Immobilization of Metal Hexacyanoferrate Ion-Exchangers for the Synthesis of Metal Ion Sorbents—A Mini-Review
Abstract
:1. Introduction
2. Synthesis and Sorption Properties of Bulk Hexacyanoferrate-Based Ion-Exchangers
2.1. Synthesis and Structure of Metal Hexacyanoferrate Ion-Exchangers

2.2. Type of Metal Hexacyanoferrate
- (a)
- (b)
- The type of ferro/ferricyanide and more specifically the presence and the type of exchangeable alkali or monovalent cation (Na+, K+ or NH4+) will orientate the use of the ion-exchanger: for example, for Cs or Tl decorporation, potassium and ammonium salts should be prohibited to limit the secondary health effects and sodium-precursor will be preferred [26,27]. This may also influence the type of mechanism involved in metal binding: for example, the presence of monovalent cation allows ion-exchange process (instead of pure surface sorption) [25].
- (c)
- The type of counter metal salt (nickel, copper, cobalt, iron, zinc) will influence the potential release of counter metal cations during the sorption process [18], but also the chemical structure [28], and the spatial arrangement (crystallographic properties) of the ion-exchanger, which, in turn, may affect the accessibility and the ion-exchange affinity (cage effect) of the material for target metal ions, but also uptake kinetics [25,29].
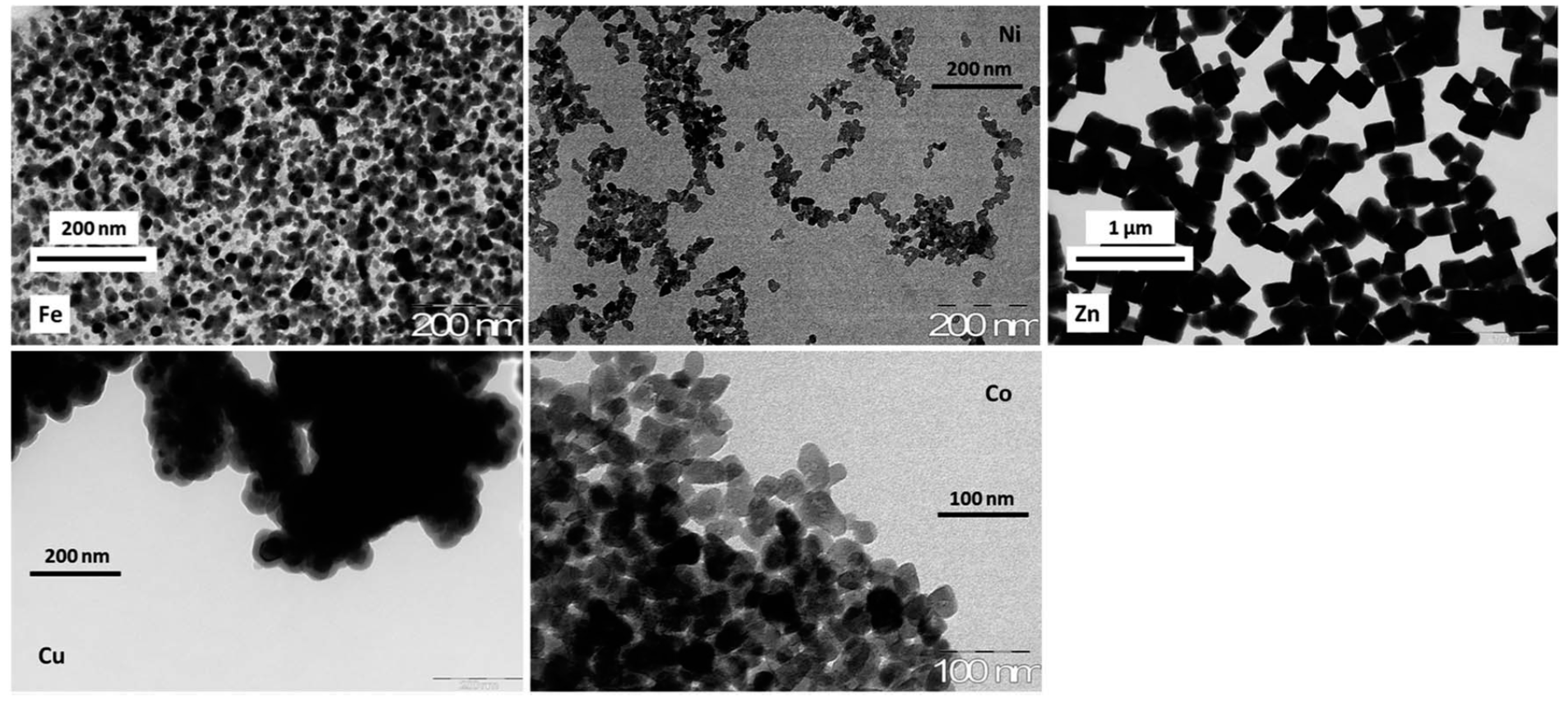
2.3. Target Metals for Ion-Exchange on Hexacyanoferrate-Based Sorbents
