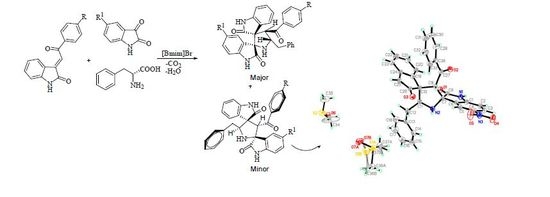
A Novel One-Pot Green Synthesis of Dispirooxindolo-pyrrolidines via 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides
Abstract
:1. Introduction
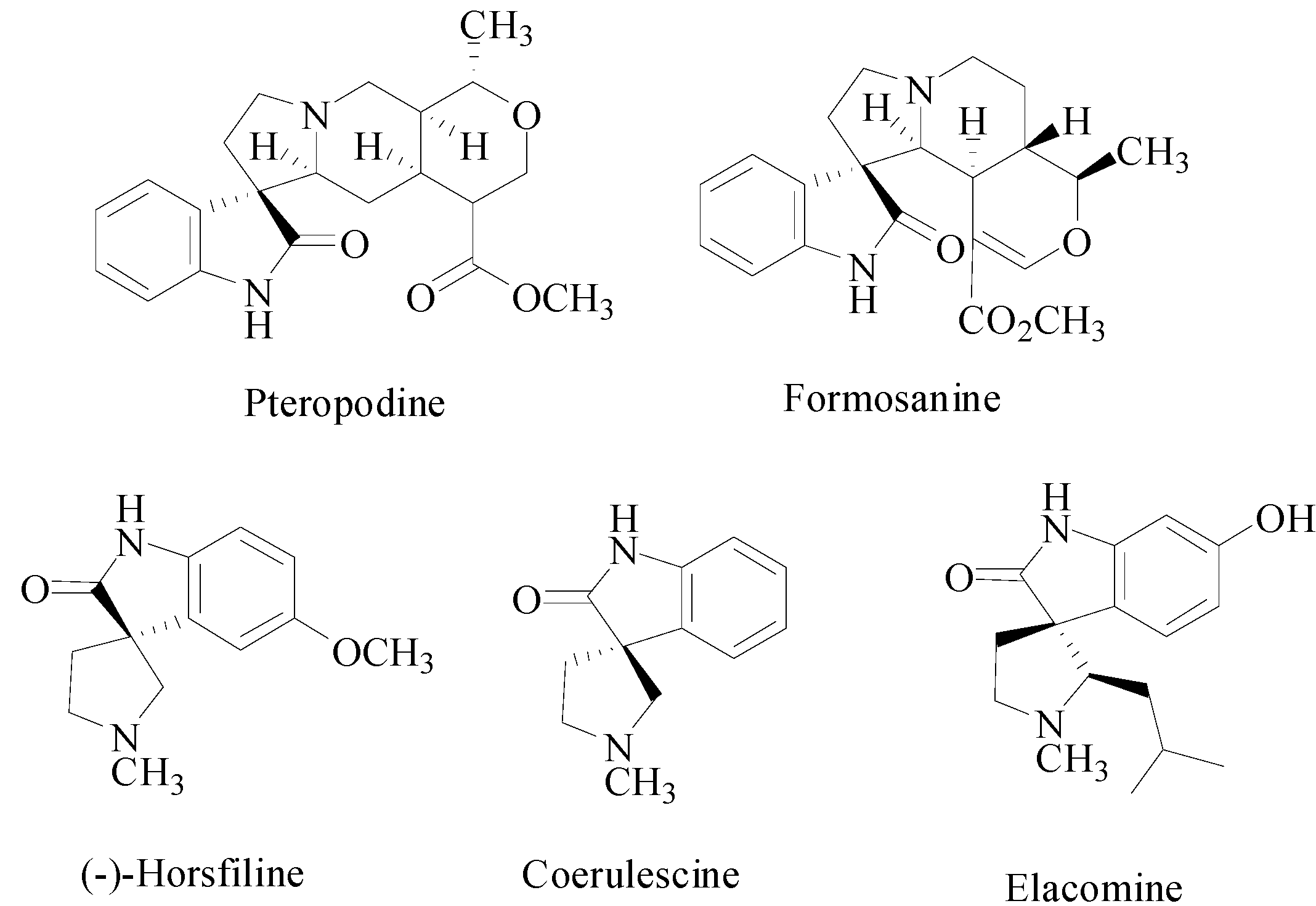
2. Results and Discussion
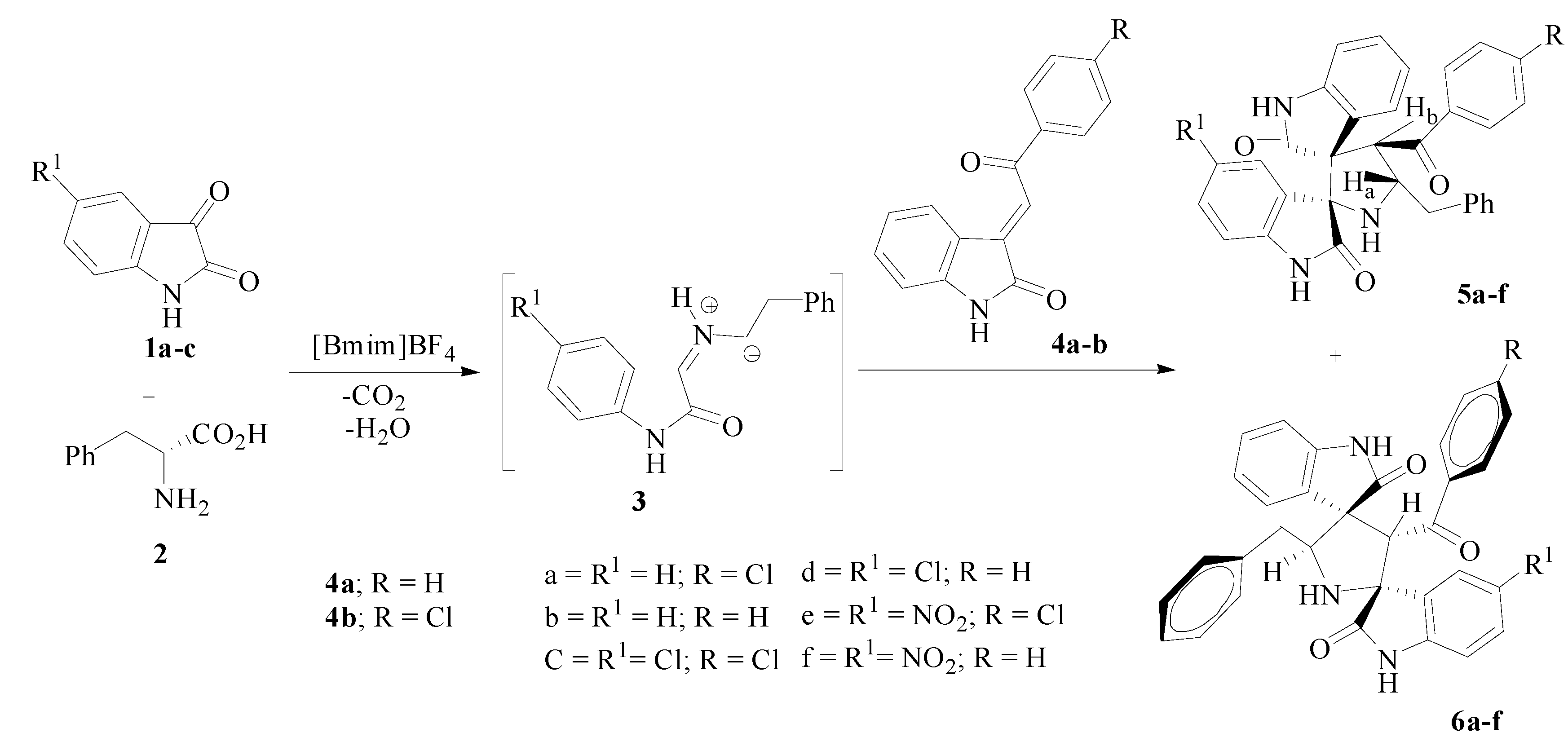
| Entry | Solvent System | Yield (%) | Time (h) | |
|---|---|---|---|---|
| 5a | 6a | |||
| 1 | Methanol | 28 | 12 | 6 |
| 2 | Ethanol | 30 | 15 | 6 |
| 3 | Dry Dioxane | 34 | 16 | 5 |
| 4 | Dioxane/Methanol | 38 | 18 | 4 |
| 5 | [bmim]Br | 69 | 8 | 2 |
| 6 | [bmim][BF4] | 77 | 7 | 2 |
| 7 | [bmim][BF4]/CuI (10 mol %) | 60 | 15 | 2 |
| 8 | [bmim][BF4]/Zn(OTf)2 (10 mol %) | 62 | 18 | 2 |
| Entry | Derivatives (a–f) | Yield of the Cycloadducts (%) # | Diastereoselectivity (5/6) | |
|---|---|---|---|---|
| 5 | 6 | |||
| 1 | a | 77 | 7 | 91/9 |
| 2 | b | 70 | 5 | 85/15 |
| 3 | c | 75 | 6 | 89/11 |
| 4 | d | 73 | 5 | 90/10 |
| 5 | e | 72 | 5 | 88/12 |
| 6 | f | 71 | 5 | 87/13 |
| Experiment | First | Second | Third | Fourth | Fifth |
|---|---|---|---|---|---|
| [Bmim]Br(Yield %) | 69:8 | 67:7 | 63:6 | 61:4 | 60:3 |
| [Bmim]BF4(Yield %) | 77:7 | 75:6 | 72:5 | 68:4 | 65:3 |
