Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Hemolytic Effect of Different α-CD Derivatives
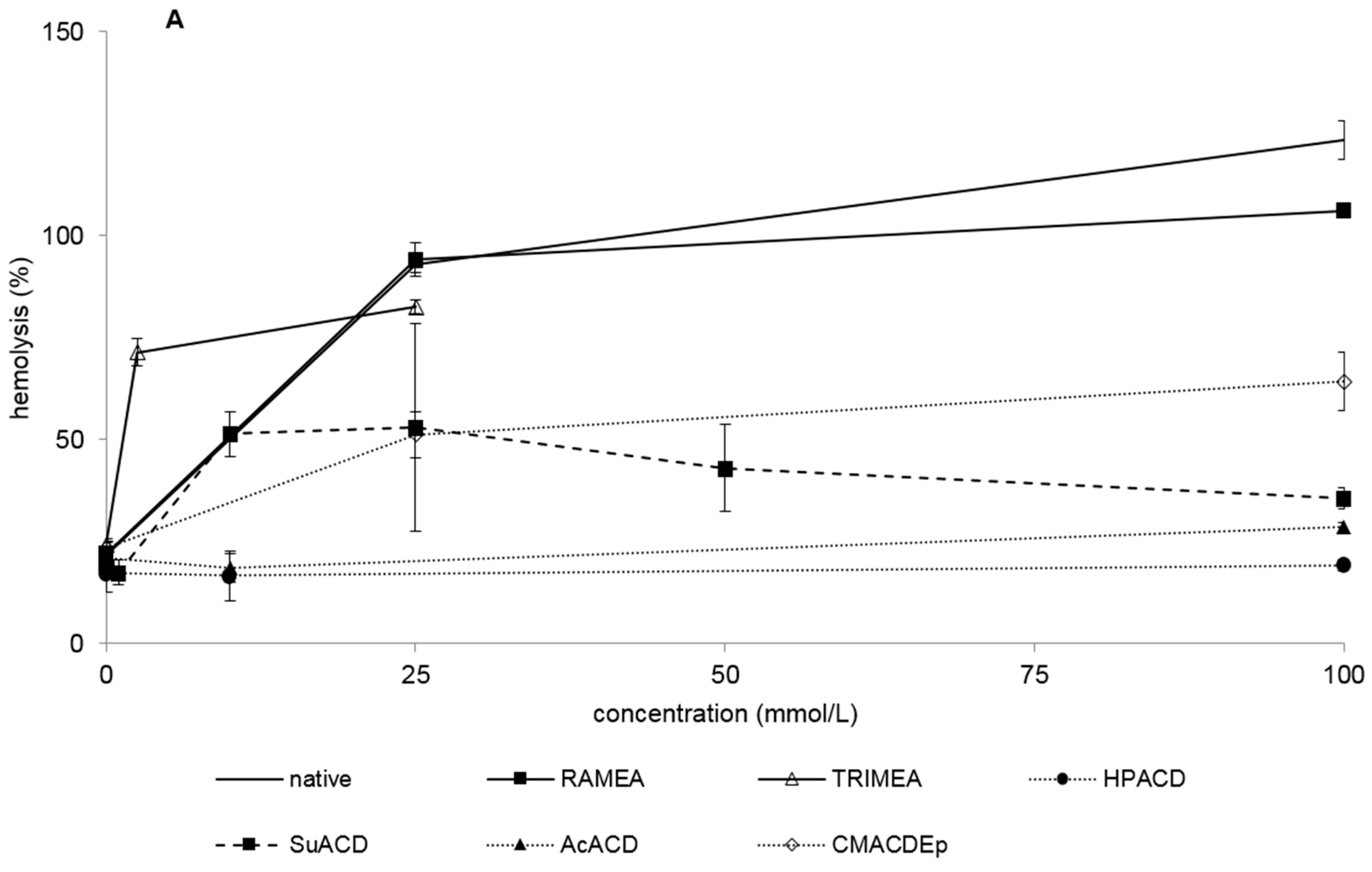
| α-Cyclodextrin Derivative | IC50 (MTT) | IC50 (RT-CES) | HC50 |
|---|---|---|---|
| native | 46.1 ± 9.2 | >25 | 16.0 ± 0.02 |
| RAMEA | 78.6 ± 15.8 | >25 | 15.5 ± 0.01 |
| TRIMEA | 1.8 ± 0.8 | >1 | 1.9 ± 0.01 |
| HPACD | >100 | >100 | >100 |
| sulfated | >100 | >10 | >100 |
| phosphated | 7.8 ± 8.6 | >10 | >100 |
| CMACD | >100 | >25 | >100 |
| SuACD | 19.0 ± 8.8 | >1 | 9.6 ± 0.03 |
| AcACD | >100 | >100 | >100 |
| CMACDEp | 58.4 ± 0.4 | >10 | 24.5 ± 0.01 |
2.1.2. Effect of α-CD Derivatives on Cell Viability

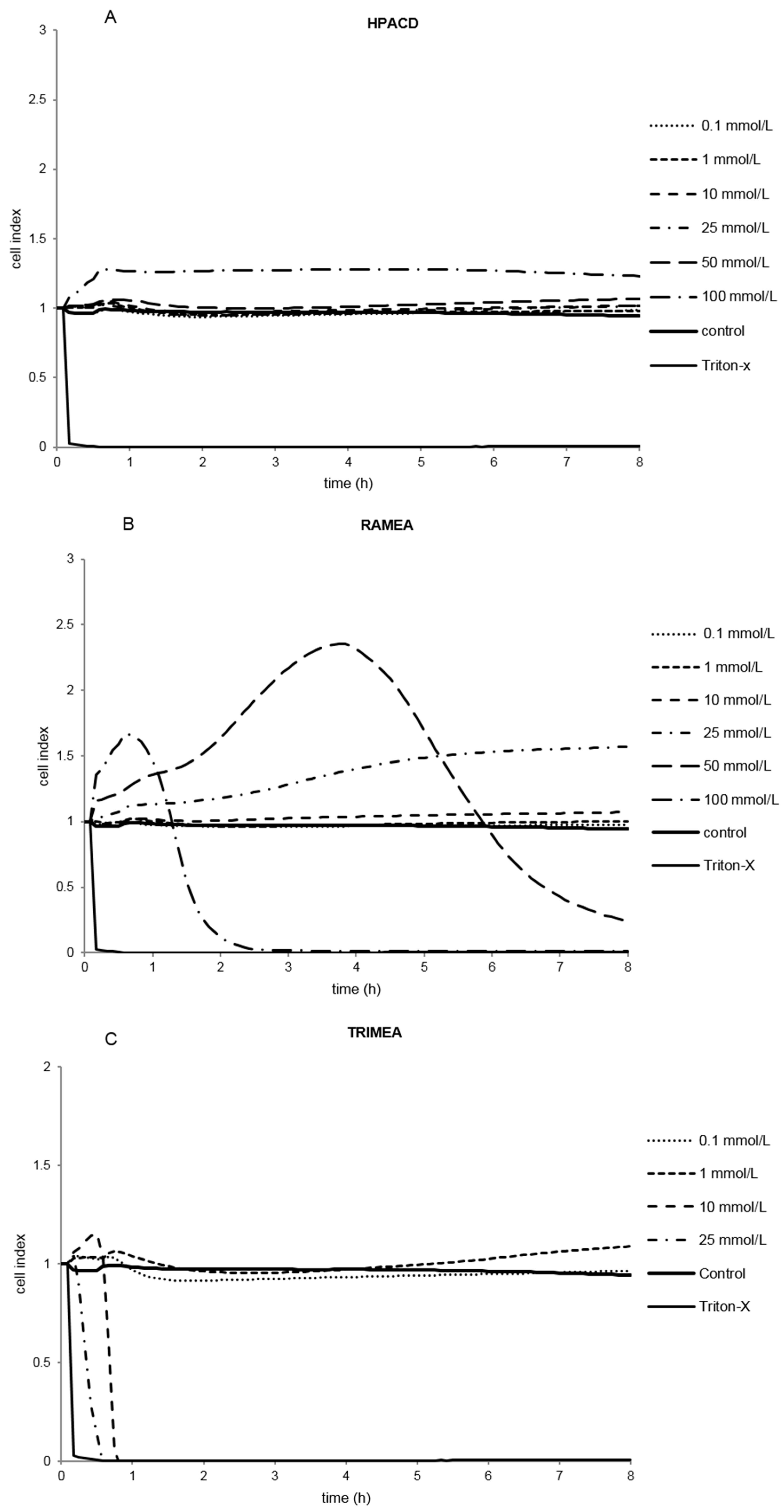
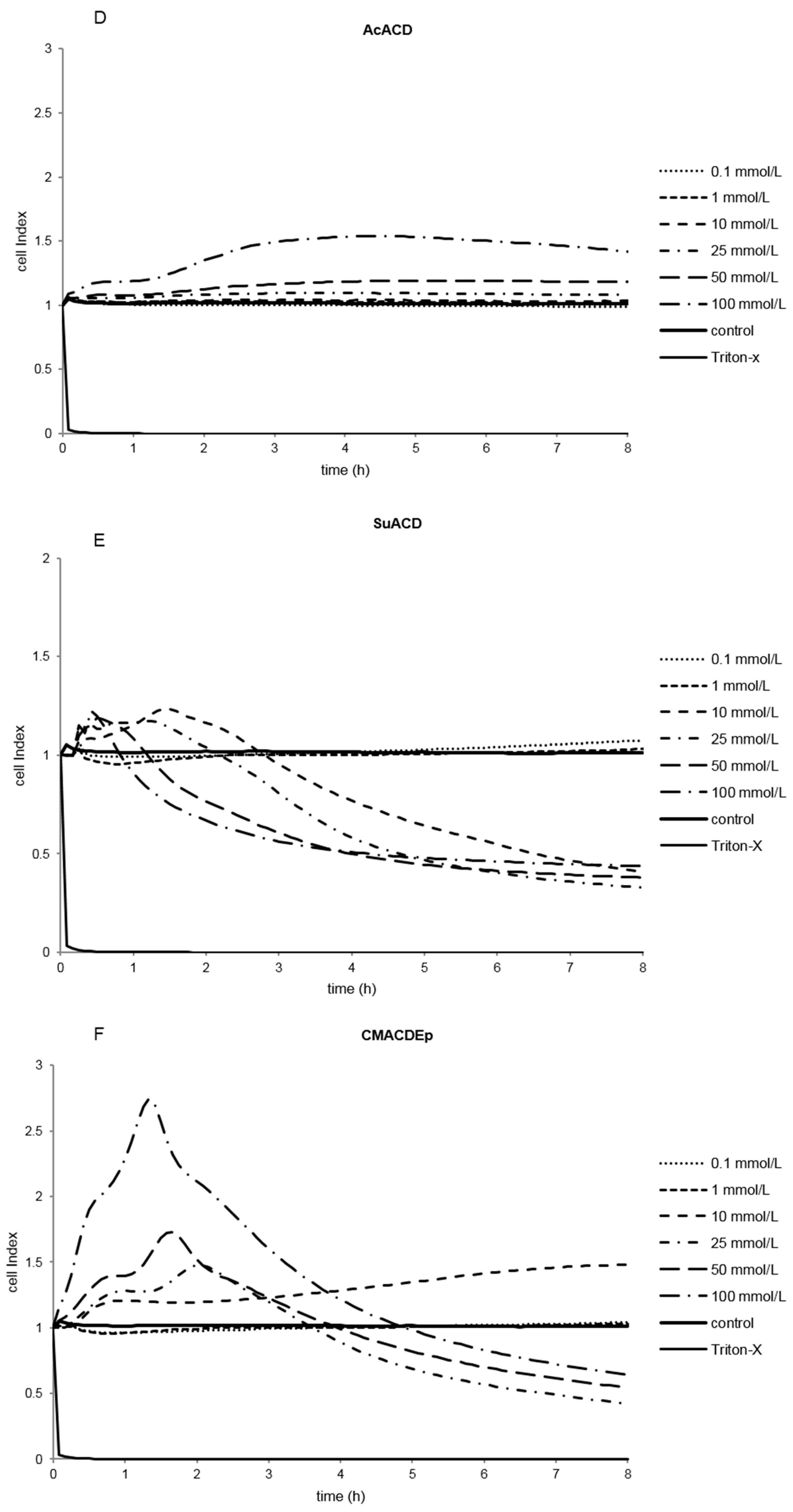
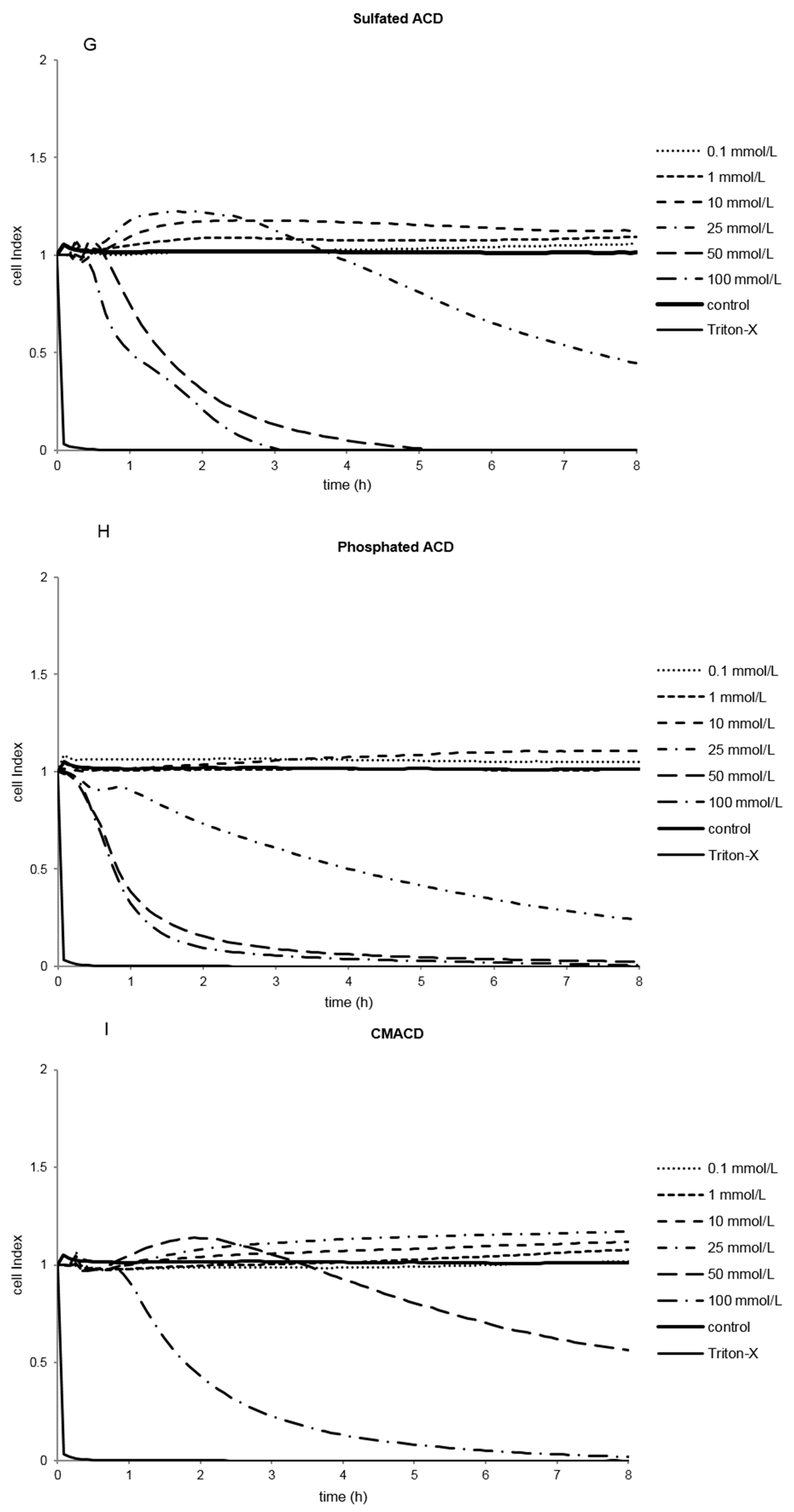
2.1.3. Indirect Verification of α-CD Cytotoxicity on Caco-2 Cells

2.2. Discussion
3. Experimental Section
3.1. Materials and Methods
| α-Cyclodextrin Derivative | Short Name | Molecular Formula | Molecular Weight | DS |
|---|---|---|---|---|
| native | C36H60O30 | 972.84 | 0 | |
| random methyl | RAMEA | C47H82O30 | 1126.9 | ~11 |
| hexakis(2,3-tri-O-methyl) | TRIMEA | C54H96O30 | 1225.4 | 18 |
| (2-hydroxy)propyl | HPACD | C49.5H87O34.5 | 1234.3 | ~4.5 |
| sulfated Na-salt | sulfated | C36H48O66S12Na12 | 2197.4 | ~12 |
| phosphated Na-salt | phosphated | C36H60O42P4Na4 | 1380.7 | ~2–6 |
| carboxymethylated Na-salt | CMACD | C48H63O36Na3 | 1212.9 | ~3.5 |
| succinylated | SuACD | C52H76O42 | 1373.2 | ~4 |
| acetylated | AcACD | C52H76O38 | 1267.1 | ~7 |
| carboxymethyl-α-CD | CMACDEp | 55 kDa | ||
| crosslinked with epichlorohydrin | ||||
3.2. Cell Culture
3.3. Hemolysis Test
3.4. MTT Cell Viability Assay
3.5. Real-Time Cell Microelectronic Sensing (RT-CES)
3.6. Statistical Analysis
| Logarithm of Concentrations | −4 | −3 | −2 | −1602 | −1301 |
|---|---|---|---|---|---|
| HPACD vs. Phosphated | ** | ||||
| HPACD vs. Polymer | * | ||||
| HPACD vs. SuACD | * | ||||