2.4. Binding Mechanisms
2.5. Performance and Process Limitations: The Rationale for Ion-Exchanger Immobilization
3. Techniques for Immobilization of Metal Hexacyanoferrates
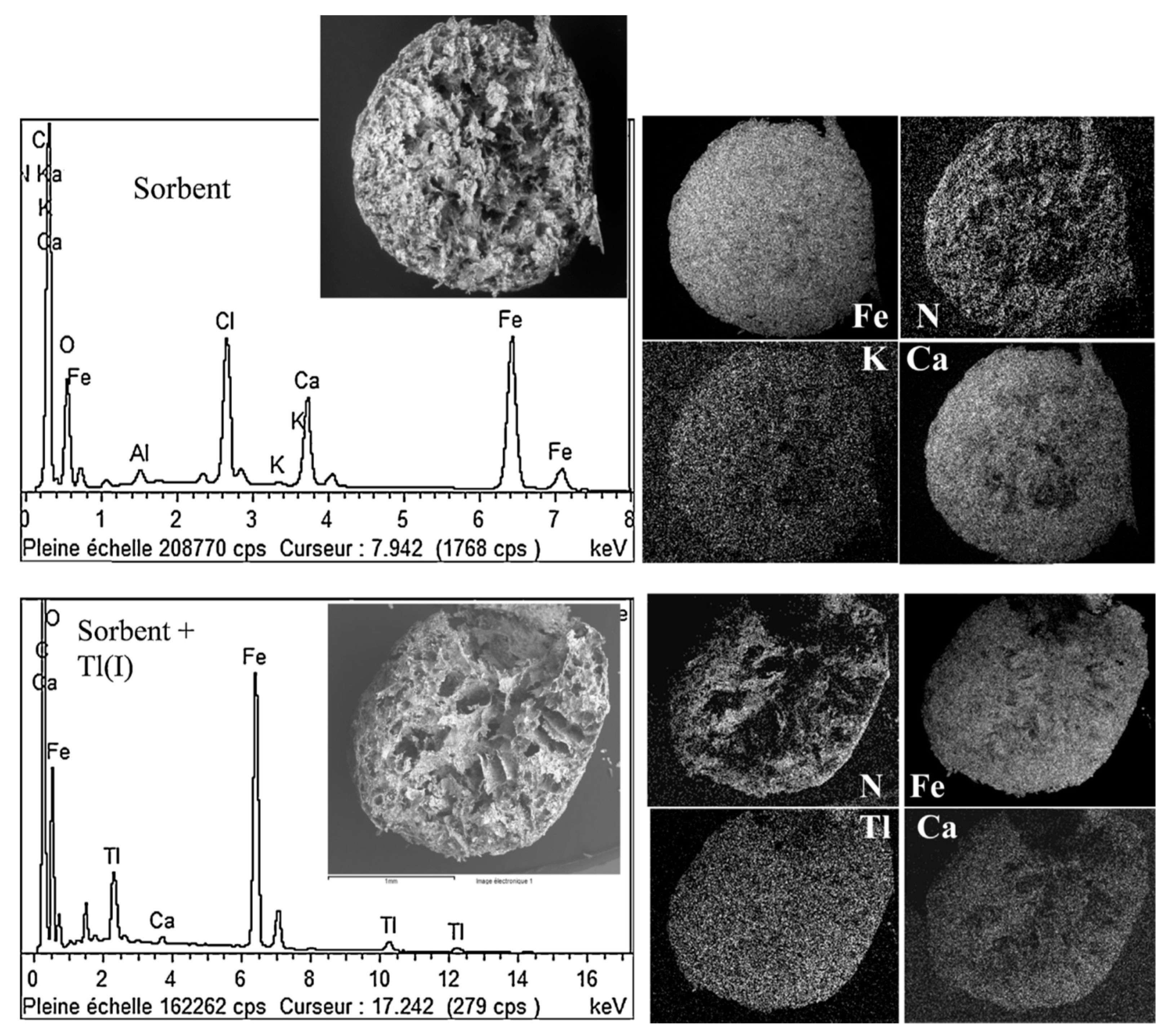
3.1. Immobilization on the Support Matrix
3.1.1. Inorganic Support
3.1.2. Polymer
3.1.3. Biopolymer
3.1.4. Carbon-Based Support
3.1.5. Miscellaneous
3.2. Encapsulation-Entrapment
3.2.1. Sol-Gel
3.2.2. Polymer
3.2.3. Biopolymer


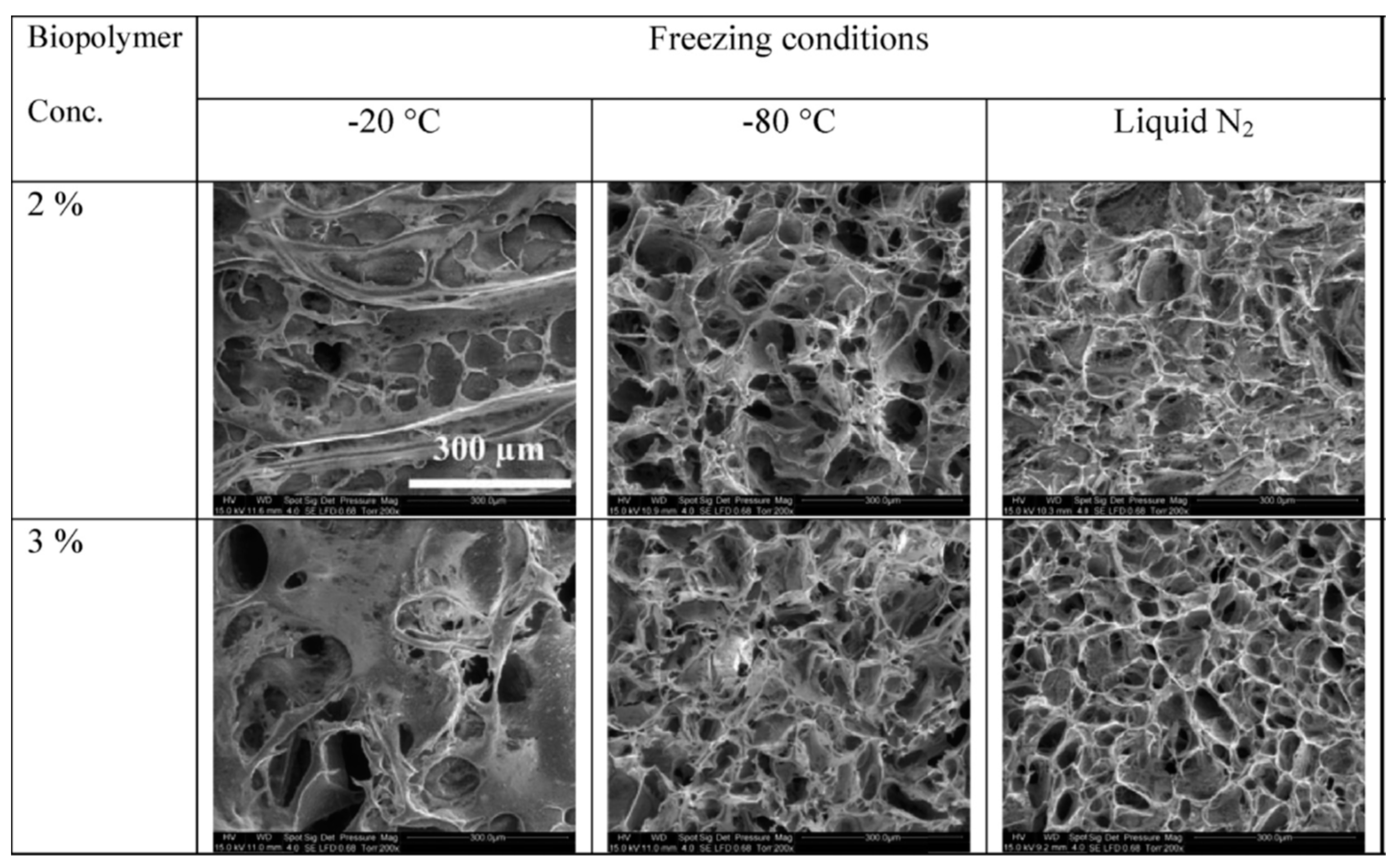

3.3. Miscellaneous–Granulation and Magnetic Particles
3.4. Stability of Structured Materials
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaur, S. Determination of Cs-137 in environmental water by ion-exchange chromatography. J. Chromatogr. A 1996, 733, 57–71. [Google Scholar] [CrossRef]
- Thompson, D.F.; Callen, E.D. Soluble or insoluble Prussian blue for radiocesium and thallium poisoning? Ann. Pharmacother. 2004, 38, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.F.; Church, C.O. Prussian blue for treatment of radiocesium poisoning. Pharmacotherapy 2001, 21, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.R.; Lipsztein, J.L.; Leggett, R.; Bertelli, L.; Guilmette, R. Efficacy of Prussian Blue on Cs-137 decorporation therapy. Health Phys. 2014, 106, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Heyltex. Radiogardase. Available online: http://www.heyltex.com/products/radiogardase (accessed on 10 August 2015).
