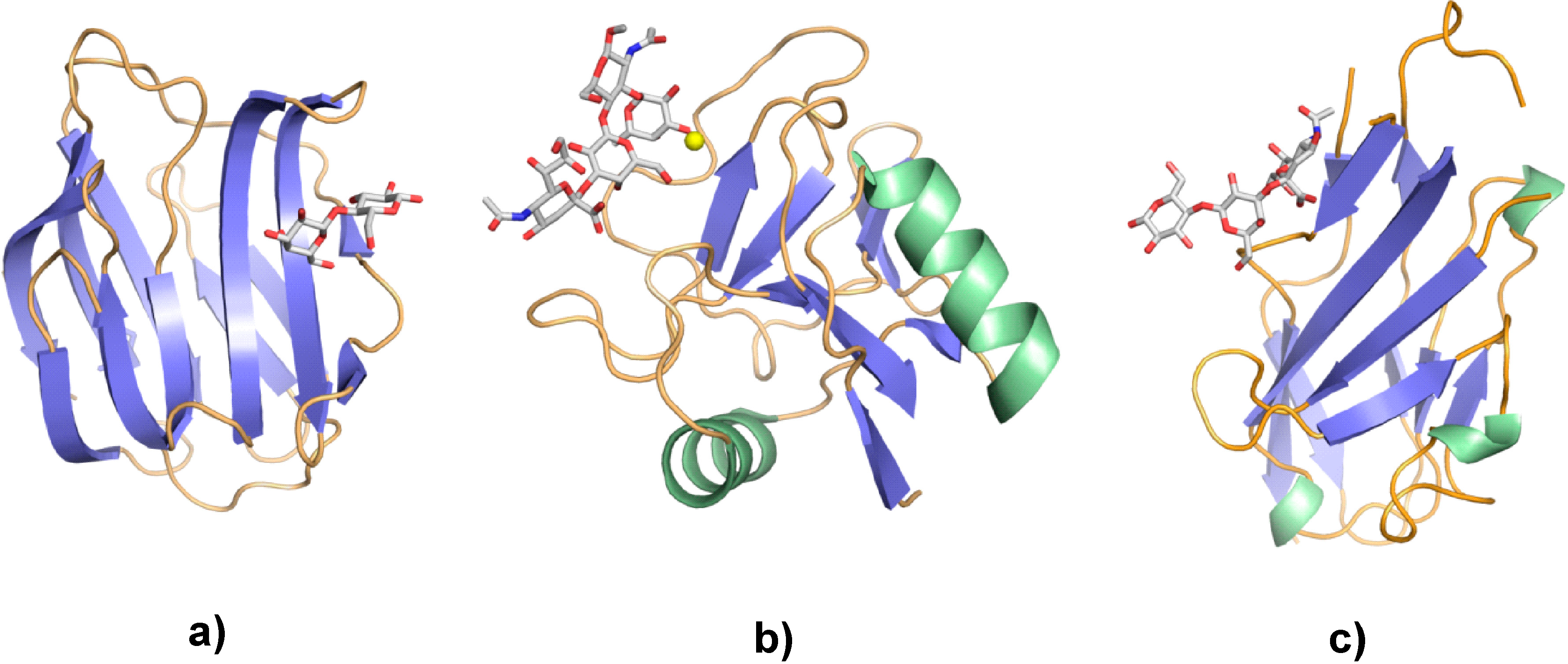

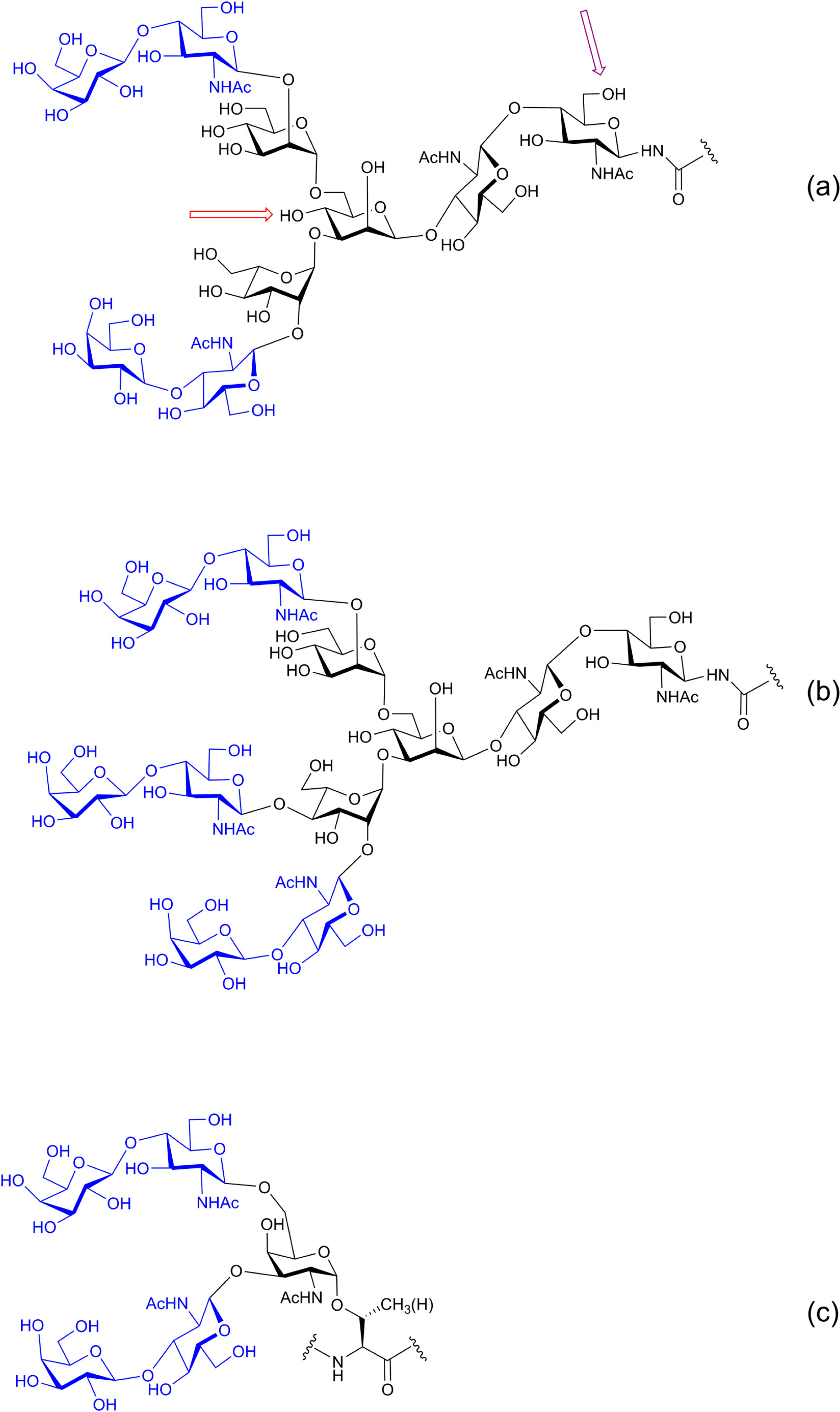
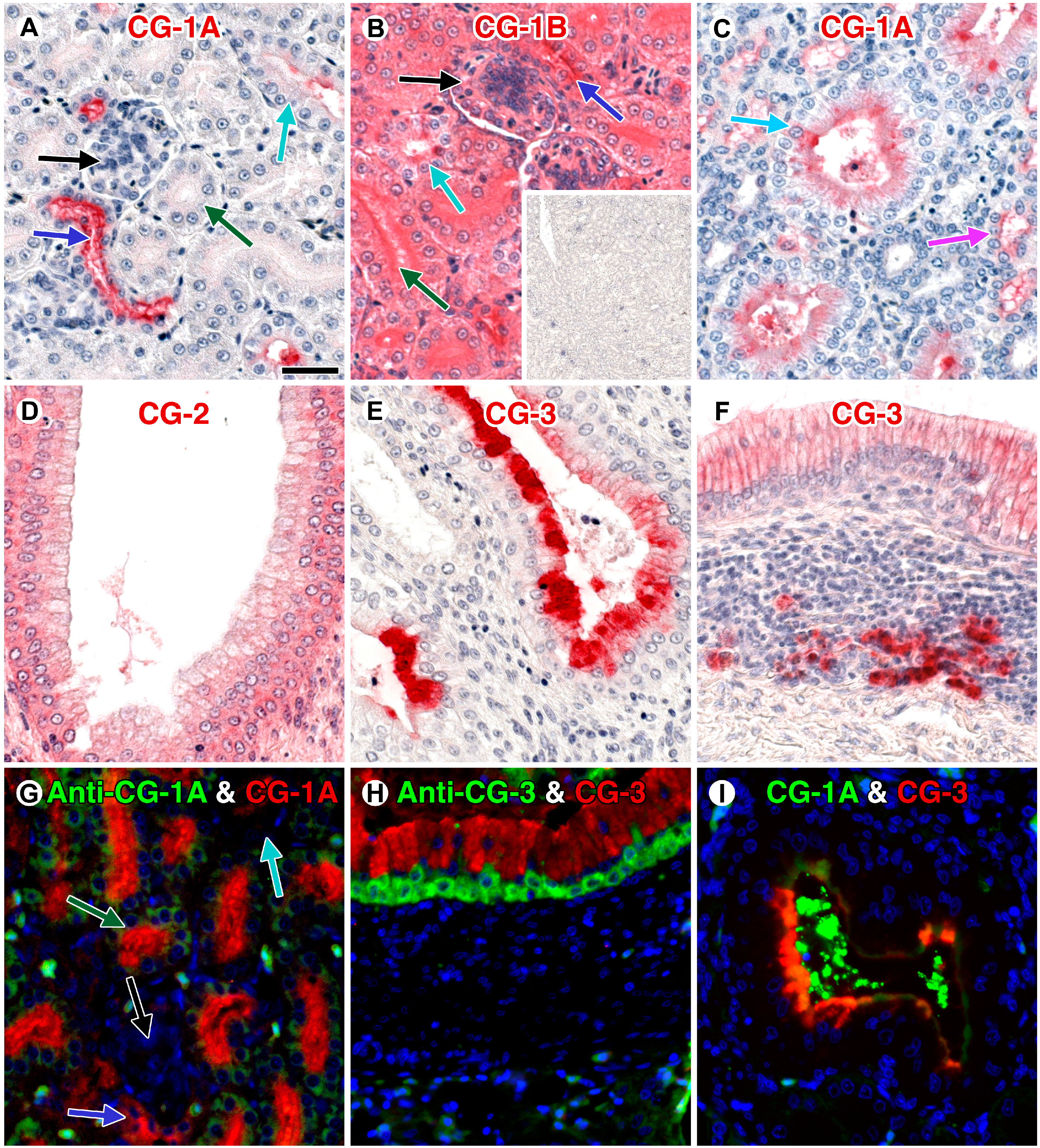
4. Galectins: A Model to Trace Lectin Divergence
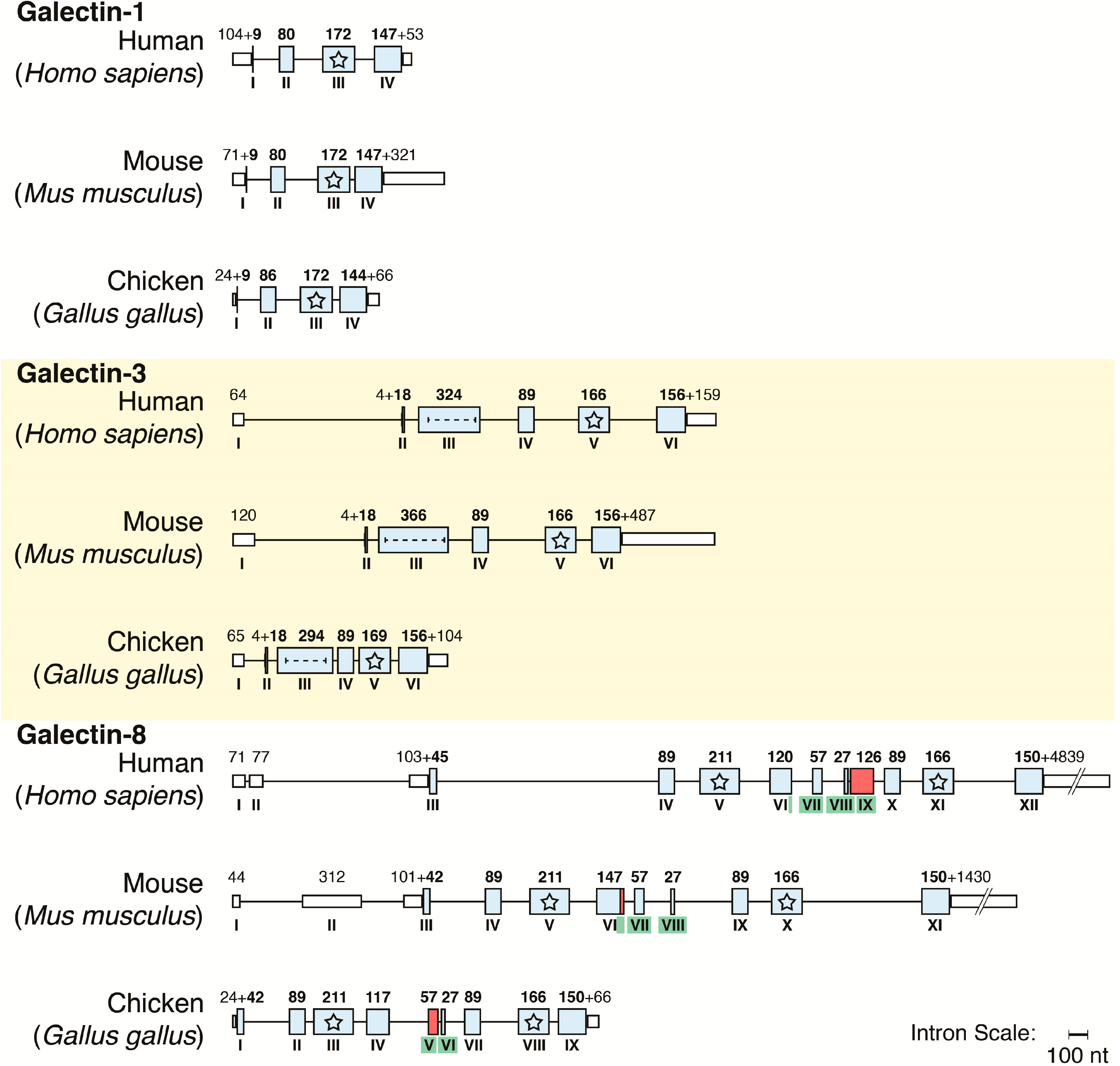
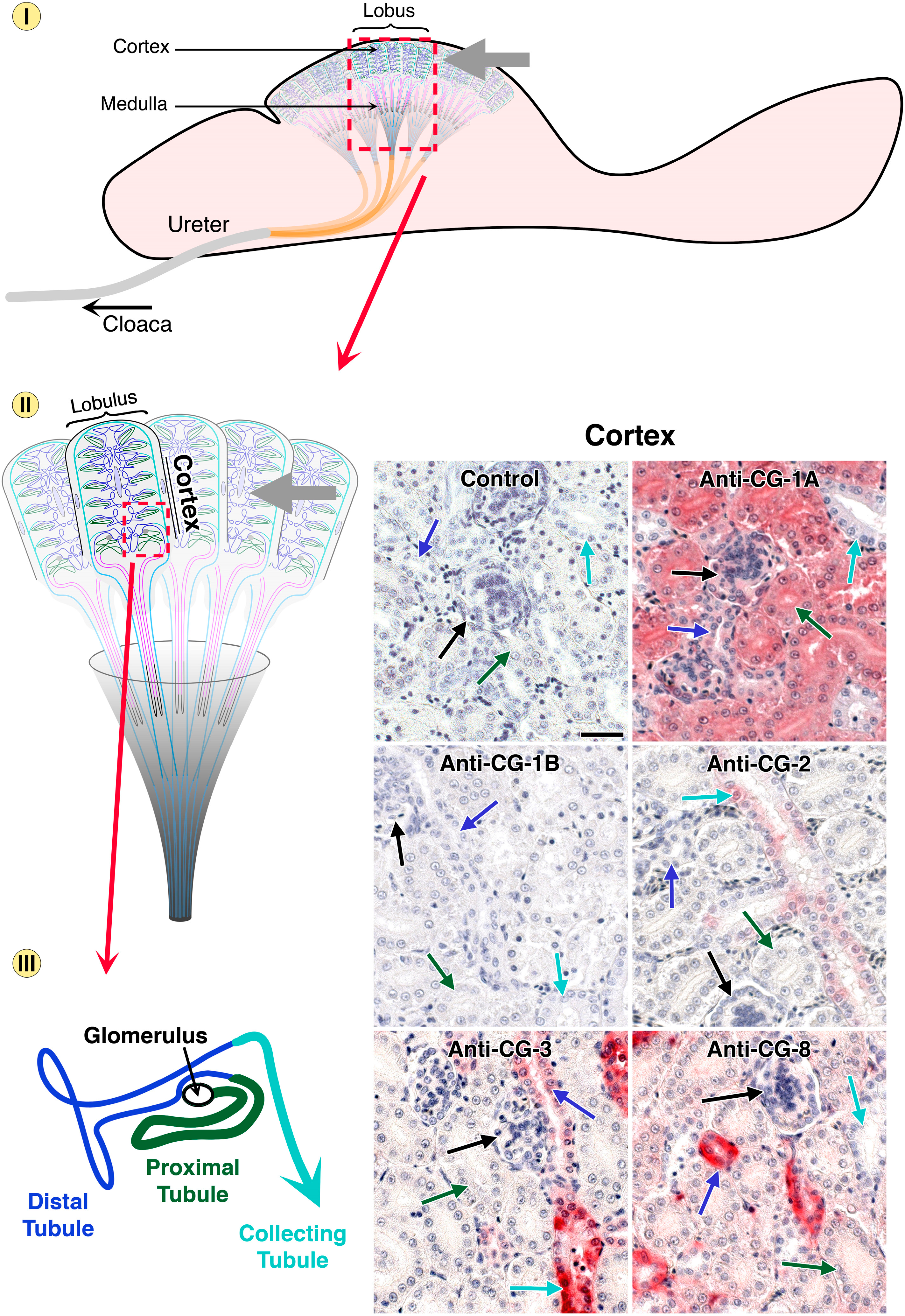

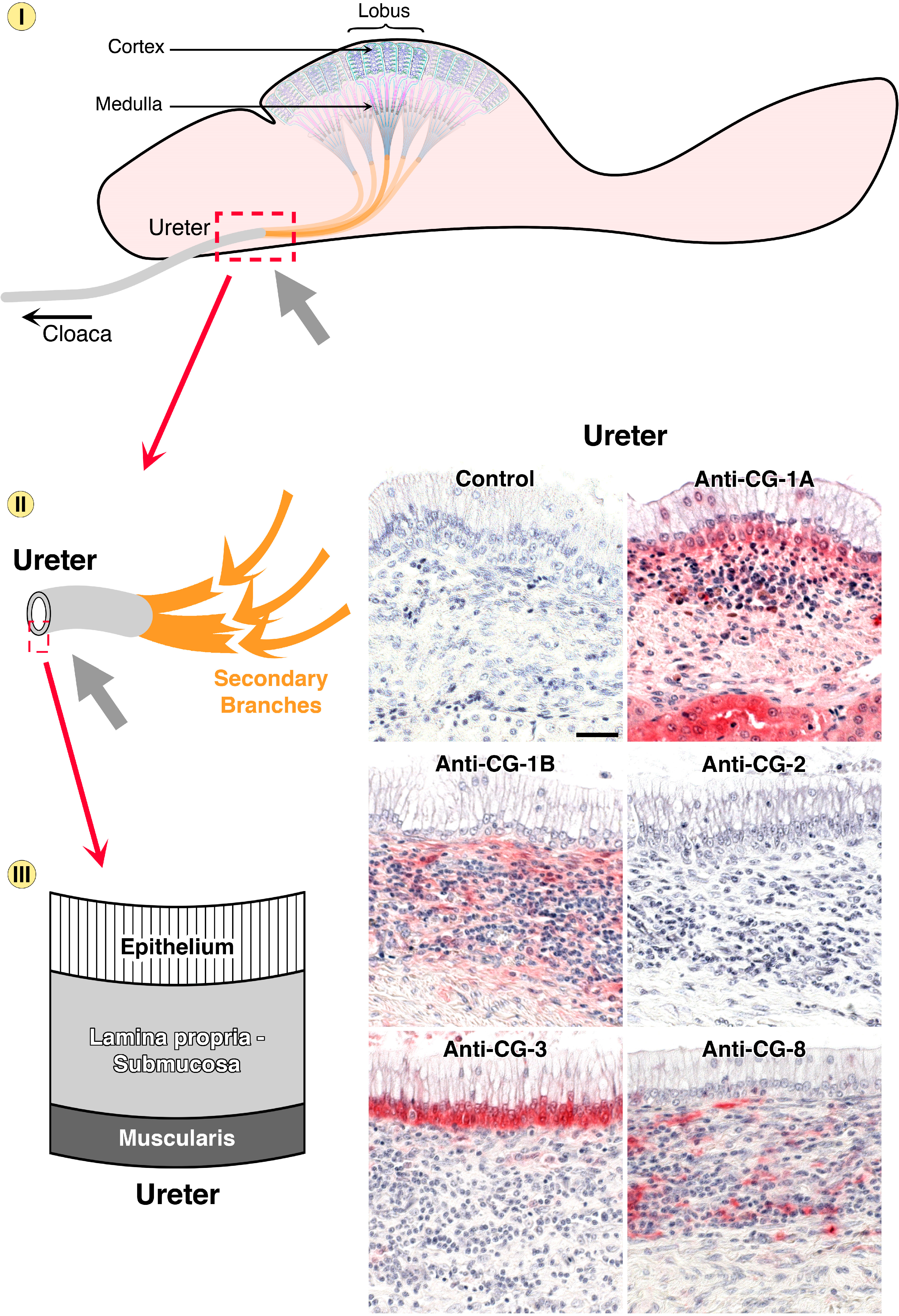
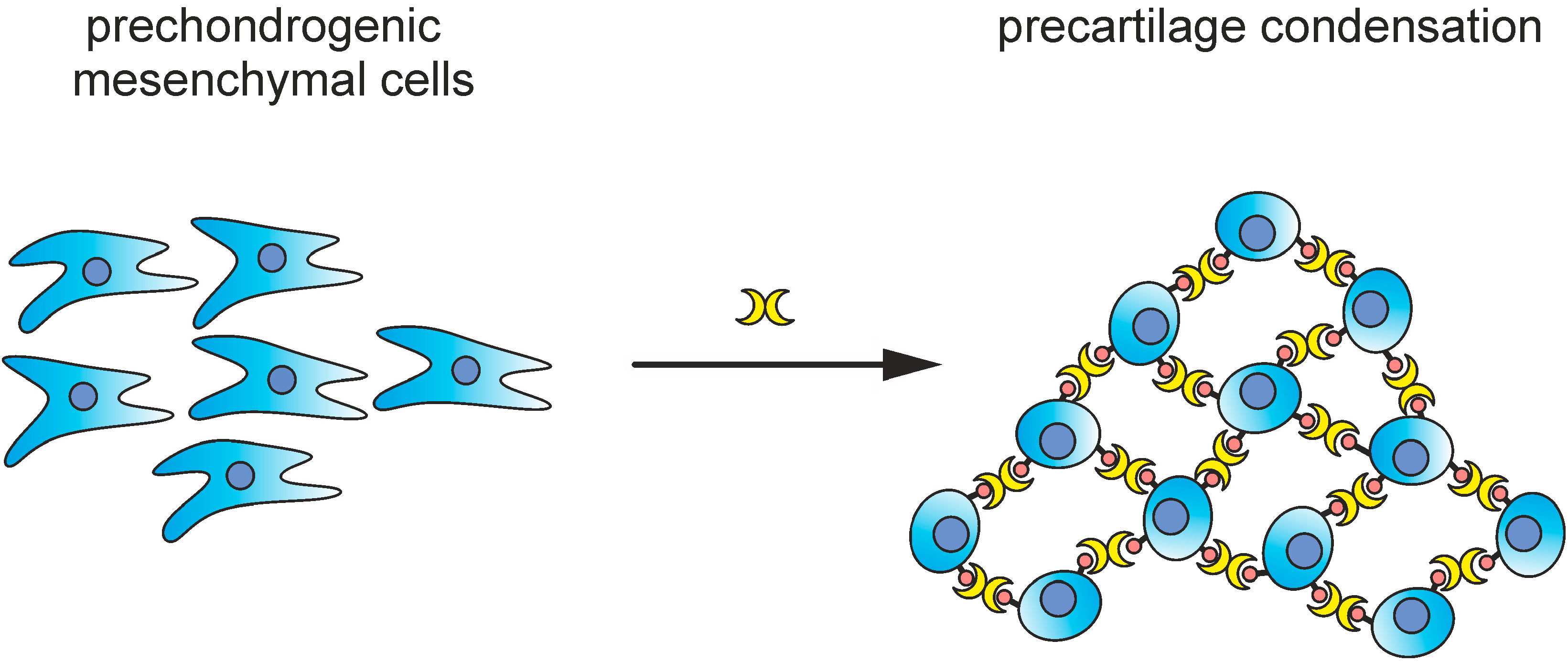
5. Endogenous Lectins as Tools and Effectors
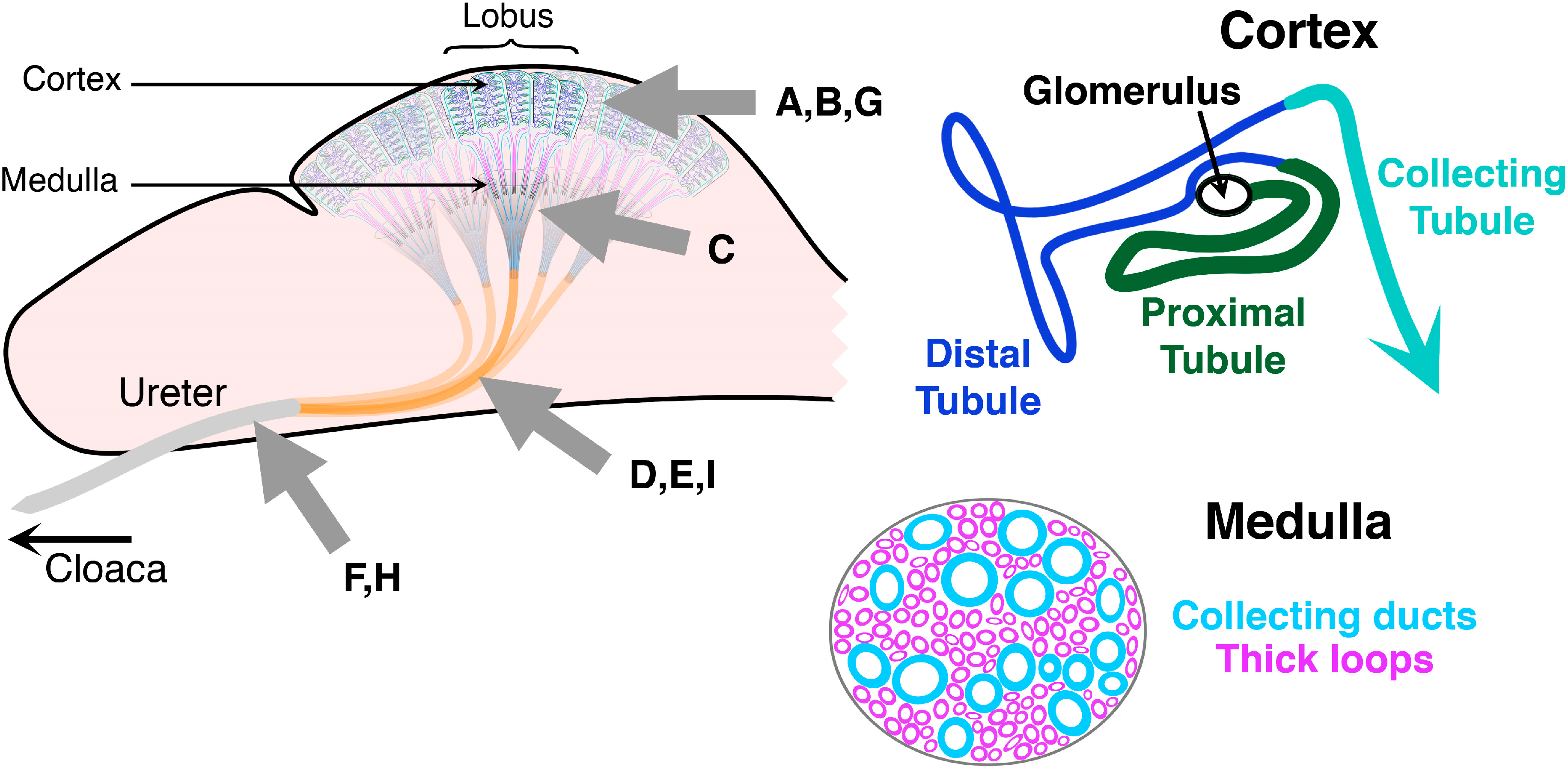
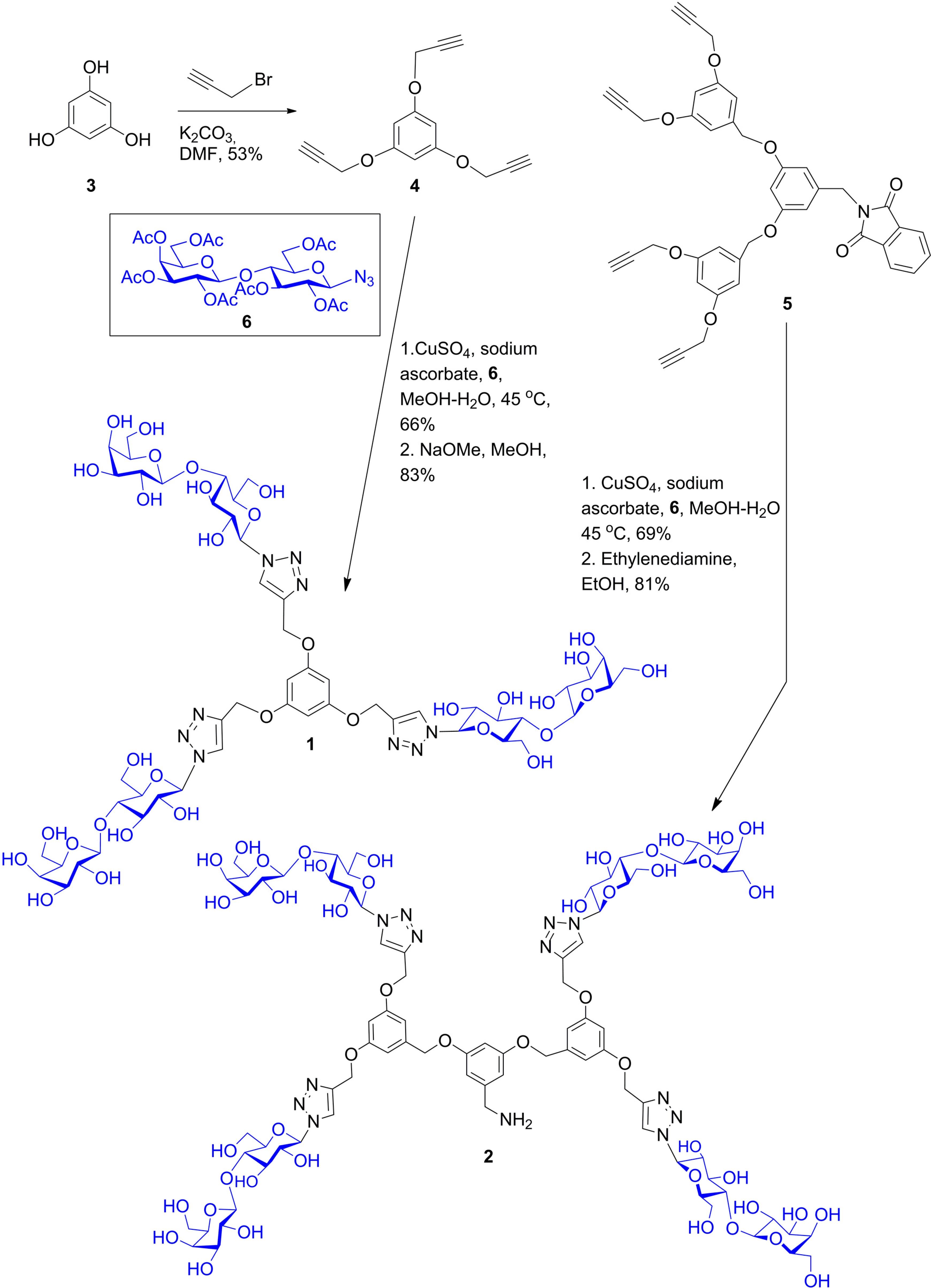
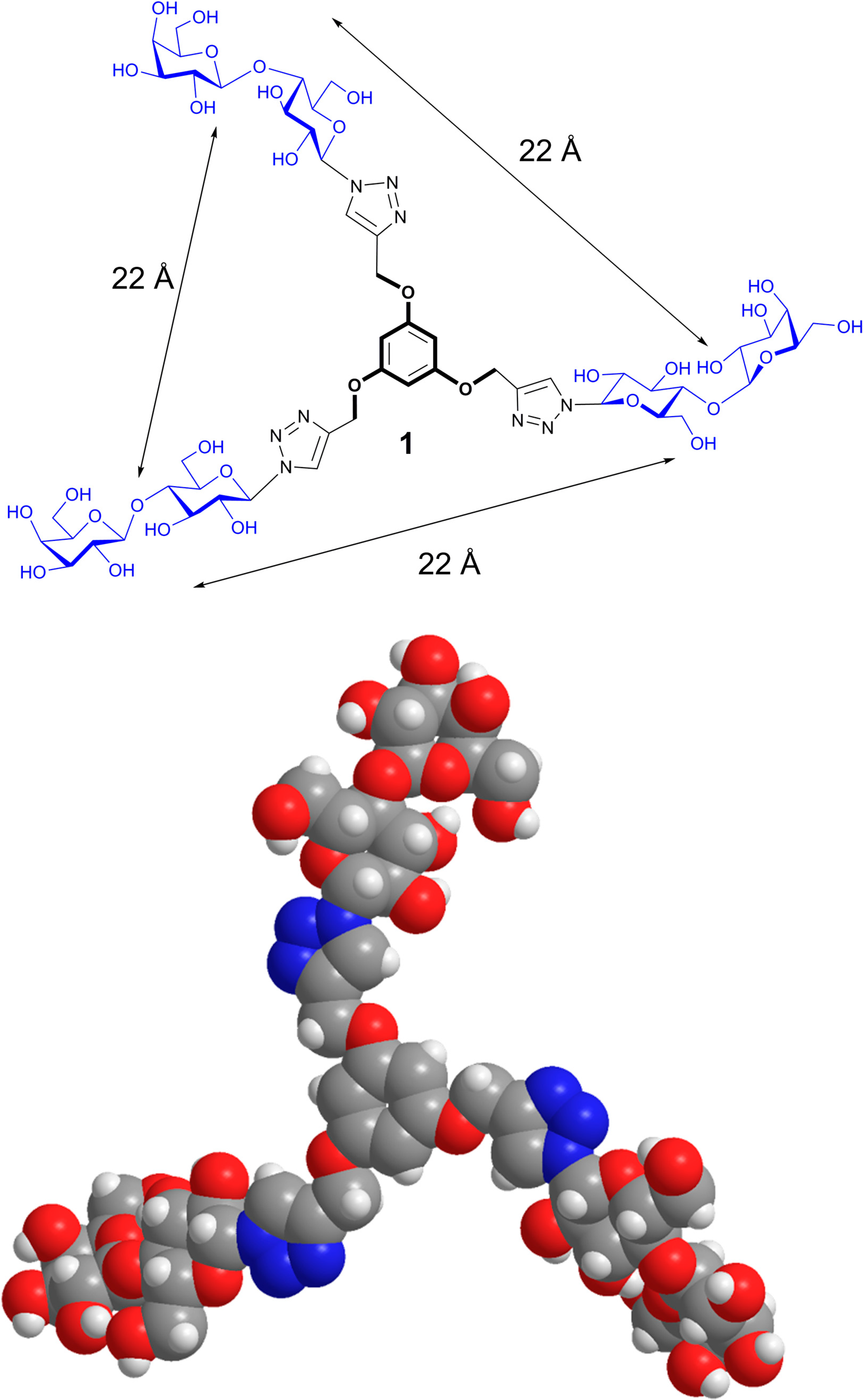
6. From Glycoclusters to Artificial Cell Surfaces
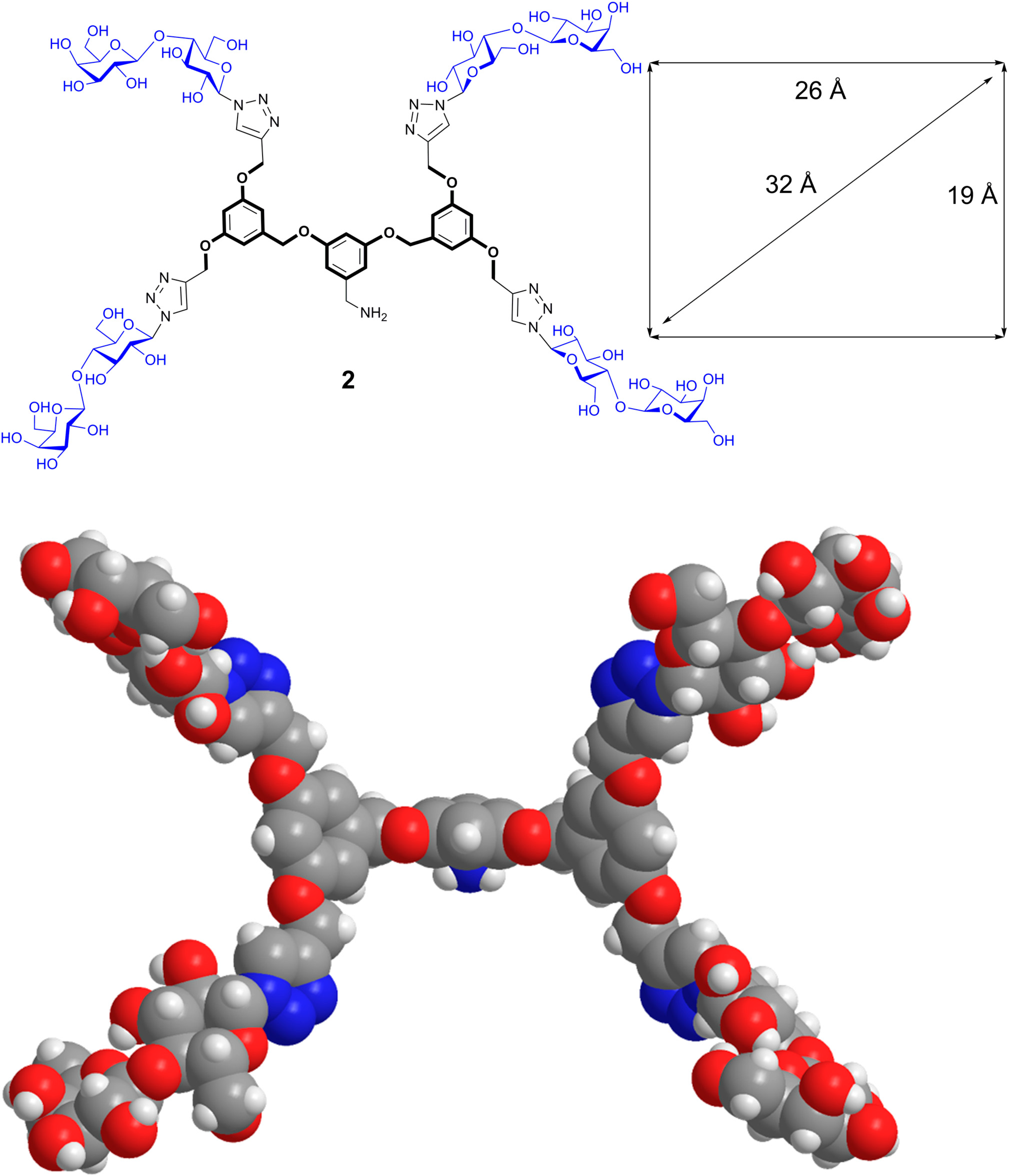
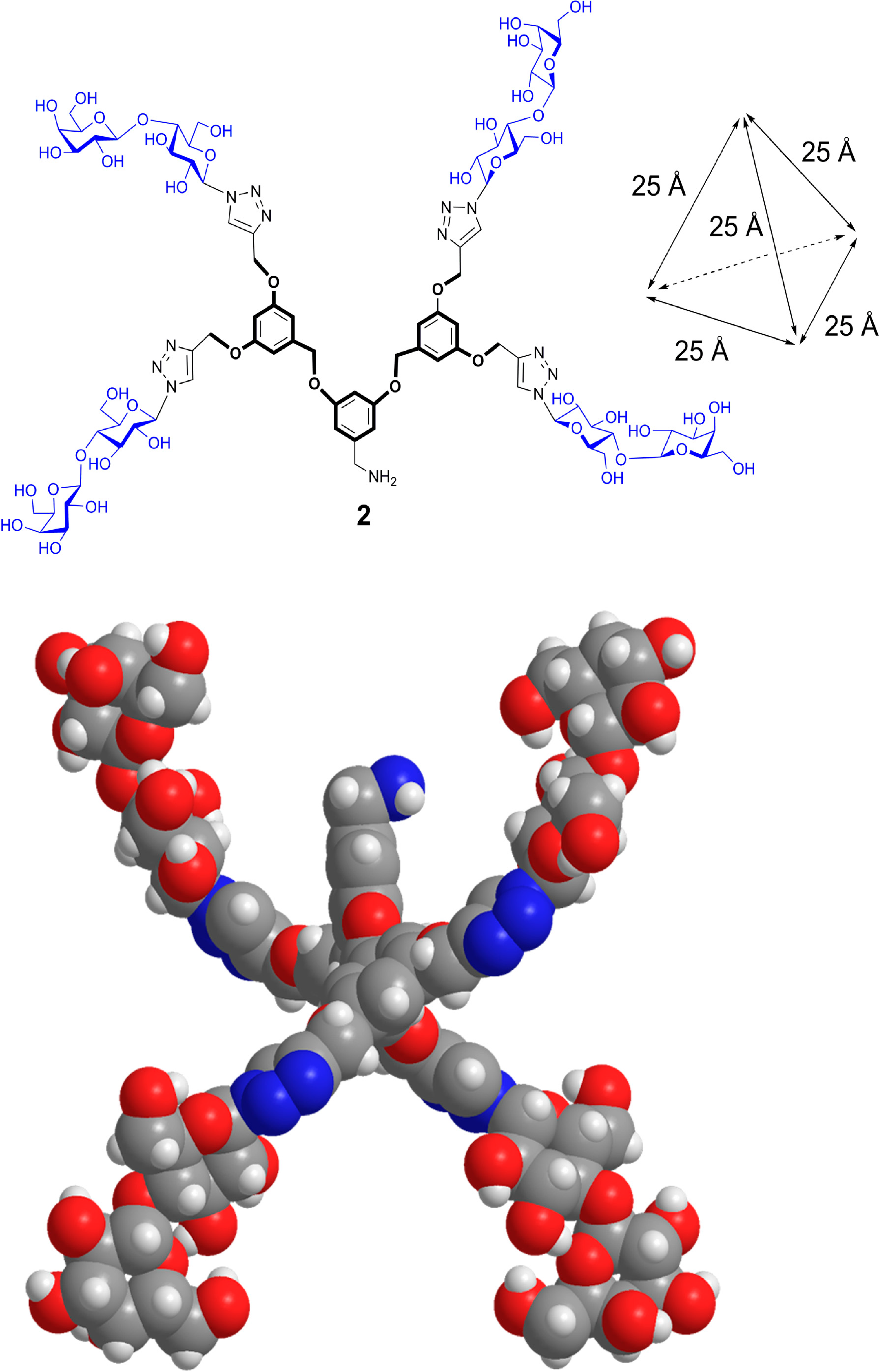

7. Conclusions
Acknowledgements
Conflicts of Interest
References
- Rüdiger, H.; Gabius, H.-J. The biochemical basis and coding capacity of the sugar code. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 3–13. [Google Scholar]
- Laine, R.A. The information-storing potential of the sugar code. In Glycosciences: Status and Perspectives; Gabius, H.-J., Gabius, S., Eds.; Chapman & Hall: London, UK, 1997; pp. 1–14. [Google Scholar]
- Brockhausen, I.; Schachter, H. Glycosyltransferases involved in N- and O-glycan biosynthesis. In Glycosciences: Status and Perspectives; Gabius, H.-J., Gabius, S., Eds.; Chapman & Hall: London, UK, 1997; pp. 79–113. [Google Scholar]
- Zuber, C.; Roth, J. N-Glycosylation. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 87–110. [Google Scholar]
- Patsos, G.; Corfield, A. O-Glycosylation: Structural diversity and function. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 111–137. [Google Scholar]
- Oriol, R.; Mollicone, R.; Cailleau, A.; Balanzino, L.; Breton, C. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates and bacteria. Glycobiology 1999, 9, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Harduin-Lepers, A.; Vallejo-Ruiz, V.; Krzewinski-Recchi, M.-A.; Samyn-Petit, B.; Julien, S.; Delannoy, P. The human sialyltransferase family. Biochimie 2001, 83, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Simala-Grant, J.L.; Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 2006, 16, 158R–184R. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S. Characterization of mouse sialyltransferase genes: Their evolution and diversity. Biosci. Biotechnol. Biochem. 2008, 72, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Jones, C.J. Fucose, placental evolution and the glycocode. Glycobiology 2012, 22, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Togayachi, A.; Narimatsu, H. Functional analysis of β1,3-N-acetylglucosaminyltransferases and regulation of immunological function by polylactosamine. Trends Glycosci. Glycotechnol. 2012, 24, 95–111. [Google Scholar] [CrossRef]
- Hemmerich, S.; Verdugo, D.; Rath, V.L. Strategies for drug discovery by targeting sulfation pathways. Drug Discov. Today 2004, 9, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.; Best, M.D.; Hanson, S.R.; Wong, C.H. Sulfotransferases: Structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. 2004, 43, 3526–3548. [Google Scholar] [CrossRef]
- Unverzagt, C.; André, S.; Seifert, J.; Kojima, S.; Fink, C.; Srikrishna, G.; Freeze, H.; Kayser, K.; Gabius, H.-J. Structure-activity profiles of complex biantennary glycans with core fucosylation and with/without additional α2,3/α2,6-sialylation: Synthesis of neoglycoproteins and their properties in lectin assays, cell binding, and organ uptake. J. Med. Chem. 2002, 45, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Schachter, H. The search for glycan function: Fucosylation of the TGF-β1 receptor is required for receptor activation. Proc. Natl. Acad. Sci. USA 2005, 102, 15721–15722. [Google Scholar] [CrossRef] [PubMed]
- Honke, K.; Taniguchi, N. Animal models to delineate glycan functionality. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 385–401. [Google Scholar]