| Sulphated vs. Phosphated | ** | **** | **** | ||
| Sulphated vs. SuACD | **** | **** | |||
| Phosphated vs. AcACD | ** | ||||
| Phosphated vs. CMACD | ** | *** | **** | ||
| Phosphated vs. SuACD | *** | ||||
| Phosphated vs. Polymer | * | ** | **** | ||
| AcACD vs. Polymer | * | ||||
| RAMEA vs. TRIMEA | * | **** | **** | ||
| RAMEA vs. HPACD | * | *** | *** | ** | |
| RAMEA vs. Sulphated | * | *** | ** | ||
| RAMEA vs. AcACD | ** | **** | **** | ** | |
| RAMEA vs. Phosphatidylcholine + RAMEA | ** | **** | *** | ** | |
| RAMEA vs. Phosphated | **** | **** | **** | ||
| RAMEA vs. SuACD | **** | **** | |||
| RAMEA vs. Polymer | * | ||||
| native vs. RAMEA | ** | *** | **** | ||
| native vs. TRIMEA | **** | **** | |||
| native vs. Phosphated | * | ||||
| native vs. SuACD | *** | ||||
| native vs. Sulphated | ** | ||||
| native vs. CMACD | ** | ||||
| native vs. Polymer | * | ||||
| TRIMEA vs. Sulphated | **** | **** | |||
| TRIMEA vs. Phosphated | *** | ||||
| TRIMEA vs. CMACD | **** | **** | |||
| TRIMEA vs. SuACD | **** | ||||
| TRIMEA vs. Polymer | **** | **** | |||
| SuACD vs. AcACD | * | ||||
| SuACD vs. Polymer | **** | **** | |||
| CMACD vs. SuACD | **** | **** |
| Logarithm of Concentration | −2 | −1602 | −1301 | −1 |
|---|---|---|---|---|
| native vs. RAMEA | * | ** | ** | |
| native vs. TRIMEA | **** | **** | **** | |
| native vs. HPACD | **** | **** | **** | **** |
| native vs. Sulfated | ** | **** | **** | **** |
| native vs. Phosphated | **** | **** | **** | |
| native vs. CMACD | **** | **** | **** | |
| native vs. SuACD | **** | **** | **** | **** |
| native vs. AcACD | ** | **** | **** | **** |
| native vs. CMACDEp | *** | **** | **** | **** |
| RAMEA vs. TRIMEA | **** | * | **** | **** |
| RAMEA vs. HPACD | **** | **** | **** | **** |
| RAMEA vs. Sulfated | **** | **** | **** | **** |
| RAMEA vs. Phosphated | **** | **** | **** | |
| RAMEA vs. CMACD | **** | **** | **** | |
| RAMEA vs. SuACD | **** | **** | **** | **** |
| RAMEA vs. AcACD | **** | **** | **** | **** |