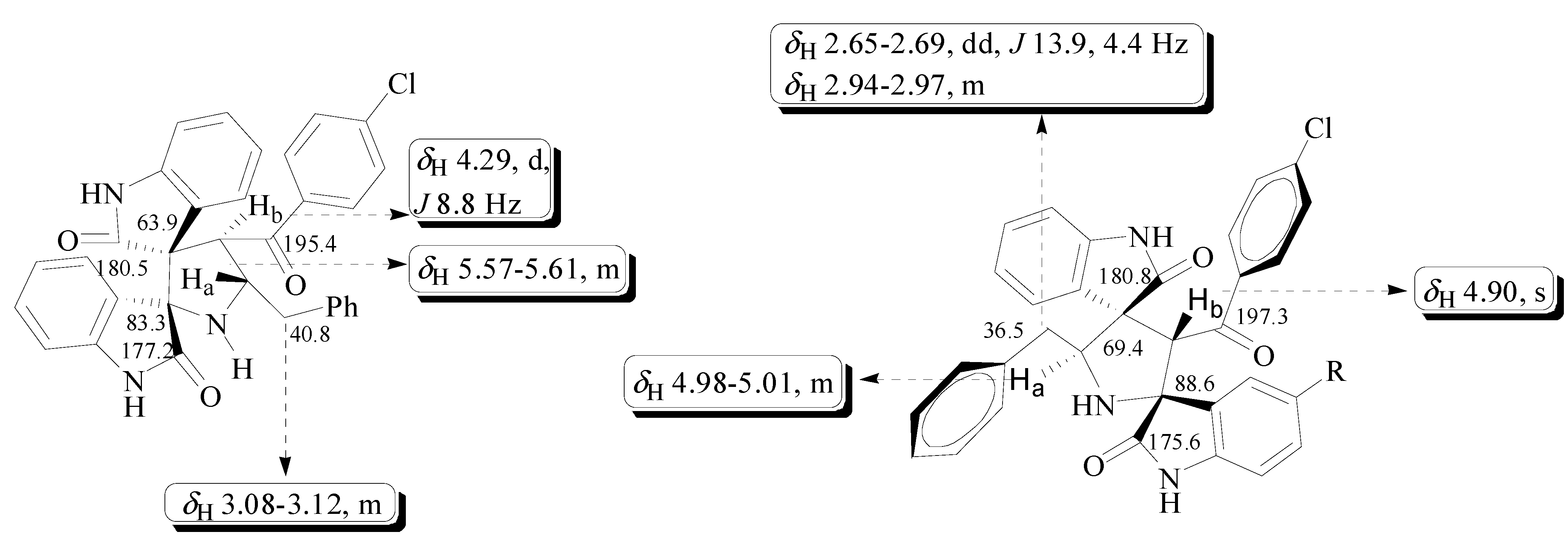
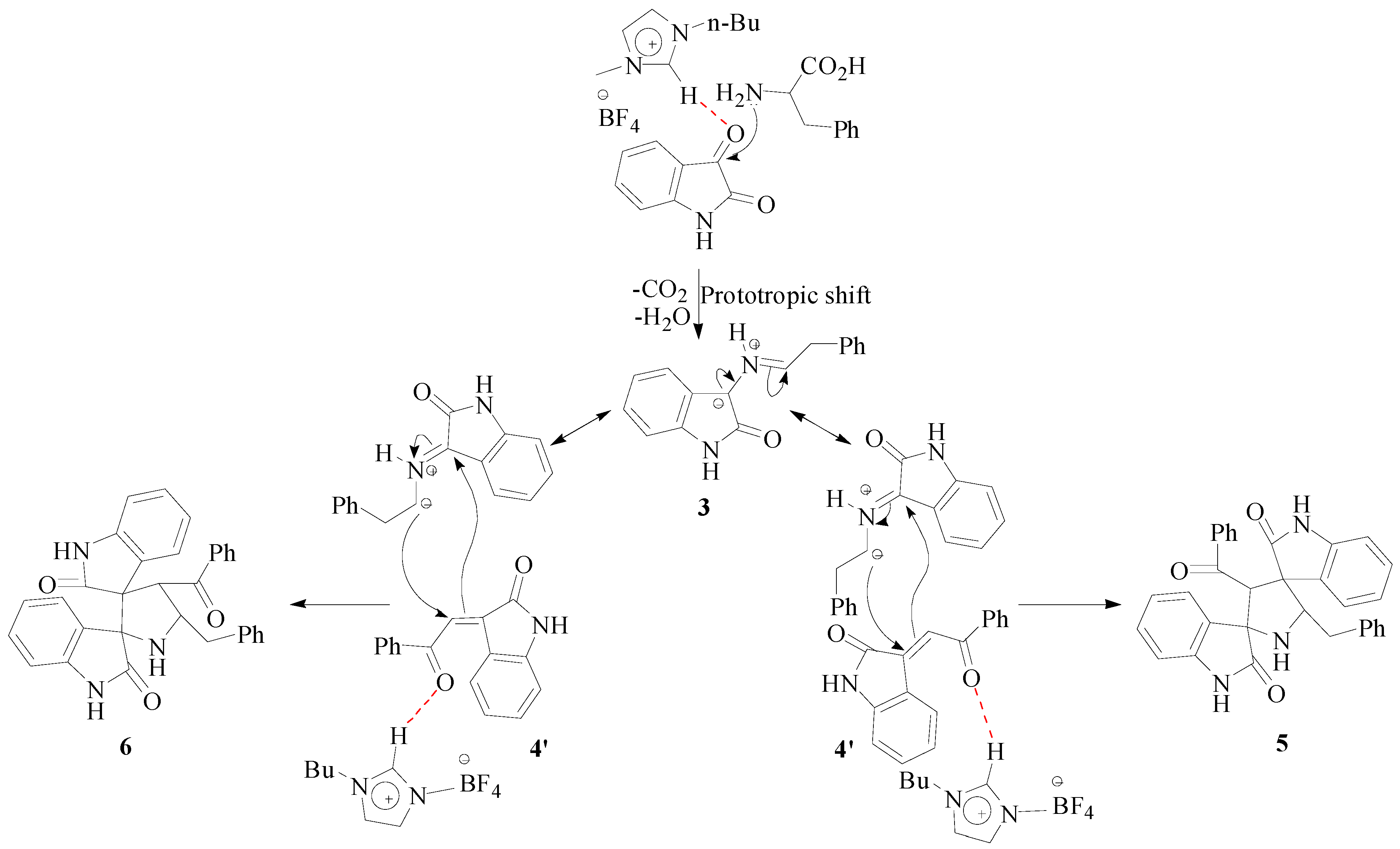
3. Experimental Section
3.1. General Methods
3.2. General Procedure for Synthesis of Dispirooxindoles 5a–f and 6a–f
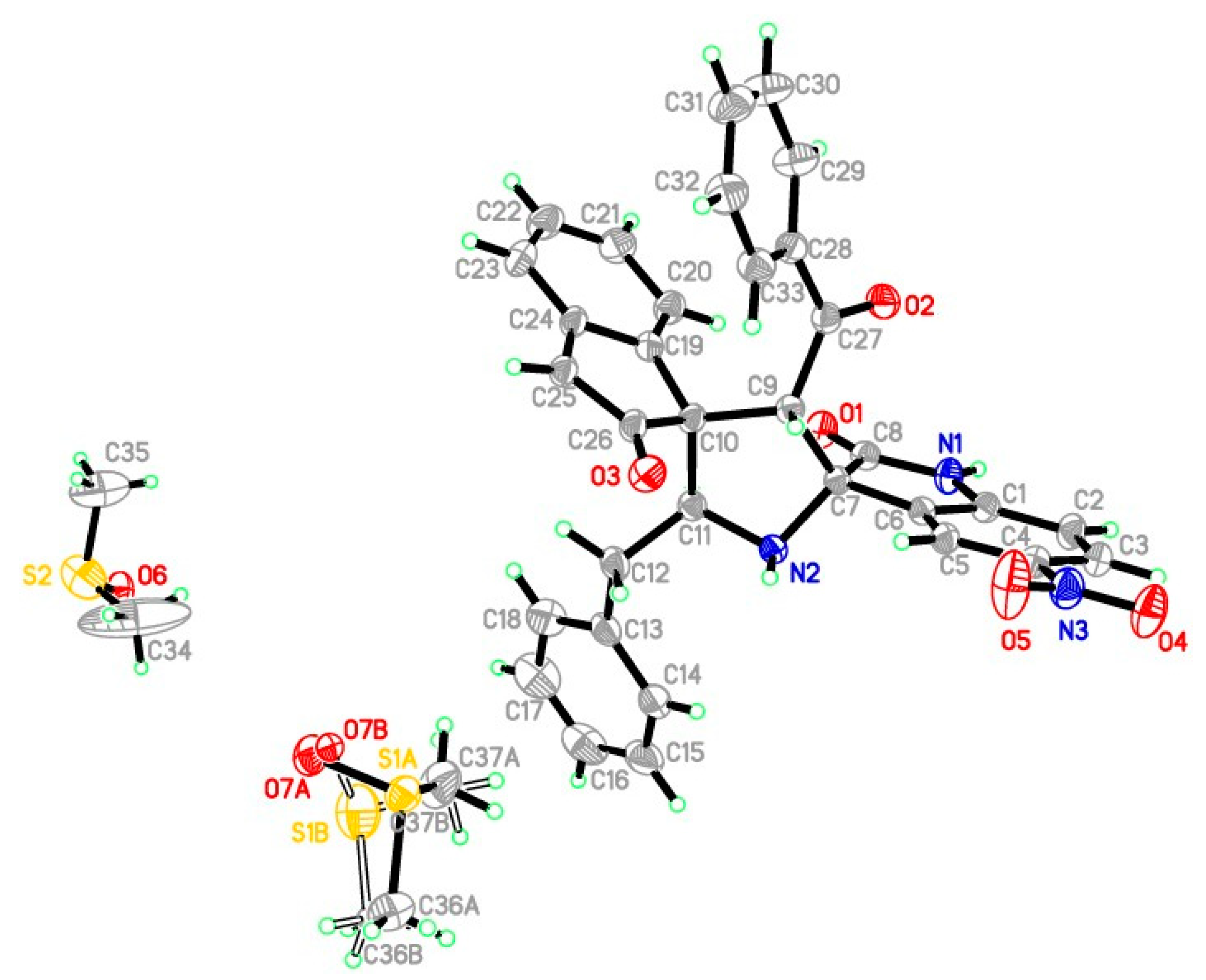
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q. OsO4 in ionic liquid [Bmim]PF6: A recyclable and reusable catalyst system for olefin dihydroxylation. Remarkable effect of DMAP. Org. Lett. 2002, 4, 2197–2199. [Google Scholar] [CrossRef]
- Kumar, A.; Pawar, S.S. Converting exo-selective Diels-Alder reaction to endo-selective in chloroloaluminate ionic liquids. J. Org. Chem. 2004, 69, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, S.A.; Frohlich, U.; Goodrich, P.; Gunaratne, H.Q.N.; Hardacre, C.; McKeown, A.; Seddon, K.R. Functionalised ionic liquids: Synthesis of ionic liquids with tethered basic groups and their use in Heck and Knoevenagel reactions. New J. Chem. 2010, 34, 723–731. [Google Scholar] [CrossRef]
- Gao, J.; Song, Q.-W.; He, L.-N.; Liu, C.; Yang, Z.-Z.; Han, X.; Li, X.-D.; Song, Q.-C. Preparation of polystyrene-supported Lewis acidic Fe(III) ionic liquid and its application in catalytic conversion of carbon dioxide. Tetrahedron 2012, 68, 3835–3842. [Google Scholar] [CrossRef]