- André, S.; Kozár, T.; Schuberth, R.; Unverzagt, C.; Kojima, S.; Gabius, H.-J. Substitutions in the N-glycan core as regulators of biorecognition: The case of core-fucose and bisecting GlcNAc moieties. Biochemistry 2007, 46, 6984–6995. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Kozár, T.; Kojima, S.; Unverzagt, C.; Gabius, H.-J. From structural to functional glycomics: Core substitutions as molecular switches for shape and lectin affinity of N-glycans. Biol. Chem. 2009, 390, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.B.H.; Paschinger, H.; Rendic, D. Glycosylation of model and “lower” organisms. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 139–154. [Google Scholar]
- Lemieux, R.U. How water provides the impetus for molecular recognition in aqueous solution. Acc. Chem. Res. 1996, 29, 373–380. [Google Scholar] [CrossRef]
- Gabius, H.-J. The how and why of protein-carbohydrate interaction: A primer to the theoretical concept and a guide to application in drug design. Pharmaceut. Res. 1998, 15, 23–30. [Google Scholar] [CrossRef]
- Carver, J.P. Experimental structure determination of oligosaccharides. Curr. Opin. Struct. Biol. 1991, 1, 716–720. [Google Scholar] [CrossRef]
- Von der Lieth, C.-W.; Siebert, H.-C.; Kozár, T.; Burchert, M.; Frank, M.; Gilleron, M.; Kaltner, H.; Kayser, G.; Tajkhorshid, E.; Bovin, N.V.; et al. Lectin ligands: New insights into their conformations and their dynamic behavior and the discovery of conformer selection by lectins. Acta Anat. 1998, 161, 91–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imberty, A.; Pérez, S. Structure, conformation, and dynamics of bioactive oligosaccharides: Theoretical approaches and experimental validations. Chem. Rev. 2000, 100, 4567–4588. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B.J. The glycosidic linkage flexibility and time-scale similarity hypotheses. J. Mol. Struct. 1997, 395–396, 187–200. [Google Scholar] [CrossRef]
- Nakagawa, H. Analytical aspects: Analysis of protein-bound glycans. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 71–83. [Google Scholar]
- Nishimura, S.-I. Toward automated glycan analysis. Adv. Carbohydr. Chem. Biochem. 2011, 65, 219–271. [Google Scholar] [PubMed]
- Corfield, T. Bacterial sialidases: Roles in pathogenicity and nutrition. Glycobiology 1992, 2, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Pshezhetsky, A.V.; Ashmarina, L.I. Desialylation of surface receptors as a new dimension in cell signaling. Biochemistry (Moscow) 2013, 78, 736–745. [Google Scholar] [CrossRef]
- Murphy, P.V.; André, S.; Gabius, H.-J. The third dimension of reading the sugar code by lectins: Design of glycoclusters with cyclic scaffolds as tools with the aim to define correlations between spatial presentation and activity. Molecules 2013, 18, 4026–4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renkonen, K.O. Studies on the nature of hemagglutinins present in seeds of some representatives of the family of leguminosae. Ann. Med. Exp. Biol. Fenn. 1948, 26, 66–72. [Google Scholar]
- Boyd, W.C. The lectins: Their present status. Vox Sang. 1963, 8, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.C.; Green, C. Lectins as blood typing reagents. Adv. Lectin Res. 1992, 5, 51–94. [Google Scholar]
- Hughes-Jones, N.C.; Gardner, B. Red cell agglutination: The first description by Creite (1869) and further observations made by Landois (1875) and Landsteiner (1901). Br. J. Haematol. 2002, 119, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.P.; Dorner, F. Karl Landsteiner and his major contributions to haematology. Br. J. Haematol. 2003, 121, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.C. The proteins of immune reactions. In The Proteins; Neurath, H., Bailey, K., Eds.; Academic Press: New York, NY, USA, 1954; Volume 2, Part 2; pp. 756–844. [Google Scholar]