| RAMEA vs. CMACDEp | **** | **** | **** | **** |
| TRIMEA vs. HPACD | **** | **** | **** | **** |
| TRIMEA vs. Sulfated | **** | **** | **** | **** |
| TRIMEA vs. Phosphated | **** | **** | **** | **** |
| TRIMEA vs. CMACD | **** | **** | **** | **** |
| TRIMEA vs. SuACD | **** | **** | **** | **** |
| TRIMEA vs. AcACD | **** | **** | **** | **** |
| TRIMEA vs. CMACDEp | **** | **** | **** | ** |
| HPACD vs. Phosphated | **** | |||
| HPACD vs. CMACD | **** | |||
| HPACD vs. SuACD | **** | **** | **** | *** |
| HPACD vs. AcACD | * | |||
| HPACD vs. CMACDEp | **** | **** | **** | |
| Sulfated vs. Phosphated | ** | |||
| Sulfated vs. CMACD | *** | |||
| Sulfated vs. SuACD | **** | **** | **** | * |
| Sulfated vs. CMACDEp | **** | **** | **** | |
| Phosphated vs. SuACD | **** | **** | **** | |
| Phosphated vs. AcACD | ** | |||
| Phosphated vs. CMACDEp | **** | **** | **** | **** |
| CMACD vs. SuACD | **** | **** | **** | |
| CMACD vs. AcACD | ** | |||
| CMACD vs. CMACDEp | **** | **** | **** | **** |
| SuACD vs. AcACD | **** | **** | **** | |
| SuACD vs. CMACDEp | **** | **** | **** | |
| AcACD vs. CMACDEp | **** | **** | **** |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vecsernyés, M.; Fenyvesi, F.; Bácskay, I.; Deli, A.M.; Szente, L.; Fenyvesi, É. Cyclodextrins, Blood-Brain-Barrier, and Treatment of Neurological Diseases. Arch. Med. Res. 2014, 45, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Medical applications of cyclodextrins. Med. Res. Rev. 1994, 14, 353–386. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quitschke, W.W.; Steinhauff, N.; Rooney, J. The effect of cyclodextrin-solubilized curcuminoids on amyloid plaques in Alzheimer transgenic mice: Brain uptake and metabolism after intravenous and subcutaneous injection. Alzheimer’s Res. Ther. 2013, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.B.; Artiss, J.D.; Jen, K.L.C.; Karakas, S.E. The beneficial effects α-cyclodextrin on blood lipids and weight loss in healthy humans. Obes. J. 2011, 19, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Quanren, H. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of α-, β-, γ-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; London, E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid extraction. Langmuir 2013, 29, 14631–14638. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, I.; Ueda, H.; Nagase, H.; Endo, T.; Kobayashi, S.; Nagai, T. Physicochemical properties and inclusion complex formation of δ-cyclodextrins. Eur. J. Pharm. Sci. 1995, 3, 153–162. [Google Scholar] [CrossRef]
- Macarak, E.J.; Kumor, K.; Weisz, P.B. Sulfation and hemolytic activity of cyclodextrin. Biochem. Pharmacol. 1991, 42, 1502–1503. [Google Scholar] [CrossRef]
- Kiss, T.; Fenyvesi, F.; Pasztor, N.; Feher, P.; Varadi, J.; Kocsan, R.; Szente, L.; Fenyvesi, E.; Szabo, G.; Vecsernyes, M.; et al. Cytotoxicity of different types of methylated β-cyclodextrins and ionic derivatives. Pharmazie 2007, 62, 557–558. [Google Scholar] [PubMed]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, E.; Iványi, R.; Szente, L.; Tósaki, A.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Debouzy, J.C.; Fauvelle, S.; Crouzy, S.; Girault, L.; Chapron, Y.; Göschl, M.; Gadelle, A. Mechanism of α-cyclodextrin Induced hemolysis. 2. A study of the Factors Controlling the Association with Serine-, Ethanolamine-, and Choline-phospholipids. J. Pharm. Sci. 1998, 87, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, L.; Toropainen, T.; Vihola, H.; Hirvonen, J.; Jarvinen, T.; Jarho, P.; Jarvinen, K. In vitro toxicity and permeation of cyclodextrins in Calu-3 cells. J. Cont. Release 2008, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Leroy-Lechat, F.; Wouessidjewe, D.; Andreux, J.P.; Puisieux, F.; Duchene, D. Evaluation of the cytotoxicity of cyclodextrins and hydroxypropylated derivatives. Int. J. Pharm. 1994, 101, 97–103. [Google Scholar] [CrossRef]