- Narayana Kumar, G.G.K.S.; Aridoss, G.; Laali, K.K. Condensation of propargylic alcohols with indoles and carbazole in [bmim][PF6]/Bi(NO3)3·5H2O: A simple high yielding propargylation method with recycling and reuse of the ionic liquid. Tetrahedron Lett. 2012, 53, 3066–3069. [Google Scholar]
- Ramon, D.J.; Yus, M. Asymmetric multicomponent reactions (AMCRs): The new frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar] [CrossRef]
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 2009, 15, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Isambert, N.; Lavilla, R. Heterocycles as key substrates in multicomponent reactions: The fast lane towards molecular complexity. Chem. Eur. J. 2008, 14, 8444–8454. [Google Scholar] [CrossRef] [PubMed]
- Orru, R.V.A.; de Greef, M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003, 1471–1499. [Google Scholar]
- Isambert, N.; Duque, M.M.S.; Plaquevent, J.-C.; Genisson, Y.; Rodriguez, J.; Constantieux, T. For a review of MCRs in ionic liquids, multicomponent reactions and ionic liquids: A perfect synergy for eco-compatible heterocyclic synthesis. Chem. Soc. Rev. 2011, 40, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Okita, T.; Isobe, M. Synthesis of the pentacyclic intermediate for dynemicin A and unusual formation of spiro-oxindole ring. Tetrahedron 1994, 50, 11143–11152. [Google Scholar] [CrossRef]
- Kornet, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Skiles, J.W.; McNeil, D. Spiro indolinone beta-lactams, inhibitors of poliovirus and rhinovirus 3C-proteinases. Tetrahedron Lett. 1990, 31, 7277–7280. [Google Scholar] [CrossRef]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sevenet, T.; Bodo, B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. Engl. 2007, 46, 8748–8758. [Google Scholar] [CrossRef] [PubMed]
- James, M.N.G.; Williams, G.J.B. The molecular and crystal structure of an oxindole alkaloid (6-hydroxy-2'-(2-methylpropyl)-3,3'-spirotetrahydropyrrolidino-oxindole). Can. J. Chem. 1972, 50, 2407–2412. [Google Scholar] [CrossRef]
- Cui, C.-B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Khafagy, M.M.; Abd El-Wahab, A.H.F.; Eid, F.A.; El-Agrody, A.M. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Matsumoto, K.; Tohda, M.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte. Eur. J. Pharmacol. 2002, 444, 39–45. [Google Scholar] [CrossRef] [PubMed]