- Watkins, W.M.; Morgan, W.T.J. Neutralisation of the anti-H agglutinin in eel serum by simple sugars. Nature 1952, 169, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Watkins, W.M. A half century of blood-group antigen research: Some personal recollections. Trends Glycosci. Glycotechnol. 1999, 11, 391–411. [Google Scholar] [CrossRef]
- Rüdiger, H.; Gabius, H.-J. The history of lectinology. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 261–268. [Google Scholar]
- Gabius, H.-J.; André, S.; Jiménez-Barbero, J.; Romero, A.; Solís, D. From lectin structure to functional glycomics: Principles of the sugar code. Trends Biochem. Sci. 2011, 36, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Solís, D.; Bovin, N.V.; Davis, A.P.; Jiménez-Barbero, J.; Romero, A.; Roy, R.; Smetana, K., Jr.; Gabius, H.-J. A guide into glycosciences: How chemistry, biochemistry and biology cooperate to crack the sugar code. Biochim. Biophys. Acta 2015, 1850, 186–235. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B.B.L.; Goldstein, I.J. Specific binding of concanavalin A to cross-linked dextran gel. Biochem. J. 1965, 96, 23c–25c. [Google Scholar]
- Loris, R. Principles of structures of animal and plant lectins. Biochim. Biophys. Acta 2002, 1572, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Edelman, G.M.; Cunningham, B.A.; Reeke, G.N., Jr.; Becker, J.W.; Waxdal, M.J.; Wang, J.L. The covalent and three-dimensional structure of concanavalin A. Proc. Natl. Acad. Sci. USA 1972, 69, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J. The how and why of Ca2+ involvement in lectin activity. Trends Glycosci. Glycotechnol. 2011, 23, 168–177. [Google Scholar] [CrossRef]
- Rice, K.G.; Weisz, O.A.; Barthel, T.; Lee, R.T.; Lee, Y.C. Defined geometry of binding between triantennary glycopeptide and the asialoglycoprotein receptor of rat hepatocytes. J. Biol. Chem. 1990, 265, 18429–18434. [Google Scholar] [PubMed]
- Gabius, H.-J. Detection and functions of mammalian lectins–with emphasis on membrane lectins. Biochim. Biophys. Acta 1991, 1071, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C. Biochemistry of carbohydrate-protein interaction. FASEB J. 1992, 6, 3193–3200. [Google Scholar] [PubMed]
- Barondes, S.H. Bifunctional properties of lectins: Lectins redefined. Trends Biochem. Sci. 1988, 13, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J. Animal lectins. Eur. J. Biochem. 1997, 243, 543–576. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.N.W. Galectinomics: Finding themes in complexity. Biochim. Biophys. Acta 2002, 1572, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Brinkman-van der Linden, E.C.M. I-type lectins. Biochim. Biophys. Acta 2002, 1572, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J.; André, S.; Kaltner, H.; Siebert, H.-C. The sugar code: Functional lectinomics. Biochim. Biophys. Acta 2002, 1572, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Gready, J.N.; Zelensky, A.N. Routes in lectin evolution: Case study on the C-type lectin-like domains. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 329–346. [Google Scholar]
- Satoh, T. Molecular and structural basis for sugar recognition by mannose-6-phosphate receptor homology domain-containing lectin and proteins. Trends Glycosci. Glycotechnol. 2012, 24, 193–202. [Google Scholar] [CrossRef]
- Gupta, G.; Surolia, A. Collectins: Sentinels of innate immunity. BioEssays 2007, 29, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, E.J.; van Eijk, M.; Haagsman, H.P. The carbohydrate recognition domain of collectins. FEBS J. 2011, 278, 3930–3941. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, T.R.; Thiel, S.; Andersen, G.R. Toward a structure-based comprehension of the lectin pathway of complement. Mol. Immunol. 2013, 56, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J.; Springer, W.R.; Barondes, S.H. Receptor for the cell binding site of discoidin I. Cell 1985, 42, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.B.; Howell, S.F. The identification of a hemagglutinin of the jack bean with concanavalin A. J. Bacteriol. 1936, 32, 227–237. [Google Scholar] [PubMed]