- Uekama, K.; Irie, T.; Sunada, M.; Otagiri, M.; Iwasaki, K.; Okano, Y.; Miyata, T.; Kase, Y. Effects of cyclodextrins on chlorpromazine-induced hemolysis and central nervous system responses. J. Pharm. Pharmacol. 1981, 33, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Puskas, I.; Pápai, K.; Salvador, E.; Roewer, N.; Förster, C.; Broscheit, J.A. Sevoflurane-Sulfobutylether-β-Cyclodextrin Complex: Preparation, Characterization, Cellular Toxicity, Molecular Modeling and Blood-Brain Barrier Transport Studies. Molecules 2015, 20, 10264–10279. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Sohajda, T.; Puskás, I.; Roewer, N.; Förster, C.; Broscheit, J.A. Ionization states, cellular toxicity and molecular modeling studies of midazolam complexed with trimethyl-β-cyclodextrin. Molecules 2014, 19, 16861–16876. [Google Scholar] [CrossRef] [PubMed]
- Valentina, V.; Carmela, C.; Maria, C.C.; Massimo, F.; Domenico, M.; Donatella, P.; Rosanna, S.; Silvana, T.; Cinzia, A.V. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Coll. Surfaces B Biointerfaces 2014, 115, 22–28. [Google Scholar] [CrossRef]
- Rahman, H.S.; Cao, S.; Steadman, S.Y.; Wei, K.J.; Parekh, M. Native and β-cyclodextrin-enclosed curcumin: Entrapment within liposomes and their in vitro cytotoxicity in lung and colon cancer. Drug Deliv. 2012, 19, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Wu, L.; Xu, L.; Lowe, T.L. β-cyclodextrin-poly(β-amino ester) nanoparticles for sustained drug delivery across the blood-brain barrier. Biomacromolecules 2012, 13, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Yaméogo, J.B.; Géze, A.; Choisnard, L.; Putaux, J.L.; Semdé, R.; Wouessidjewe, D. Progress in developing amphiphilic cyclodextrin-based nanodevices for drug delivery. Curr. Top. Med. Chem. 2014, 14, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Marminon, C.; Zeinyeh, W.; Nebois, P.; Bollacke, A.; Jose, J.; Parrot-Lopez, H.; le Borgne, M. Preparation and characterization of CK2 inhibitor-loaded cyclodextrin nanoparticles for drug delivery. Int. J. Pharm. 2013, 441, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [PubMed]