- García Prado, E.; García Gimenez, M.D.; de la Puerta Vázquez, R.; Espartero Sánchez, J.L.; Sáenz Rodríguez, M.T. Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 2007, 14, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Raghunathan, R. Facile synthesis of β-lactam-grafted spirooxindolopyrrolidine through regioselective 1,3-dipolar cycloaddition reaction. Synth. Commun. 2011, 41, 2747–2755. [Google Scholar] [CrossRef]
- Arumugam, N.; Jayashankaran, J.; Rathna Durga, R.S.M.; Raghunathan, R. A novel access to highly functionalised β-lactams by regio- and stereoselective 1,3-dipolar cycloaddition reaction. Tetrahedron 2005, 61, 8512–8516. [Google Scholar] [CrossRef]
- Arumugam, N.; Raghunathan, R.; Shanmugaiah, V.; Mathivanan, N. Synthesis of novel β-lactam fused spiroisoxazolidine chromanones and tetralones as potent antimicrobial agent for human and plant pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Periyasami, G.; Raghunathan, R.; Kamalraj, S.; Muthumary, J. Synthesis and antimicrobial activity of highly functionalised novel β-lactam grafted spiropyrrolidines and pyrrolizidines. Eur. J. Med. Chem. 2011, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Raghunathan, R.; Almansour, A.I.; Karama, U. An efficient synthesis of highly functionalized novel chromeno[4,3-b]pyrroles and indolizino[6,7-b]indoles as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Perumal, S.; Ghabbour, H.A.; Fun, H.-K. A 1,3-dipolar cycloaddition–annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron Lett. 2013, 55, 2515–2519. [Google Scholar] [CrossRef]
- Suresh Babu, A.R.; Raghunathan, R. ZrOCl·8H2O mediated microwave induced [3+2] cycloaddition of azomethine ylides-a facile one-pot synthesis of novel dispiroheterocycles. Tetrahedron Lett. 2007, 48, 305–308. [Google Scholar] [CrossRef]
- Suresh Babu, A.R.; Raghunathan, R. Ultrasonic assisted-silica mediated [3+2] cycloaddition of azomethine ylides-a facile multicomponent one-pot synthesis of novel dispiroheterocycles. Tetrahedron Lett. 2007, 48, 6809–6813. [Google Scholar] [CrossRef]
- Suresh Babu, A.R.; Raghunathan, R. An easy access to novel steroidal dispiropyrrolidines through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett. 2008, 49, 4618–4620. [Google Scholar] [CrossRef]
- Crystallographic data (excluding structure factors) for dispiro compound 6f in this article have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 1026186–1026187. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44 (0)1223 336033 or e-mail: [email protected]].
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Periyasami, G.; A. Ghabbour, H.; Fun, H.-K. A Novel One-Pot Green Synthesis of Dispirooxindolo-pyrrolidines via 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides. Molecules 2015, 20, 780-791. https://doi.org/10.3390/molecules20010780
Almansour AI, Arumugam N, Kumar RS, Periyasami G, A. Ghabbour H, Fun H-K. A Novel One-Pot Green Synthesis of Dispirooxindolo-pyrrolidines via 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides. Molecules. 2015; 20(1):780-791. https://doi.org/10.3390/molecules20010780
Chicago/Turabian StyleAlmansour, Abdulrahman I., Natarajan Arumugam, Raju Suresh Kumar, Govindasami Periyasami, Hazem A. Ghabbour, and Hoong-Kun Fun. 2015. "A Novel One-Pot Green Synthesis of Dispirooxindolo-pyrrolidines via 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides" Molecules 20, no. 1: 780-791. https://doi.org/10.3390/molecules20010780