- Gupta, G.; Surolia, A.; Sampathkumar, S.G. Lectin microarrays for glycomic analysis. Omics 2010, 14, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Donczo, B.; Kerekgyarto, J.; Szurmai, Z.; Guttman, A. Glycan microarrays: New angles and new strategies. Analyst 2014, 139, 2650–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, S.S.; Schulte, B.A. Diversity of cell glycoconjugates shown histochemically: A perspective. J. Histochem. Cytochem. 1992, 40, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Danguy, A.; Akif, F.; Pajak, B.; Gabius, H.-J. Contribution of carbohydrate histochemistry to glycobiology. Histol. Histopathol. 1994, 9, 155–171. [Google Scholar] [PubMed]
- Roth, J. Protein glycosylation in the endoplasmic reticulum and the Golgi apparatus and cell-type specificity of cell surface glycoconjugate expression: Analysis by protein A-gold and lectin-gold techniques. Histochem. Cell Biol. 1996, 106, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Roth, J. Lectins for histochemical demonstration of glycans. Histochem. Cell Biol. 2011, 136, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. Lectins as tools for glycoconjugate purification and characterization. In Glycosciences: Status and Perspectives; Gabius, H.-J., Gabius, S., Eds.; Chapman & Hall: London, UK, 1997; pp. 191–199. [Google Scholar]
- Mo, H.; Winter, H.C.; Goldstein, I.J. Purification and characterization of a Neu5Acα2–6Galβ1–4Glc/GlcNAc-specific lectin from the fruiting body of the polypore mushroom Polyporus squamosus. J. Biol. Chem. 2000, 275, 10623–10629. [Google Scholar] [CrossRef] [PubMed]
- Toma, V.; Zuber, C.; Winter, H.C.; Goldstein, I.J.; Roth, J. Application of a lectin from the mushroom Polysporus squamosus for the histochemical detection of the NeuAcα2,6Galβ1,4Glc/GlcNAc sequence of N-linked oligosaccharides: A comparison with the Sambucus nigra lectin. Histochem. Cell Biol. 2001, 116, 183–193. [Google Scholar] [PubMed]
- Kadirvelraj, R.; Grant, O.C.; Goldstein, I.J.; Winter, H.C.; Tateno, H.; Fadda, E.; Woods, R.J. Structure and binding analysis of Polyporus squamosus lectin in complex with the Neu5Acα2–6Galβ1–4GlcNAc human-type influenza receptor. Glycobiology 2011, 21, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Patsos, G.; André, S.; Roeckel, N.; Gromes, R.; Gebert, J.; Kopitz, J.; Gabius, H.-J. Compensation of loss of protein function in microsatellite-unstable colon cancer cells (HCT116): A gene-dependent effect on the cell surface glycan profile. Glycobiology 2009, 19, 726–734. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Sanchez-Ruderisch, H.; Nakagawa, H.; Buchholz, M.; Kopitz, J.; Forberich, P.; Kemmner, W.; Böck, C.; Deguchi, K.; Detjen, K.M.; et al. Tumor suppressor p16INK4a: Modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007, 274, 3233–3256. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Singh, T.; Lacal, J.C.; Smetana, K., Jr.; Gabius, H.-J. Rho GTPase Rac1: Molecular switch within the galectin network and for N-glycan α2,6-sialylation/O-glycan core 1 sialylation in colon cancer in vitro. Folia Biol. (Praha) 2014, 60, 95–107. [Google Scholar]
- Morell, A.G.; van den Hamer, C.J.A.; Scheinberg, I.H.; Ashwell, G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal d-galactose residues. J. Biol. Chem. 1966, 241, 3745–3749. [Google Scholar] [PubMed]
- Morell, A.G.; Irvine, R.A.; Sternlieb, I.; Scheinberg, I.H.; Ashwell, G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J. Biol. Chem. 1968, 243, 155–159. [Google Scholar] [PubMed]
- Hudgin, R.L.; Pricer, W.E.J.; Ashwell, G.; Stockert, R.J.; Morell, A.G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J. Biol. Chem. 1974, 249, 5536–5543. [Google Scholar] [PubMed]