- Seithel, A.; Karlsson, J.; Hilgendorf, C.; Björquist, A.; Ungeil, A.L. Variability in mRNA expression of ABC- and SCL-transporters in human intestinal cells: Comparison between human segments and Caco-2 cells. Eur. J. Pharm. Sci. 2006, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Bourrié, M.; Berger, Y.; Fabre, G. The human intestinal epithelial cell line Caco-2, pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—Fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.Y.; Chen, Y.H.; Liu, T.; Li, C.; Cui, X.; White, R.E.; Cheng, K.C. Evaluation of a novel in vitro Caco-2 hepatocyte hybrid system for predicting in vivo oral bioavailability. Drug Metab. Dispos. 2004, 32, 937–942. [Google Scholar] [PubMed]
- Konsoula, R.; Barile, F.A. Correlation of in vitro cytotoxicity with paracellular permeability in Caco-2 cells. Toxicol. Vitro 2005, 19, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays. Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kürti, L.; Veszelka, S.; Bocsik, A.; Dung, N.T.K.; Ózsvári, B.; Puskás, L.G.; Kittel, Á.; Szabó-Révész, P.; Deli, M.A. The effect of sucrose esters on a culture model of the nasal barrier. Toxicol. In Vitro 2012, 26, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ózsvári, B.; Puskás, G.L.; Nagy, L.I.; Kanizsai, I.; Gyuris, M.; Madácsi, R.; Fehér, L.Z.; Gerö, D.; Szabó, C. A cell-microelectronic sensing technique for the screening of cytoprotective compounds. Int. J. Mol. Med. 2010, 25, 525–530. [Google Scholar] [PubMed]
- Boyd, J.M.; Huang, L.; Xie, L.; Moe, B.; Gabos, S.; Li, X.F. A cell-microelectronic sensing technique for profiling cytotoxicity of chemicals. Anal. Chim. Acta 2008, 615, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.Z.; Zhu, L.; Gabos, S.; Xie, L. Microelectronic cell sensor detection of cytotoxicity and prediction of acute toxicity. Toxicol. In