- Ding, D.; Zhao, Y.; Yang, S.; Shi, W.; Zhang, Z.; Lei, Z.; Yang, Y. Adsorption of cesium from aqueous solution using agricultural residue-Walnut shell: Equilibrium, kinetic and thermodynamic modeling studies. Water Res. 2013, 47, 2563–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milyutin, V.; Gelis, V. Optimal conditions for coprecipitation of cesium radionuclides with nickel ferrocyanide. Radiochemistry 2008, 50, 64–66. [Google Scholar] [CrossRef]
- Milyutin, V.; Mikheev, S.; Gelis, V.; Kononenko, O. Coprecipitation of microamounts of cesium with precipitates of transition metal ferrocyanides in alkaline solutions. Radiochemistry 2009, 51, 295–297. [Google Scholar] [CrossRef]
- Lehto, J.; Haukka, S.; Harjula, R.; Blomberg, M. Mechanism of cesium ion-exchange on potassium cobalt hexacyanoferrates(II). J. Chem. Soc., Dalton Trans. 1990, 1007–1011. [Google Scholar] [CrossRef]
- Haas, P.A. A review of information on ferrocyanide solids for removal of cesium from solutions. Sep. Sci. Technol. 1993, 28, 2479–2506. [Google Scholar] [CrossRef]
- Ayrault, S.; Loos-Neskovic, C.; Fedoroff, M.; Garnier, E. Copper hexacyanoferrates—Preparation, composition and structure. Talanta 1994, 41, 1435–1452. [Google Scholar] [CrossRef]
- Ismail, I.; El-Sourougy, M.; Moneim, N.; Aly, H. Preparation, characterization, and utilization of potassium nickel hexacyanoferrate for the separation of cesium and cobalt from contaminated waste water. J. Radioanal. Nucl. Chem. 1998, 237, 97–103. [Google Scholar] [CrossRef]
- Ayrault, S.; Loos-Neskovic, C.; Fedoroff, M.; Garnier, E.; Jones, D.J. Compositions and structures of copper hexacyanoferrates(II) and (III): Experimental results. Talanta 1995, 42, 1581–1593. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Fedoroff, M.; Garnier, E. Preparation, composition and structure of some nickel and zinc ferrocyanides: Experimental results. Talanta 1989, 36, 749–759. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Fedoroff, M.; Garnier, E.; Gravereau, P. Zinc and nickel ferrocyanides—Preparation, composition and structure. Talanta 1984, 31, 1133–1147. [Google Scholar] [CrossRef]
- Lee, E.F.T.; Streat, M. Sorption of cesium by complex hexacyanoferrates. 5. A comparison of some cyanoferrates. J. Chem. Technol. Biotechnol. A 1983, 33, 333–338. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Fedoroff, M. Fixation mechanisms of cesium on nickel and zinc ferrocyanides. Solvent Extr. Ion Exch. 1989, 7, 131–158. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Barre, Y.; Guari, Y.; Le Saout, G.; Guibal, E. Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: Synthesis and characterization. J. Mater. Chem. A 2014, 2, 10007–10021. [Google Scholar] [CrossRef]
- Mimura, H.; Kageyama, N.; Akiba, K.; Yoneya, M.; Miyamoto, Y. Ion-exchange properties of potassium nickel hexacyanoferrate(II) compounds. Solvent Extr. Ion Exch. 1998, 16, 1013–1031. [Google Scholar] [CrossRef]
- Ishfaq, M.M.; Karim, H.M.A.; Khan, M.A. Preparation and characterization of potassium copper nickel hexacyanoferrate(II) as an ion exchanger for cesium. J. Radioanal. Nucl. Chem. 1992, 159, 335–342. [Google Scholar] [CrossRef]
- Gaffar, M.A.; Omar, M.H. Thermal analytical study of different phases of potassium hexacyanoferrate(II) crystal—Effects of growth conditions, heat treatment and gamma-irradiation on the unit cell parameters. J. Therm. Anal. Calorim. 2005, 81, 477–487. [Google Scholar] [CrossRef]
- Dwivedi, C.; Kumar, A.; Singh, K.K.; Juby, A.K.; Kumar, M.; Wattal, P.K.; Bajaj, P.N. Copper hexacyanoferrate-polymer composite beads for cesium ion removal: Synthesis, characterization, sorption, and kinetic studies. J. Appl. Polym. Sci. 2013, 129, 152–160. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pathak, S.K.; Kumar, M.; Tripathi, S.C.; Bajaj, P.N. Potassium cobalthexacyanoferrate-gel beads for cesium removal: Kinetics and sorption studies. RSC Adv. 2013, 3, 22102–22110. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, J.; Reguera, E.; Lima, E.; Balmaseda, J.; Martinez-Garcia, R.; Yee-Madeira, H. An atypical coordination in hexacyanometallates: Structure and properties of hexagonal zinc phases. J. Phys. Chem. Solids 2007, 68, 1630–1642. [Google Scholar] [CrossRef]
- Ayrault, S.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.J.; Loos-Neskovic, C. Sorption mechanisms of cesium on CuII2FeII(CN)6 and CuII3[FeIII(CN)6]2: Hexacyanoferrates and their relation to the crystalline structure. J. Solid State Chem. 1998, 141, 475–485. [Google Scholar] [CrossRef]
- Faustino, P.J.; Yang, Y.; Progar, J.J.; Brownell, C.R.; Sadrieh, N.; May, J.C.; Leutzinger, E.; Place, D.A.; Duffy, E.P.; Houn, F.; et al. Quantitative determination of cesium binding to ferric hexacyanoferrate: Prussian blue. J. Pharm. Biomed. Anal. 2008, 47, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, B.; Taran, F.; Renault, D.; Wilk, J.C.; Ansoborlo, E. Comparison of Prussian blue and apple-pectin efficacy on 137Cs decorporation in rats. Biochimie 2006, 88, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- De Tacconi, N.R.; Rajeshwar, K.; Lezna, R.O. Metal hexacyanoferrates: Electrosynthesis, in situ characterization, and applications. Chem. Mater. 2003, 15, 3046–3062. [Google Scholar] [CrossRef]
- Grandjean, A.; Delchet, C.; Causse, J.; Barré, Y.; Guari, Y.; Larionova, J. Effect of the chemical nature of different transition metal ferrocyanides to entrap Cs. J. Radioanal. Nucl. Chem. 2015. [Google Scholar] [CrossRef]
- Avramenko, V.; Bratskaya, S.; Zheleznov, V.; Sheveleva, I.; Marinin, D.; Sergienko, V. Latex particles functionalized with transition metals ferrocyanides for cesium uptake and decontamination of solid bulk materials. In Proceedings of the 13th International Conference on Environmental Remediation and Radioactive Waste Management, ICEM2010, Tsukuba, Japan, 3–7 October 2010; ASME: Tsukuba, Japan, 2011; pp. 253–258. [Google Scholar]
- Avramenko, V.; Bratskaya, S.; Zheleznov, V.; Sheveleva, I.; Voitenko, O.; Sergienko, V. Colloid stable sorbents for cesium removal: Preparation and application of latex particles functionalized with transition metals ferrocyanides. J. Hazard. Mater. 2011, 186, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Bondar, Y.; Kuzenko, S.; Han, D.H.; Cho, H.K. Development of novel nanocomposite adsorbent based on potassium nickel hexacyanoferrate-loaded polypropylene fabric. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Bykov, G.; Milyutin, V.; Ershov, B.; Korchagin, Y.; Gelis, V.; Bessonov, A. Radiation resistance of a composite ferrocyanide-silica gel sorbent. Radiochemistry 2011, 53, 191–195. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chau, L.K.; Hu, W.P.; Wang, C.Y.; Liao, J.H. Nickel hexacyanoferrate multilayers on functionalized mesoporous silica supports for selective sorption and sensing of cesium. Microporous Mesoporous Mater. 2008, 109, 505–512. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. Removal of radionuclide Sr2+ ions from aqueous solution using synthesized magnetic chitosan beads. Nucl. Eng. Des. 2012, 242, 445–451. [Google Scholar] [CrossRef]