- Caron, M.; Bladier, D.; Joubert, R. Soluble galactoside-binding vertebrate lectins: A protein family with common properties. Int. J. Biochem. 1990, 22, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J. Recent topics on galectins. Trends Glycosci. Glycotechnol. 1997, 9, 1–180. [Google Scholar] [CrossRef]
- Wang, J.L.; Gray, R.M.; Haudek, K.C.; Patterson, R.J. Nucleocytoplasmic lectins. Biochim. Biophys. Acta 2004, 1673, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Villalobo, A.; Nogales-Gonzáles, A.; Gabius, H.-J. A guide to signaling pathways connecting protein-glycan interaction with the emerging versatile effector functionality of mammalian lectins. Trends Glycosci. Glycotechnol. 2006, 18, 1–37. [Google Scholar] [CrossRef]
- Smetana, K., Jr.; André, S.; Kaltner, H.; Kopitz, J.; Gabius, H.-J. Context-dependent multifunctionality of galectin-1: A challenge for defining the lectin as therapeutic target. Expert Opin. Ther. Targets 2013, 17, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H. Galectins: A personal review. Trends Glycosci. Glycotechnol. 1997, 9, 1–7. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A β-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Raschta, A.-S.; Manning, J.C.; Gabius, H.-J. Copy-number variation of functional galectin genes: Studying animal galectin-7 (p53-induced gene 1 in man) and tandem-repeat-type galectins-4 and -9. Glycobiology 2013, 23, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.-I.-I.; Hirabayashi, J. Galectins: A family of animal lectins that decipher glycocodes. J. Biochem. 1996, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Houzelstein, D.; Gonçalves, I.R.; Fadden, A.J.; Sidhu, S.S.; Cooper, D.N.W.; Drickamer, K.; Leffler, H.; Poirier, F. Phylogenetic analysis of the vertebrate galectin family. Mol. Biol. Evol. 2004, 21, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Gabius, H.-J. A toolbox of lectins for translating the sugar code: The galectin network in phylogenesis and tumors. Histol. Histopathol. 2012, 27, 397–416. [Google Scholar] [PubMed]
- Varela, P.F.; Solís, D.; Díaz-Mauriño, T.; Kaltner, H.; Gabius, H.-J.; Romero, A. The 2.15 Å crystal structure of CG-16, the developmentally regulated homodimeric chicken galectin. J. Mol. Biol. 1999, 294, 537–549. [Google Scholar] [CrossRef] [PubMed]
- López-Lucendo, M.F.; Solís, D.; Sáiz, J.L.; Kaltner, H.; Russwurm, R.; André, S.; Gabius, H.-J.; Romero, A. Homodimeric chicken galectin CG-1B (C-14): Crystal structure and detection of unique redox-dependent shape changes involving inter- and intrasubunit disulfide bridges by gel filtration, ultracentrifugation, site-directed mutagenesis, and peptide mass fingerprinting. J. Mol. Biol. 2009, 386, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.M.; Fernandez, I.S.; Lopez-Merino, L.; Lagartera, L.; Kaltner, H.; Menendez, M.; André, S.; Solis, D.; Gabius, H.-J.; Romero, A. Fine-tuning of prototype chicken galectins: Structure of CG-2 and structure-activity correlations. Acta Crystallogr. 2013, D69, 1665–1676. [Google Scholar]
- Sakakura, Y.; Hirabayashi, J.; Oda, Y.; Ohyama, Y.; Kasai, K.-I.-I. Structure of chicken 16-kDa β-galactoside-binding lectin. Complete amino acid sequence, cloning of cDNA and production of recombinant lectin. J. Biol. Chem. 1990, 265, 21573–21579. [Google Scholar] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Müller, W.E.G.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Singh, T.; Liu, J.-H.; Krzeminski, M.; Russwurm, R.; Siebert, H.-C.; Bonvin, A.M.J.J.; André, S.; Gabius, H.-J. Activity-structure correlations in divergent lectin evolution: Fine specificity of chicken galectin CG-14 and computational analysis of flexible ligand docking for CG-14 and the closely related CG-16. Glycobiology 2007, 17, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Göhler, A.; Buchner, C.; Doose, S.; Kaltner, H.; Gabius, H.