Vitro 2006, 20, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, X.; Xu, X.; Abassi, Y.A. Dynamic and label-free monitoring of natural killer cell cytotoxic activity using cell sensor arrays. J. Immunol. Methods 2006, 309, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Arima, H.; Hirayama, F.; Uekama, K. A moderate Interaction of Maltosyl-α-cyclodextrin with Caco-2 Cells in Comparison with the Parent Cyclodextrin. Biol. Pharm. Bull. 2001, 24, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Laza-Knoerr, A.L.; Gref, R.; Couvreur, P. Cyclodextrins for drug delivery. J. Drug Target 2010, 18, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical Applications of Cyclodextrins. III. Toxicological Issues and Safety Evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Réti-Nagy, K.; Bacsó, Z.; Gutay-Tóth, Z.; Malanga, M.; Fenyvesi, É.; Szente, L.; Váradi, J.; Ujhelyi, Z.; Fehér, P.; et al. Fluorescently Labeled Methyl-β-Cyclodextrin Enters Intestinal Epithelial Caco-2 Cells by Fluid-Phase Endocytosis. PLoS ONE 2014, 9, e84856. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Arima, H.; Toyodome, H.; Irie, T.; Hirayama, F.; Uekama, K. Effect of 2,6-d-O-methyl-α-cyclodextrin on hemolysis and morphological change in rabbit’s red blood cells. Eur. J. Pharm. Sci. 2006, 29, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Laine, V.; Pilard, F.; Gadelle, A.; Defaye, J.; Perly, B. The hemolytic properties of chemically modified cyclodextrins. J. Incl. Phenom. Mol. Recognit. Chem. 1997, 29, 57–63. [Google Scholar] [CrossRef]
- Saarinen-Savolainen, P.; Juarvinen, T.; Araki-Sasaki, K.; Watanabe, H.; Urtti, A. Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cylodextrins in an immortalized human corneal eiphelial cell line. Pharm. Res. 1998, 15, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Bigansoli, E.; Cavenaghi, L.A.; Rossi, R.; Brunati, M.C.; Nolli, M.L. Use of a Caco-2 cell culture model for the characterization of intestinal absorption of antibiotics. Farmaco 1999, 54, 594–599. [Google Scholar] [CrossRef]