- Du, Z.; Jia, M.; Wang, X. Cesium removal from solution using PAN-based potassium nickel hexacyanoferrate(II) composite spheres. J. Radioanal. Nucl. Chem. 2013, 298, 167–177. [Google Scholar] [CrossRef]
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nucl. Chem. 2012, 1–21. [Google Scholar] [CrossRef]
- Ishfaq, M.M.; Karim, H.M.A.; Khan, M.A. A radiochemical study on the thermodynamics of cesium adsorption on potassium copper nickel hexacyanoferrate(II) from aqueous solutions. J. Radioanal. Nucl. Chem. 1997, 222, 177–181. [Google Scholar] [CrossRef]
- Ismail, I.M.; El-Sourougy, M.R.; El-Moneim, N.A.; Aly, H.F. The sorption of cobalt from aqueous solutions by potassium nickel hexacyanoferrate complex. Solvent Extr. Ion Exch. 2002, 20, 589–600. [Google Scholar] [CrossRef]
- Jalali-Rad, R.; Ghafourian, H.; Asef, Y.; Dalir, S.T.; Sahafipour, M.H.; Gharanjik, B.M. Biosorption of cesium by native and chemically modified biomass of marine algae: introduce the new biosorbents for biotechnology applications. J. Hazard. Mater. 2004, 116, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jeerage, K.M.; Steen, W.A.; Schwartz, D.T. Charge-density-dependent partitioning of Cs+ and K+ into nickel hexacyanoferrate matrixes. Langmuir 2002, 18, 3620–3625. [Google Scholar] [CrossRef]
- Karadas, F.; El-Faki, H.; Deniz, E.; Yavuz, C.T.; Aparicio, S.; Atilhan, M. CO2 adsorption studies on Prussian blue analogues. Microporous Mesoporous Mater. 2012, 162, 91–97. [Google Scholar] [CrossRef]
- Kazemian, H.; Zakeri, H.; Rabbani, M.S. Cs and Sr removal from solution using potassium nickel hexacyanoferrate impregnated zeolites. J. Radioanal. Nucl. Chem. 2006, 268, 231–236. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicki, Z.; Kubica, B. Hexacyanoferrate composite sorbent in removal of anionic species from waters and waste waters. Sep. Sci. Technol. 2012, 47, 1361–1368. [Google Scholar] [CrossRef]
- Kopyrin, A.A.; Pyartman, A.K.; Keskinov, V.A.; Pleshkov, M.A.; Sobolev, I.A.; Dmitriev, S.A. Cesium-selective composites. I. Synthesis and ion-exchange properties of sorbents based on AV-17 anion exchanger and double ferrocyanides of transition metals and potassium. Radiochemistry 1999, 41, 247–249. [Google Scholar]
- Kopyrin, A.A.; Pyartman, A.K.; Keskinov, V.A.; Pleshkov, M.A.; Sobolev, I.A.; Dmitriev, S.A. Cesium-selective composites. II. Synthesis and ion-exchange properties of sorbents based on VP-1Ap anion exchanger and double ferrocyanides of transition metals and potassium. Radiochemistry 1999, 41, 250–252. [Google Scholar]
- Kopyrin, A.A.; Pyartman, A.K.; Keskinov, V.A.; Pleshkov, M.A.; Sobolev, I.A.; Dmitriev, S.A. Cesium-selective composites: III. Synthesis and ion-exchange properties of sorbents based on AMP anion exchanger and double ferrocyanides of transition metals and potassium. Radiochemistry 2000, 42, 83–85. [Google Scholar]
- Kubica, B.; Tuteja-Krysa, M.; Misiak, R.; My, T.T.T.; Kubica, M.; Stobinski, M.; Godunowa, H. The behavior of Ba and Sr on inorganic and organic ion-exchangers from sulphuric acid solutions—Preliminary experiments. J. Radioanal. Nucl. Chem. 2003, 258, 167–170. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Dierkes, M.H.; Jackwerth, E.; Fedoroff, M.; Garnier, E. FIixation of palladium on insoluble simple or complex cyano compounds. Hydrometallurgy 1993, 32, 345–363. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Pedoroff, M. Exchange mechanisms of silver on nickel and zinc ferrocyanides. Solvent Extr. Ion Exch. 1987, 5, 757–780. [Google Scholar] [CrossRef]
- Milyutin, V.; Kononenko, O.; Mikheev, S.; Gelis, V. Sorption of cesium on finely dispersed composite ferrocyanide sorbents. Radiochemistry 2010, 52, 281–283. [Google Scholar] [CrossRef]
- Mimura, H.; Kimura, M.; Akiba, K.; Onodera, Y. Selective removal of cesium from sodium nitrate solutions by potassium nickel hexacyanoferrate-loaded chabazites. Sep. Sci. Technol. 1999, 34, 17–28. [Google Scholar] [CrossRef]
- Mimura, H.; Kimura, M.; Akiba, K.; Onodera, Y. Selective removal of cesium from highly concentrated sodium nitrate neutral solutions by potassium nickel hexacyanoferrate(II)-loaded silica gels. Solvent Extr. Ion Exch. 1999, 17, 403–417. [Google Scholar] [CrossRef]
- Mostafa, M.; El-Absy, M.A.; Amin, M.; El-Amir, M.A.; Farag, A.B. Partial purification of neutron-activation (99)Mo from cross-contaminant radionuclides onto potassium nickel hexacyanoferrate(II) column. J. Radioanal. Nucl. Chem. 2011, 285, 579–588. [Google Scholar] [CrossRef]
- Orechovska, J.; Rajec, P. Sorption of cesium on composite sorbents based on nickel ferrocyanide. J. Radioanal. Nucl. Chem. 1999, 242, 387–390. [Google Scholar] [CrossRef]
- Parab, H.; Sudersanan, M. Engineering a lignocellulosic biosorbent—Coir pith for removal of cesium from aqueous solutions: Equilibrium and kinetic studies. Water Res. 2010, 44, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Rajec, P.; Orechovska, J.; Novak, I. NIFSIL: A new composite sorbent for cesium. J. Radioanal. Nucl. Chem. 2000, 245, 317–321. [Google Scholar] [CrossRef]
- Ramaswamy, M. Sorption of cesium by hexacyanoferrate composites from neutral and acidic media. Solvent Extr. Ion Exch. 1997, 15, 1119–1131. [Google Scholar] [CrossRef]
- Sharygin, L.; Muromskiy, A.; Kalyagina, M.; Borovkov, S. A granular inorganic cation-exchanger selective to cesium. J. Nucl. Sci. Technol. 2007, 44, 767–773. [Google Scholar] [CrossRef]
- Sheha, R.R. Synthesis and characterization of magnetic hexacyanoferrate(II) polymeric nanocomposite for separation of cesium from radioactive waste solutions. J. Colloid Interface Sci. 2012, 388, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, A.; Agulhon, P.; Long, J.; Quignard, F.; Robitzer, M.; Ferreira, R.A.S.; Carlos, L.D.; Larionova, J.; Guerin, C.; Guari, Y. Synthesis and study of Prussian blue type nanoparticles in an alginate matrix. J. Mater. Chem. 2012, 22, 20232–20242. [Google Scholar] [CrossRef]
- Voronina, A.V.; Semenishchev, V.S.; Nogovitsyna, E.V.; Betenekov, N.D. Peculiarities of sorption isotherm and sorption chemisms of caesium by mixed nickel-potassium ferrocyanide based on hydrated titanium dioxide. J. Radioanal. Nucl. Chem. 2013, 298, 67–75. [Google Scholar] [CrossRef]
- Vrtoch, Ľ.; Pipíška, M.; Horník, M.; Augustín, J.; Lesný, J. Sorption of cesium from water solutions on potassium nickel hexacyanoferrate-modified Agaricus bisporus mushroom biomass. J. Radioanal. Nucl. Chem. 2011, 287, 853–862. [Google Scholar] [CrossRef]