-J. Analysis of homodimeric avian and human galectins by two methods based on fluorescence spectroscopy: Different structural alterations upon oxidation and ligand binding. Biochimie 2012, 94, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Solís, D.; Kopitz, J.; Lensch, M.; Lohr, M.; Manning, J.C.; Mürnseer, M.; Schnölzer, M.; André, S.; Sáiz, J.L.; et al. Prototype chicken galectins revisited: Characterization of a third protein with distinctive hydrodynamic behaviour and expression pattern in organs of adult animals. Biochem. J. 2008, 409, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Solís, D.; André, S.; Lensch, M.; Manning, J.C.; Mürnseer, M.; Saíz, J.L.; Gabius, H.-J. Unique chicken tandem-repeat-type galectin: Implications of alternative splicing and a distinct expression profile compared to those of the three proto-type proteins. Biochemistry 2009, 48, 4403–4416. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Kübler, D.; López-Merino, L.; Lohr, M.; Manning, J.C.; Lensch, M.; Seidler, J.; Lehmann, W.D.; André, S.; Solís, D.; et al. Toward comprehensive analysis of the galectin network in chicken: Unique diversity of galectin-3 and comparison of its localization profile in organs of adult animals to the other four members of this lectin family. Anat. Rec. 2011, 294, 427–444. [Google Scholar] [CrossRef]
- Lu, Y.; Lotan, R. Transcriptional regulation by butyrate of mouse galectin-1 gene in embryonal carcinoma cells. Biochim. Biophys. Acta 1999, 1444, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, Y.; Obinata, A.; Hirabayashi, J.; Sakakura, Y.; Endo, H.; Kasai, K.-I.-I.; Hirano, H. Secretion of endogenous 16-kDa β-galactoside-binding lectin from vitamin A-pretreated chick embryonic cultured skin. Exp. Cell Res. 1993, 205, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Katzenmaier, E.M.; André, S.; Kopitz, J.; Gabius, H.-J. Impact of sodium butyrate on the network of adhesion/growth-regulatory galectins in human colon cancer in vitro. Anticancer Res. 2014, 34, 5429–5438. [Google Scholar] [PubMed]
- Nio, J.; Kon, Y.; Iwanaga, T. Differential cellular expression of galectin family mRNAs in the epithelial cells of the mouse digestive tract. J. Histochem. Cytochem. 2005, 53, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J. Histochem. Cytochem. 2009, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J.; Kayser, K. Introduction to glycopathology: The concept, the tools and the perspectives. Diagn Pathol. 2014, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Gitt, M.A.; Wiser, M.F.; Leffler, H.; Herrmann, J.; Xia, Y.; Massa, S.M.; Cooper, D.N.W.; Lusis, A.J.; Barondes, S.H. Sequence and mapping of galectin-5, a β-galactoside-binding lectin, found in rat erythrocytes. J. Biol. Chem. 1995, 270, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Kanwar, Y.S. Identification and characterization of galectin-9, a novel β-galactoside-binding mammalian lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef] [PubMed]
- Lensch, M.; Lohr, M.; Russwurm, R.; Vidal, M.; Kaltner, H.; André, S.; Gabius, H.-J. Unique sequence and expression profiles of rat galectins-5 and -9 as a result of species-specific gene divergence. Int. J. Biochem. Cell Biol. 2006, 38, 1741–1758. [Google Scholar] [CrossRef] [PubMed]
- Houzelstein, D.; Goncalves, I.R.; Orth, A.; Bonhomme, F.; Netter, P. Lgals6, a 2-million-year-old gene in mice: A case of positive Darwinian selection and presence/absence polymorphism. Genetics 2008, 178, 1533–1545. [Google Scholar] [CrossRef]