- Fagerholm, U. Prediction of human pharmacokinetics-gastrointestinal absorption. J. Pharm. Pharmacol. 2007, 59, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Nornoo, A.O.; Osborne, D.W.; Chow, D.S.L. Cremophor-free intravenous microemulsions for paclitaxel I: Formulation, cytotoxicity and hemolysis. Int. J. Pharm. 2008, 349, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, N.; Garrigue, J.S.; Razafindratsita, A.; Lambert, G.; Benita, S. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J. Pharm. Sci. 2003, 92, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Walter, F.R.; Bocsik, A.; Veszelka, S.; Ozsvári, B.; Puskás, L.G.; Szabó-Révész, P.; Deli, M.A. Kinetic analysis of the toxicity of pharmaceutical excipients Cremophor EL and RH40 on endothelial and epithelial cells. J. Pharm. Sci. 2013, 29. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Cyclodextrin samples used in the above mentioned experiments are available at Cyclolab Ltd. (Illatos út 7, Budapest H-1097, Hungary).
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Róka, E.; Ujhelyi, Z.; Deli, M.; Bocsik, A.; Fenyvesi, É.; Szente, L.; Fenyvesi, F.; Vecsernyés, M.; Váradi, J.; Fehér, P.; et al. Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes. Molecules 2015, 20, 20269-20285. https://doi.org/10.3390/molecules201119694
Róka E, Ujhelyi Z, Deli M, Bocsik A, Fenyvesi É, Szente L, Fenyvesi F, Vecsernyés M, Váradi J, Fehér P, et al. Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes. Molecules. 2015; 20(11):20269-20285. https://doi.org/10.3390/molecules201119694
Chicago/Turabian StyleRóka, Eszter, Zoltán Ujhelyi, Mária Deli, Alexandra Bocsik, Éva Fenyvesi, Lajos Szente, Ferenc Fenyvesi, Miklós Vecsernyés, Judit Váradi, Pálma Fehér, and et al. 2015. "Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes" Molecules 20, no. 11: 20269-20285. https://doi.org/10.3390/molecules201119694