- Watari, K.; Imai, K.; Ohmomo, Y.; Muramatsu, Y.; Nishimura, Y.; Izawa, M.; Baciles, L.R. Simultaneous adsorption of Cs-137 and I-131 from water and milk on “metal ferrocyanide-anion exchange resin”. J. Nucl. Sci. Technol. 1988, 25, 495–499. [Google Scholar] [CrossRef]
- Won, H.J.; Moon, J.K.; Jung, C.H.; Chung, W.Y. Evaluation of ferrocyanide anion exchange resins regarding the uptake of Cs+ ions and their regeneration. Nucl. Eng. Technol. 2008, 40, 489–496. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, Y.; Wang, X.; Yan, Q.; Yuan, J.; Shao, Y.; Niu, L.; Huang, S. Controllable synthesis of coaxial nickel hexacyanoferrate/carbon nanotube nanocables as advanced supercapcitors materials. Electrochim. Acta 2015, 167, 364–371. [Google Scholar] [CrossRef]
- Chen, R.; Tanaka, H.; Kawamoto, T.; Asai, M.; Fukushima, C.; Kurihara, M.; Watanabe, M.; Arisaka, M.; Nankawa, T. Preparation of a film of copper hexacyanoferrate nanoparticles for electrochemical removal of cesium from radioactive wastewater. Electrochem. Commun. 2012, 25, 23–25. [Google Scholar] [CrossRef]
- Clarke, T.D.; Wai, C.M. Selective removal of cesium from acid solutions with immobilized copper ferrocyanide. Anal. Chem. 1998, 70, 3708–3711. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, G.-H.; Gu, P. Removal of cesium from simulated liquid waste with countercurrent two-stage adsorption followed by microfiltration. J. Hazard. Mater. 2012, 225–226, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, G.-H.; Gu, P. Adsorption kinetics and equilibrium modeling of cesium on copper ferrocyanide. J. Radioanal. Nucl. Chem. 2013, 295, 369–377. [Google Scholar] [CrossRef]
- Ishfaq, M.M.; Karim, H.M.A.; Khan, M.A. Adsorption studies of cesium on potassium copper-nickel hexacyanoferrate(II) from aqueous solutions. J. Radioanal. Nucl. Chem. 1993, 170, 321–331. [Google Scholar] [CrossRef]
- Jimenez-Gallegos, J.; Rodriguez-Hernandez, J.; Yee-Madeira, H.; Reguera, E. Structure of porous copper prussian blue analogues: Nature of their high H-2 storage capacity. J Phys. Chem. C 2010, 114, 5043–5048. [Google Scholar] [CrossRef]
- Kubica, B. Sorption of lead(II) on copper(II) and nickel-potassium hexacyanoferrates and magnetite-loaded resin from inorganic acid solutions. Nukleonika 2010, 55, 163–168. [Google Scholar]
- Loos-Neskovic, C.; Ayrault, S.; Badillo, V.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.J.; Merinov, B. Structure of copper-potassium hexacyanoferrate (II) and sorption mechanisms of cesium. J. Solid State Chem. 2004, 177, 1817–1828. [Google Scholar] [CrossRef]
- Milyutin, V.; Mikheev, S.; Gelis, V.; Kozlitin, E. Sorption of cesium on ferrocyanide sorbents from highly saline solutions. Radiochemistry 2009, 51, 298–300. [Google Scholar] [CrossRef]
- Nilchi, A.; Malek, B.; Maragheh, M.G.; Khanchi, A. Investigation of the resistance of the potassium copper nickel hexacyanoferrate(II) ion exchanger against gamma irradiation. Radiat. Phys. Chem. 2003, 68, 837–842. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Moradi, M.; Azizpour, H.; Zarghami, R. Adsorption of cesium on copper hexacyanoferrate-PAN composite ion exchanger from aqueous solution. Chem. Eng. J. 2011, 172, 572–580. [Google Scholar] [CrossRef]
- Rumyantseva, E.; Veleshko, A.; Kulyukhin, S.; Veleshko, I.; Shaitura, D.; Rozanov, K.; Dmitrieva, N. Preparation and properties of modified spherically granulated chitosan for sorption of 137Cs from solutions. Radiochemistry 2009, 51, 496–501. [Google Scholar] [CrossRef]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C.; Yantasee, W. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, M.; Liu, C.; Zhao, Y.; Li, S.; Wang, H.; Yan, L.; Tian, G.; Li, S. Supporting of potassium copper hexacyanoferrate on porous activated carbon substrate for cesium separation. Sep. Sci. Technol. 2009, 44, 4023–4035. [Google Scholar] [CrossRef]
- Watari, K.; Imai, K.; Izawa, M. Isolation of 137Cs with copper ferrocyanide-anion exchange resin. J. Nucl. Sci. Technol. 1967, 4, 190–194. [Google Scholar] [CrossRef]
- Ali, I.O.; Salama, T.M.; Thabet, M.S.; El-Nasser, K.S.; Hassan, A.M. Encapsulation of ferro- and ferricyanide complexes inside ZSM-5 zeolite synthesized from rice straw: Implications for synthesis of Prussian blue pigment. Mater. Chem. Phys. 2013, 140, 81–88. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Oh, W.-K.; Shin, K.-Y.; Kwon, O.S.; Son, S.; Jang, J. Spatially controlled carbon sponge for targeting internalized radioactive materials in human body. Biomaterials 2012, 33, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Fugetsu, B.; Yu, H.; Abe, Y. Prussian blue caged in spongiform adsorbents using diatomite and carbon nanotubes for elimination of cesium. J. Hazard. Mater. 2012, 217–218, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew. Chem. Int. Ed. 2012, 51, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Kawatake, K.; Shigemoto, N. Preparation of potassium iron(III) hexacyanoferrate(II) supported on activated carbon and Cs uptake performance of the adsorbent. J. Nucl. Sci. Technol. 2012, 49, 1048–1056. [Google Scholar] [CrossRef]
- Kitajima, A.; Tanaka, H.; Minami, N.; Yoshino, K.; Kawamoto, T. Efficient cesium adsorbent using Prussian Blue nanoparticles immobilized on cotton matrices. Chem. Lett. 2012, 41, 1473–1474. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Liang, C.; Liu, P.; Xu, J.; Fang, J.; Shen, W.; Zhao, J. Morphologies and magnetic properties of cobalt-iron Prussian Blue analogues nanoparticles synthesized in microemulsion. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2010, 40, 805–811. [Google Scholar]
- Namiki, Y.; Namiki, T.; Ishii, Y.; Koido, S.; Nagase, Y.; Tsubota, A.; Tada, N.; Kitamoto, Y. Inorganic-organic magnetic nanocomposites for use in preventive medicine: A rapid and reliable elimination system for cesium. Pharm. Res. 2012, 29, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Pyrasch, M.; Toutianoush, A.; Jin, W.Q.; Schnepf, J.; Tieke, B. Self-assembled films of Prussian blue and analogues: Optical and electrochemical properties and application as ion-sieving membranes. Chem. Mater. 2003, 15, 245–254. [Google Scholar] [CrossRef]
- Taj, S.; Ashraf Chaudhry, M.; Mazhar, M. Potassium iron(III)hexacyanoferrate(II) supported on polymethylmethacrylate ion-exchanger for removal of strontium(II). J. Radioanal. Nucl. Chem. 2009, 281, 393–403. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Imura, M.; Naito, M.; Yamauchi, Y. Large Cs adsorption capability of nanostructured Prussian Blue particles with high accessible surface areas. J. Mater. Chem. 2012, 22, 18261–18267. [Google Scholar] [CrossRef]