- Cao, H.; de Bono, B.; Belov, K.; Wong, E.S.; Trowsdale, J.; Barrow, A.D. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics 2009, 61, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Kaltner, H.; Schwartz-Albiez, R.; Sinowatz, F.; Gabius, H.-J. Towards functional glycomics by lectin histochemistry: Strategic probe selection to monitor core and branch-end substitutions and detection of cell-type and regional selectivity in adult mouse testis and epididymis. Anat. Histol. Embryol. 2010, 39, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Plzák, J.; Betka, J.; Smetana, K., Jr.; Chovanec, M.; Kaltner, H.; André, S.; Kodet, R.; Gabius, H.-J. Galectin-3: An emerging prognostic indicator in advanced head and neck carcinoma. Eur. J. Cancer 2004, 40, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; André, S.; Karamitopoulou, E.; Zlobec, I.; Gabius, H.-J. The growing galectin network in colon cancer and clinical relevance of cytoplasmic galectin-3 reactivity. Anticancer Res. 2013, 33, 3053–3059. [Google Scholar] [PubMed]
- Ohannesian, D.W.; Lotan, D.; Thomas, P.; Jessup, J.M.; Fukuda, M.; Gabius, H.-J.; Lotan, R. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells. Cancer Res. 1995, 55, 2191–2199. [Google Scholar] [PubMed]
- Bhat, R.; Lerea, K.M.; Peng, H.; Kaltner, H.; Gabius, H.-J.; Newman, S.A. A regulatory network of two galectins mediates the earliest steps of avian limb skeletal morphogenesis. BMC Dev. Biol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Toegel, S.; Bieder, D.; André, S.; Kayser, K.; Walzer, S.M.; Hobusch, G.; Windhager, R.; Gabius, H.-J. Human osteoarthritic knee cartilage: Fingerprinting of adhesion/growth-regulatory galectins in vitro and in situ indicates differential upregulation in severe degeneration. Histochem. Cell Biol. 2014, 142, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, H.F.; Pace, K.E.; Cabrera, P.V.; White, R.; Porvari, K.; Kaija, H.; Vihko, P.; Baum, L.G. O-Glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res. 2007, 67, 6155–6162. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G.; André, S.; Bleich, D.; Huet, G.; Kaltner, H.; Kopitz, J.; Gabius, H.-J. Beyond glycoproteins as galectin counterreceptors: Tumor/effector T cell growth control via ganglioside GM1. Ann. N. Y. Acad. Sci. 2012, 1253, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Kopitz, J.; Bergmann, M.; Gabius, H.-J. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: Case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life 2010, 62, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Pace, K.E.; Baum, L.G. Induction of T lymphocyte apoptosis: A novel function for galectin-1. Trends Glycosci. Glycotechnol. 1997, 9, 21–29. [Google Scholar] [CrossRef]
- Gabius, H.-J. Probing the cons and pros of lectin-induced immunomodulation: Case studies for the mistletoe lectin and galectin-1. Biochimie 2001, 83, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Rappl, G.; Abken, H.; Muche, J.M.; Sterry, W.; Tilgen, W.; André, S.; Kaltner, H.; Ugurel, S.; Gabius, H.-J.; Reinhold, U. CD4+CD7- leukemic T cells from patients with Sézary syndrome are protected from galectin-1-triggered T cell death. Leukemia 2002, 16, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Sanchez-Ruderisch, H.; Welzel, M.; Wiedenmann, B.; Sakai, T.; André, S.; Gabius, H.-J.; Khachigian, L.; Detjen, K.; Rosewicz, S. Galectin-1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 2005, 280, 37266–37277. [Google Scholar] [CrossRef] [PubMed]
- Blaskó, A.; Fajka-Boja, R.; Ion, G.; Monostori, E. How does it act when soluble? Critical evaluation of mechanism of galectin-1-induced T-cell apoptosis. Acta Biol. Hung. 2011, 62, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruderisch, H.; Detjen, K.M.; Welzel, M.; André, S.; Fischer, C.; Gabius, H.-J.; Rosewicz, S. Galectin-1 sensitizes carcinoma cells to anoikis via the fibronectin receptor α5β1-integrin. Cell Death Differ. 2011, 18, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Dimitroff, C.J. Galectin-1 research in T cell immunity: Past, present and future. Clin. Immunol. 2012, 142, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Lensch, M.; André, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.