- Tsuji, H.; Kondo, Y.; Suzuki, Y.; Yasutaka, T. Development of a method for rapid and simultaneous monitoring of particulate and dissolved radiocesium in water with nonwoven fabric cartridge filters. J. Radioanal. Nucl. Chem. 2012. [Google Scholar] [CrossRef]
- Vipin, A.K.; Hu, B.; Fugetsu, B. Prussian blue caged in alginate/calcium beads as adsorbents for removal of cesium ions from contaminated water. J. Hazard. Mater. 2013, 258, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Faustino, P.J.; Progar, J.J.; Brownell, C.R.; Sadrieh, N.; May, J.C.; Leutzinger, E.; Place, D.A.; Duffy, E.P.; Yu, L.X.; et al. Quantitative determination of thallium binding to ferric hexacyanoferrate: Prussian blue. Int. J. Pharm. 2008, 353, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yasutaka, T.; Kawamoto, T.; Kawabe, Y.; Sato, T.; Sato, M.; Suzuki, Y.; Nakamura, K.; Komai, T. Rapid measurement of radiocesium in water using a Prussian blue impregnated nonwoven fabric: Fukushima NPP Accident Related. J. Nucl. Sci. Technol. 2013, 50, 674–681. [Google Scholar] [CrossRef]
- Terada, K.; Hayakawa, H.; Sawada, K.; Kiba, T. Silica gel as a support for inorganic ion-exchangers for the determination of caesium-137 in natural waters. Talanta 1970, 17, 955–963. [Google Scholar] [CrossRef]
- Ca, D.V.; Cox, J.A. Solid phase extraction of cesium from aqueous solution using sol-gel encapsulated cobalt hexacyanoferrate. Microchim. Acta 2004, 147, 31–37. [Google Scholar]
- Delchet, C.; Tokarev, A.; Dumail, X.; Toquer, G.; Barre, Y.; Guari, Y.; Guerin, C.; Larionova, J.; Grandjean, A. Extraction of radioactive cesium using innovative functionalized porous materials. RSC Adv. 2012, 2, 5707–5716. [Google Scholar] [CrossRef]
- Hirayama, Y.; Okamura, Y.; Fujiwara, K.; Sugo, T.; Umeno, D.; Saito, K. Effect of salt concentration of cesium solution on cesium-binding capacity of potassium cobalt-hexacyanoferrate-impregnated fiber. Kagaku Kogaku Ronbunshu 2013, 39, 28–32. [Google Scholar] [CrossRef]
- Lehto, J.; Harjula, R. Separation of cesium from nuclear waste solutions with hexacyanoferrate(II)s and ammonium phosphomolybdate. Solvent Extr. Ion Exch. 1987, 5, 343–352. [Google Scholar] [CrossRef]
- Liang, C.; Liu, P.; Xu, J.; Wang, H.; Wang, W.; Fang, J.; Wang, Q.; Shen, W.; Zhao, J. A simple method for the synthesis of Fe-Co Prussian Blue analogue with novel morphologies, different structures, and dielectric properties. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2011, 41, 1108–1113. [Google Scholar] [CrossRef]
- Liu, H.D.; Li, F.Z.; Zhao, X.; Yun, G.C. Preparing high-loaded potassium cobalt hexacyanoferrate/silica composite for radioactive wastewater treatment. Nucl. Technol. 2009, 165, 200–208. [Google Scholar]
- Valsala, T.P.; Joseph, A.; Shah, J.G.; Raj, K.; Venugopal, V. Synthesis and characterization of cobalt ferrocyanides loaded on organic anion exchanger. J. Nucl. Mater. 2009, 384, 146–152. [Google Scholar] [CrossRef]
- Vo, V.; Van Minh, N.; Lee, H.I.; Kim, J.M.; Kim, Y.; Kim, S.J. Synthesis and characterization of Co-Fe Prussian blue nanoparticles within MCM-41. Mater. Res. Bull. 2009, 44, 78–81. [Google Scholar] [CrossRef]
- Li, B.; Liao, J.; Wu, J.; Zhang, D.; Zhao, J.; Yang, Y.; Cheng, Q.; Feng, Y.; Liu, N. Removal of radioactive cesium from solutions by zinc ferrocyanide. Nucl. Sci. Tech. 2008, 19, 88–92. [Google Scholar] [CrossRef]
- Nilchi, A.; Hadjmohammadi, M.R.; Garmarodi, S.R.; Saberi, R. Studies on the adsorption behavior of trace amounts of (90)Sr2+, (140)La3+, (60)Co2+, Ni2+ and Zr4+ cations on synthesized inorganic ion exchangers. J. Hazard. Mater. 2009, 167, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Shakir, K.; Sohsah, M.; Soliman, M. Removal of cesium from aqueous solutions and radioactive waste simulants by coprecipitate flotation. Sep. Purif. Technol. 2007, 54, 373–381. [Google Scholar] [CrossRef]
- Chen, R.; Tanaka, H.; Kawamoto, T.; Asai, M.; Fukushima, C.; Na, H.; Kurihara, M.; Watanabe, M.; Arisaka, M.; Nankawa, T. Selective removal of cesium ions from wastewater using copper hexacyanoferrate nanofilms in an electrochemical system. Electrochim. Acta 2013, 87, 119–125. [Google Scholar] [CrossRef]
- Egorin, A.M.; Avramenko, V.A. Dynamics of sorption of cesium radionuclides on selective ferrocyanide sorbents. Distribution of the 137Cs activity in the stationary phase. Radiochemistry 2012, 54, 483–488. [Google Scholar] [CrossRef]
- Epimakhov, V.; Moskvin, L.; Chetverikov, V.; Epimakhov, T.; Ganyushkin, A.; Prokhorkin, S. Treatment of water from spent nuclear fuel storage basins with ion-exchange resins modified with transition metal hexacyanoferrates. Radiochemistry 2010, 52, 610–612. [Google Scholar] [CrossRef]
- Harjula, R.; Lehto, J.; Paajanen, A.; Brodkin, L.; Tusa, E. Removal of radioactive cesium from nuclear waste solutions with the transition metal hexacyanoferrate ion exchanger CsTreat. Nucl. Sci. Eng. 2001, 137, 206–214. [Google Scholar]
- Ishfaq, M.M.; Safdar, M. A radiochemical study of the kinetics and mechanism of caesium ion adsorption on potassium copper nickel hexacyanoferrate(II). Adsorpt. Sci. Technol. 1999, 17, 689–701. [Google Scholar]
- Kamenik, J.; Dulaiova, H.; Sebesta, F.; St’astna, K. Fast concentration of dissolved forms of cesium radioisotopes from large seawater samples. J. Radioanal. Nucl. Chem. 2013, 296, 841–846. [Google Scholar] [CrossRef]
- Mimura, H.; Kimura, M.; Akiba, K.; Onodera, Y. Separation of Cesium and Strontium by Potassium Nickel. J. Nucl. Sci. Technol. 1999, 36, 307–310. [Google Scholar] [CrossRef]
- Peterskova, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Extraction of valuable metal ions (Cs, Rb, Li, U) from reverse osmosis concentrate using selective sorbents. Desalination 2012, 286, 316–323. [Google Scholar] [CrossRef]
- Sheveleva, I.; Avramenko, V.; Bratskaya, S.; Zheleznov, V.; Modin, E.; Sergienko, V. Composite sorbents for recovery of cesium radionuclides. Russ. J. Appl. Chem. 2010, 83, 2115–2120. [Google Scholar] [CrossRef]
- Sheveleva, I.; Zheleznov, V.; Bratskaya, S.; Avramenko, V.; Kuryavyi, V. Sorption of cesium radionuclides with composite carbon fibrous materials. Russ. J. Appl. Chem. 2011, 84, 1152–1157. [Google Scholar] [CrossRef]
- Sun, B.; Hao, X.G.; Wang, Z.D.; Guan, G.Q.; Zhang, Z.L.; Li, Y.B.; Liu, S.B. Separation of low concentration of cesium ion from wastewater by electrochemically switched ion exchange method: Experimental adsorption kinetics analysis. J. Hazard. Mater. 2012, 233, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Taj, S.; Muhammad, D.; Chaudhry, M.A.; Mazhar, M. Lithium, rubidium and cesium ion removal using potassium iron(III) hexacyanoferrate(II) supported on polymethylmethacrylate. J. Radioanal. Nucl. Chem. 2011, 288, 79–88. [Google Scholar] [CrossRef]
- Tsuruoka, S.; Fugetsu, B.; Khoerunnisa, F.; Minami, D.; Takeuchi, K.; Fujishige, M.; Hayashi, T.; Kim, Y.A.; Park, K.C.; Asai, M.; et al. Intensive synergetic Cs adsorbent incorporated with polymer spongiform for scalable purification without post filtration. Mater. Express 2013, 3, 21–29. [Google Scholar] [CrossRef]
- Valsala, T.P.; Roy, S.C.; Shah, J.G.; Gabriel, J.; Raj, K.; Venugopal, V. Removal of radioactive caesium from low level radioactive waste (LLW) streams using cobalt ferrocyanide impregnated organic anion exchanger. J. Hazard. Mater. 2009, 166, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.K.; Ling, S.; Fugetsu, B. Sodium cobalt hexacyanoferrate encapsulated in alginate vesicle with CNT for both cesium and strontium removal. Carbohydr. Polym. 2014, 111, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Voronina, A.; Semenishchev, V.; Nogovitsyna, E.; Betenekov, N. A study of ferrocyanide sorbents on hydrated titanium dioxide support using physicochemical methods. Radiochemistry 2012, 54, 69–74. [Google Scholar] [CrossRef]
- Voronina, A.V.; Semenishchev, V.S. Sorption-active matrix based on titanium hydroxide for concentration and joint immobilization of caesium and strontium radionuclides. J. Radioanal. Nucl. Chem. 2015, 303, 229–236. [Google Scholar] [CrossRef]
- Watari, K.; Imai, K.; Izawa, M. Radiochemical application of “Iron Ferrocyanide-Anion Exchange Resin”. J. Nucl. Sci. Technol. 1968, 5, 309–312. [Google Scholar] [CrossRef]
- Vashnia, S.; Tavakoli, H.; Cheraghali, R.; Sepehrian, H. Supporting of lead hexacyanoferrate on mesoporous MCM-41 and its use as effective adsorbent for strontium: Equilibrium, kinetic, and thermodynamic studies. Sep. Sci. Technol. 2014, 49, 241–248. [Google Scholar] [CrossRef]
- Gogoi, D.; Shanmugamani, A.G.; Rao, S.V.S.; Kumar, T.; Shreekumar, B.; Sinha, P.K. Studies on adsorptive removal of radioactive cobalt from alkaline waste generated in sodium cooled fast breeder reactors. J. Radioanal. Nucl. Chem. 2013, 295, 1531–1535. [Google Scholar] [CrossRef]
- Harish, S.; Joseph, J.; Phani, K.L.N. Interaction between gold(III) chloride and potassium hexacyanoferrate(II/III)-Does it lead to gold analogue of Prussian blue? Electrochim. Acta 2011, 56, 5717–5721. [Google Scholar] [CrossRef]
- Mimura, H.; Sakakibara, T.; Yan, W.; Niibori, Y.; Koyama, S.I.; Ohnishi, T. Selective uptake of palladium from high-level liquid wastes by hybrid microcapsules enclosed with insoluble ferrocyanides. In Proceedings of the 13th International Conference on Environmental Remediation and Radioactive Waste Management ICEM2010, Tsukuba, Japan, 3–7 October 2010; ASME: Tsukuba, Japan, 2011; pp. 265–272. [Google Scholar]
- Sheha, R.R. Preparation and performance of a novel composite as a reactive resin for copper retention. Chem. Eng. J. 2012, 213, 163–174. [Google Scholar] [CrossRef]
- Rykov, A.I.; Wang, J.; Zhang, T.; Nomura, K. Cs sorption by “soluble” and “insoluble” iron hexacyanocobaltates probed by Mössbauer spectroscopy. Hyperfine Interact. 2013, 218, 53–58. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Pholosi, A.; Naidoo, E.B. Kinetics and competitive modeling of cesium biosorption onto iron(III) hexacyanoferrate modified pine cone powder. Int. Biodeterior. Biodegrad. 2014, 92, 71–78. [Google Scholar] [CrossRef]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Qing, Y.; Li, J.; Kang, B.; Chang, S.; Dai, Y.; Long, Q.; Yuan, C. Selective sorption mechanism of Cs+ on potassium nickel hexacyanoferrate(II) compounds. J. Radioanal. Nucl. Chem. 2015, 304, 527–533. [Google Scholar] [CrossRef]
- Nilchi, A.; Atashi, H.; Javid, A.H.; Saberi, R. Preparations of PAN-based adsorbers for separation of cesium and cobalt from radioactive wastes. Appl. Radiat. Isot. 2007, 65, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.K.; Lee, E.H.; Kim, H.T. Ion exchange of Cs ion in acid solution with potassium cobalt hexacyanoferrate. Korean J. Chem. Eng. 2004, 21, 1026–1031. [Google Scholar] [CrossRef]
- Takahatake, Y.; Watanabe, S.; Shibata, A.; Nomura, K.; Koma, Y. Decontamination of radioactive liquid waste with hexacyanoferrate(II). Procedia Chemistry 2012, 7, 610–615. [Google Scholar] [CrossRef]
- Sinha, P.K.; Amalraj, R.V.; Krishnasamy, V. Flocculation studies on freshly precipitated copper ferrocyanide for the removal of caesium from radioactive liquid waste. Waste Manag. 1993, 13, 341–350. [Google Scholar] [CrossRef]
- Hu, L.; Mei, J.Y.; Chen, Q.W.; Zhang, P.; Yan, N. Magnetically separable Prussian blue analogue Mn-3 Co(CN)(6) (2)center dot nH(2)O porous nanocubes as excellent absorbents for heavy metal ions. Nanoscale 2011, 3, 4270–4274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Wei, J.; Li, F. Sorption behavior of cesium from aqueous solution on magnetic hexacyanoferrate materials. Nucl. Eng. Des. 2014, 275, 322–328. [Google Scholar] [CrossRef]
- Dashtinejad, M.; Samadfam, M.; Fasihi, J.; Fumeshkenar, F.G.; Sepehrian, H. Synthesis, characterization, and cesium sorption performance of potassium nickel hexacyanoferrate-loaded granular activated carbon. Part. Sci. Technol. 2014, 32, 348–354. [Google Scholar] [CrossRef]
- Liu, H.-D.; Li, F.-Z.; Zhao, X. Preparation of high surface area porous potassium titanium hexacynoferrate/SiO2 bead for radioactive waste water treatment. Chin. J. Inorg. Chem. 2008, 24, 1657–1663. [Google Scholar] [CrossRef]
- Folch, B.; Larionova, J.; Guari, Y.; Molvinger, K.; Luna, C.; Sangregorio, C.; Innocenti, C.; Caneschi, A.; Guerin, C. Synthesis and studies of water-soluble Prussian Blue-type nanoparticles into chitosan beads. Phys. Chem. Chem. Phys. 2010, 12, 12760–12770. [Google Scholar] [CrossRef] [PubMed]
- De Mattos, I.L.; Gorton, L. Metal-hexacyanoferrate films: A tool in analytical chemistry. Quim. Nova 2001, 24, 200–205. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Miecznikowski, K.; Chojak, M.; Malik, M.A.; Zamponi, S.; Marassi, R. Electrochromic features of hybrid films composed of polyaniline and metal hexacyanoferrate. Electrochim. Acta 2001, 46, 4371–4378. [Google Scholar] [CrossRef]
- Narang, J.; Chauhan, N.; Pundir, C.S. Construction of triglyceride biosensor based on nickel oxide-chitosan/zinc oxide/zinc hexacyanoferrate film. Int. J. Biol. Macromol. 2013, 60, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.S.; Lal, K.B.; Narasimhan, S.V.; Ahmed, J. Copper ferrocyanide-polyurethane foam as a composite ion exchanger for removal of radioactive cesium. J. Radioanal. Nucl. Chem. 1999, 240, 269–276. [Google Scholar] [CrossRef]
- Rao, S.V.S.; Narasimhan, S.V.; Lal, K.B. Composite CFC-PU foam ion exchanger in the removal of radioactive cesium. J. Radioanal. Nucl. Chem. 2003, 256, 137–141. [Google Scholar] [CrossRef]