-J. Human galectin-2: Novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Eriksson, H.; Manning, J.C.; Detjen, K.M.; André, S.; Nishimura, S.-I.; Lehtiö, J.; Gabius, H.-J. Tumour suppressor p16INK4a: Anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J. 2012, 279, 4062–4080. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruderisch, H.; Fischer, C.; Detjen, K.M.; Welzel, M.; Wimmel, A.; Manning, J.C.; André, S.; Gabius, H.-J. Tumor suppressor p16INK4a: Downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010, 277, 3552–3563. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.M.; Roy, R. The chemist’s way to prepare multivalency. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 53–70. [Google Scholar]
- Wang, G.-N.; André, S.; Gabius, H.-J.; Murphy, P.V. Bi- to tetravalent glycoclusters: Synthesis, structure-activity profiles as lectin inhibitors and impact of combining both valency and headgroup tailoring on selectivity. Org. Biomol. Chem. 2012, 10, 6893–6907. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Cho, M.; Cummings, R.D.; Brewer, C.F. Thermodynamics of carbohydrate binding to galectin-1 from Chinese hamster ovary cells and two mutants. A comparison with four galactose-specific plant lectins. Biochemistry 1996, 35, 15236–15243. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.K.; Gabius, H.-J.; André, S.; Kaltner, H.; Lensch, M.; Brewer, C.F. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Wang, G.-N.; Gabius, H.-J.; Murphy, P.V. Combining glycocluster synthesis with protein engineering: An approach to probe into the significance of linker length in a tandem-repeat-type lectin (galectin-4). Carbohydr. Res. 2014, 389, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Stechly, L.; Morelle, W.; Dessein, A.F.; André, S.; Grard, G.; Trinel, D.; Dejonghe, M.J.; Leteurtre, E.; Drobecq, H.; Trugnan, G.; et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic 2009, 10, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.; Díez-Revuelta, N.; Hernández-Iglesias, T.; Kaltner, H.; André, S.; Gabius, H.-J.; Abad-Rodriguez, J. Neuronal galectin-4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J. Neurochem. 2013, 125, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Jose, B.; Mallon, C.T.; Forster, R.J.; Blackledge, C.; Keyes, T.E. Lipid bilayer assembly at a gold nanocavity array. Chem. Commun. 2011, 47, 12530–12532. [Google Scholar] [CrossRef]
- Percec, V.; Leowanawat, P.; Sun, H.J.; Kulikov, O.; Nusbaum, C.D.; Tran, T.M.; Bertin, A.; Wilson, D.A.; Peterca, M.; Zhang, S.; et al. Modular synthesis of amphiphilic Janus glycodendrimers and their self-assembly into glycodendrimersomes and other complex architectures with bioactivity to biomedically relevant lectins. J. Am. Chem. Soc. 2013, 135, 9055–9077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Moussodia, R.O.; Sun, H.J.; Leowanawat, P.; Muncan, A.; Nusbaum, C.D.; Chelling, K.M.; Heiney, P.A.; Klein, M.L.; André, S.; et al. Mimicking biological membranes with programmable glycan ligands self-assembled from amphiphilic Janus glycodendrimers. Angew. Chem. Int. Ed. 2014, 53, 10899–10903. [Google Scholar] [CrossRef]
- Sharon, N. Glycoproteins now and then: A personal account. Acta Anat. 1998, 161, 7–17. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, S.; Kaltner, H.; Manning, J.C.; Murphy, P.V.; Gabius, H.-J. Lectins: Getting Familiar with Translators of the Sugar Code. Molecules 2015, 20, 1788-1823. https://doi.org/10.3390/molecules20021788
André S, Kaltner H, Manning JC, Murphy PV, Gabius H-J. Lectins: Getting Familiar with Translators of the Sugar Code. Molecules. 2015; 20(2):1788-1823. https://doi.org/10.3390/molecules20021788
Chicago/Turabian StyleAndré, Sabine, Herbert Kaltner, Joachim C. Manning, Paul V. Murphy, and Hans-Joachim Gabius. 2015. "Lectins: Getting Familiar with Translators of the Sugar Code" Molecules 20, no. 2: 1788-1823. https://doi.org/10.3390/molecules20021788