- Rao, S.V.S.; Lekshmi, R.; Mani, A.G.S.; Sinha, P.K. Treatment of low level radioactive liquid wastes using composite ion-exchange resins based on polyurethane foam. J. Radioanal. Nucl. Chem. 2010, 283, 379–384. [Google Scholar] [CrossRef]
- Kuang, J.; Yuk, K.Y.; Huh, K.M. Polysaccharide-based superporous hydrogels with fast swelling and superabsorbent properties. Carbohydr. Polym. 2011, 83, 284–290. [Google Scholar] [CrossRef]
- Vincent, C.; Hertz, A.; Vincent, T.; Barré, Y.; Guibal, E. Immobilization of inorganic ion-exchanger into biopolymer foams—Application to cesium sorption. Chem. Eng. J. 2014, 236, 202–211. [Google Scholar] [CrossRef]
- Vincent, C.; Barré, Y.; Vincent, T.; Taulemesse, J.M.; Robitzer, M.; Guibal, E. Chitin-Prussian blue sponges for Cs(I) recovery: From synthesis to application in the treatment of accidental dumping of metal-bearing solutions. J. Hazard. Mater. 2015, 287, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Folch, B.; Guari, Y.; Larionova, J.; Luna, C.; Sangregorio, C.; Innocenti, C.; Caneschi, A.; Guerin, C. Synthesis and behaviour of size controlled cyano-bridged coordination polymer nanoparticles within hybrid mesoporous silica. New J. Chem. 2008, 32, 273–282. [Google Scholar] [CrossRef]
- Thammawong, C.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Lin, Y.; Fryxell, G.E.; Wu, H.; Engelhard, M. Selective sorption of cesium using self-assembled monolayers on mesoporous supports. Environ. Sci. Technol. 2001, 35, 3962–3966. [Google Scholar] [CrossRef] [PubMed]
- Voronina, A.V.; Semenishchev, V.S. Effect of surface modification of hydrated titanium dioxide on its selectivity to strontium. Radiochemistry 2013, 55, 94–97. [Google Scholar] [CrossRef]
- Sharygin, L.M.; Muromskii, A.Y. New inorganic sorbent for ion-selective purification of liquid radioactive wastes. At. Energ. 2000, 89, 658–662. [Google Scholar] [CrossRef]
- Sharygin, L.M.; Muromskii, A.Y. Inorganic sorbent for selective treatment of liquid radioactive wastes. Radiochemistry 2004, 46, 185–189. [Google Scholar] [CrossRef]
- Jeerage, K.M.; Schwartz, D.T. Characterization of cathodically deposited nickel hexacyanoferrate for electrochemically switched ion exchange. Sep. Sci. Technol. 2000, 35, 2375–2392. [Google Scholar] [CrossRef]
- Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Selective removal of cesium from aqueous solutions with nickel(II) hexacyanoferrate(III) functionalized agricultural residue-walnut shell. J. Hazard. Mater. 2014, 270, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Causse, J.; Tokarev, A.; Ravaux, J.; Moloney, M.; Barre, Y.; Grandjean, A. Facile one-pot synthesis of copper hexacyanoferrate nanoparticle functionalised silica monoliths for the selective entrapment of Cs-137. J. Mater. Chem. A 2014, 2, 9461–9464. [Google Scholar] [CrossRef]
- Yi, K.; Jin, R.G. Study on optimum coagulation conditions of high molecular weight PAN fiber in wet spinning by orthogonal experimental design. Fibers Polym. 2012, 13, 1259–1265. [Google Scholar] [CrossRef]
- Vincent, T.; Taulemesse, J.M.; Dauvergne, A.; Chanut, T.; Testa, F.; Guibal, E. Thallium(I) sorption using prussian blue immobilized in alginate capsules. Carbohydr. Polym. 2013, 99, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Nilchi, A.; Malek, B.; Ghanadi Maragheh, M.; Khanchi, A. Exchange properties of cyanide complexes. J. Radioanal. Nucl. Chem. 2003, 258, 457–462. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Abousahl, S.; Fedoroff, M. Column-usable inorganic fixator preparation by localized growth on a solid alkaline ferrocyanide. J. Mater. Sci. 1990, 25, 677–682. [Google Scholar] [CrossRef]
- Hu, M.; Torad, N.L.K.; Chiang, Y.-D.; Wu, K.C.W.; Yamauchi, Y. Size- and shape-controlled synthesis of Prussian Blue nanoparticles by a polyvinylpyrrolidone-assisted crystallization process. Crystengcomm 2012, 14, 3387–3396. [Google Scholar] [CrossRef]
- Andersen, T.; Melvik, J.E.; Gasered, O.; Alsberg, E.; Christensen, B.E. Ionically gelled alginate foams: Physical properties controlled by operational and macromolecular parameters. Biomacromolecules 2012, 13, 3703–3710. [Google Scholar] [CrossRef] [PubMed]
- Galambos, M.; Dano, M.; Rosskopfova, O.; Sersen, F.; Kufcakova, J.; Adamcova, R.; Rajec, P. Effect of gamma-irradiation on adsorption properties of Slovak bentonites. J. Radioanal. Nucl. Chem. 2012, 292, 481–492. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Chaki, T.K.; Khastgir, D.; Pinto, R. Gamma Irradiation effects on optical, thermal, and mechanical properties of polysulfone/MWCNT nanocomposites in argon atmosphere. J. Appl. Polym. Sci. 2015, 132. [Google Scholar]
- Islam, M.M.; Khan, M.A.; Rahman, M.M. Preparation of gelatin based porous biocomposite for bone tissue engineering and evaluation of gamma irradiation effect on its properties. Mater. Sci. Eng. C 2015, 49, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.S.R.; Bhatt, H.; Deo, M.N.; Verma, R.; Reddy, A.V.R. Effect of gamma irradiation on the ion exchange capacity of polyaniline. Radiat. Phys. Chem. 2014, 96, 75–80. [Google Scholar] [CrossRef]
- Olatunji, M.A.; Khandaker, M.U.; Mahmud, H.; Amin, Y.M. Influence of adsorption parameters on cesium uptake from aqueous solutions- a brief review. RSC Adv. 2015, 5, 71658–71683. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Motkuri, R.K.; Fernandez, C.A.; McGrail, B.P.; Behrooz, G.S. Prussian Blue analogues for CO2 and SO2 capture and separation applications. Inorg. Chem. 2010, 49, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Chelebaeva, E.; Larionova, J.; Guari, Y.; Ferreira, R.A.S.; Carlos, L.D.; Trifonov, A.A.; Kalaivani, T.; Lascialfari, A.; Guerin, C.; Molvinger, K.; et al. Nanoscale coordination polymers exhibiting luminescence properties and NMR relaxivity. Nanoscale 2011, 3, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Vallat, R.; Ferreira, R.A.S.; Carlos, L.D.; Almeida Paz, F.A.; Guari, Y.; Larionova, J. A bifunctional luminescent single-ion magnet: Towards correlation between luminescence studies and magnetic slow relaxation processes. Chem. Commun. 2012, 48, 9974–9976. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, T.; Vincent, C.; Guibal, E. Immobilization of Metal Hexacyanoferrate Ion-Exchangers for the Synthesis of Metal Ion Sorbents—A Mini-Review. Molecules 2015, 20, 20582-20613. https://doi.org/10.3390/molecules201119718
Vincent T, Vincent C, Guibal E. Immobilization of Metal Hexacyanoferrate Ion-Exchangers for the Synthesis of Metal Ion Sorbents—A Mini-Review. Molecules. 2015; 20(11):20582-20613. https://doi.org/10.3390/molecules201119718
Chicago/Turabian StyleVincent, Thierry, Chloë Vincent, and Eric Guibal. 2015. "Immobilization of Metal Hexacyanoferrate Ion-Exchangers for the Synthesis of Metal Ion Sorbents—A Mini-Review" Molecules 20, no. 11: 20582-20613. https://doi.org/10.3390/molecules201119718




