Stem Bark Extract and Fraction of Persea americana (Mill.) Exhibits Bactericidal Activities against Strains of Bacillus cereus Associated with Food Poisoning
Abstract
:1. Introduction
2. Results
| Bacterial Codes | Zones of Inhibition (mm) ** | ||||
|---|---|---|---|---|---|
| BL (10 mg/mL) | AQ (10 mg/mL) | HX (10 mg/mL) | CL (10 mg/mL) | EA (10 mg/mL) | |
| B1 | 20 ± 0.94 | 0 | 0 | 0 | 0 |
| B2 | 19 ± 0.82 | 0 | 0 | 0 | 0 |
| B3 | 22 ± 0.00 | 0 | 12.67 ± 0.94 | 0 | 14.67 ± 0.94 |
| B4 | 17 ± 0.62 | 0 | 0 | 0 | 0 |
| B5 | 24 ± 0.94 | 00 ± 0.00 | 0 | 0 | 0 |
| B6 | 12 ± 0.00 | 0 | 0 | 0 | 0 |
| B7 | 13 ± 0.24 | 11.67 ± 0.47 | 0 | 0 | 0 |
| B8 | 16 ± 0.41 | 10.67 ± 0.94 | 0 | 0 | 0 |
| B9 | 17 ± 0.82 | 0 | 0 | 0 | 0 |
| B10 | 16 ± 0.24 | 0 | 0 | 0 | 0 |
| B11 | 16 ± 0.00 | 0 | 0 | 0 | 0 |
| B12 | 18 ± 0.47 | 0 | 0 | 0 | 0 |
| B13 | 14 ± 0.62 | 0 | 0 | 0 | 0 |
| B14 | 19 ± 0.82 | 0 | 0 | 0 | 0 |
| B15 | 14 ± 0.00 | 0 | 0 | 0 | 0 |
| B16 | 18 ± 0.00 | 0 | 0 | 0 | 0 |
| B17 | 24 ± 0.94 | 0 | 0 | 0 | 0 |
| B18 | 25 ± 0.94 | 0 | 0 | 0 | 0 |
| B19 | 14 ± 0.00 | 0 | 0 | 0 | 0 |
| B20 | 15 ± 0.94 | 0 | 0 | 0 | 0 |
| B21 | 22 ± 0.00 | 10.67 ± 0.94 | 0 | 0 | 0 |
| B22 | 25 ± 0.47 | 0 | 0 | 0 | 0 |
| B23 | 23 ± 0.94 | 0 | 0 | 0 | 0 |
| B24 | 19 ± 0.82 | 0 | 0 | 0 | 0 |
| B25 | 23 ± 0.82 | 0 | 0 | 0 | 0 |
| B26 | 16 ± 0.00 | 0 | 0 | 0 | 0 |
| B27 | 26 ± 0.47 | 0 | 0 | 0 | 0 |
| B28 | 16 ± 0.94 | 0 | 0 | 0 | 0 |
| B29 | 18 ± 0.94 | 0 | 0 | 0 | 0 |
| B30 | 23 ± 0.47 | 0 | 0 | 0 | 0 |
| B31 | 22 ± 0.47 | 0 | 0 | 0 | 0 |
| B32 | 17 ± 0.82 | 0 | 0 | 0 | 0 |
| B33 | 20 ± 0.00 | 0 | 0 | 0 | 0 |
| Bacterial Code | Zones of Inhibition (mm) ** | ||
|---|---|---|---|
| Crude Extract (25 mg/mL) | Streptomycin (1 mg/mL) | Ampicillin (1 mg/mL) | |
| B1 | 22 ± 0.41 | 23 ± 0.47 | 22 ± 0.00 |
| B2 | 17 ± 0.47 | 22 ± 0.00 | 25 ± 0.94 |
| B3 | 15 ± 0.94 | 20 ± 0.47 | 26 ± 0.00 |
| B4 | 16 ± 0.94 | 24 ± 0.94 | 28 ± 0.00 |
| B5 | 10 ± 0.47 | 28 ± 0.00 | 26 ± 0.00 |
| B6 | 11 ± 0.94 | 27 ± 0.82 | 24 ± 0.00 |
| B7 | 13 ± 0.82 | 20 ± 0.94 | 24 ± 0.00 |
| B8 | 11 ± 0.47 | 20 ± 0.47 | 24 ± 0.82 |
| B9 | 16 ± 0.00 | 26 ± 0.00 | 25 ± 0.82 |
| B10 | 13 ± 0.94 | 24 ± 0.00 | 24 ± 0.00 |
| B11 | 17 ± 0.94 | 28 ± 0.00 | 23 ± 0.82 |
| B12 | 16 ± 0.00 | 25 ± 0.47 | 20 ± 0.00 |
| B13 | 15 ± 0.47 | 25 ± 0.47 | 22 ± 0.94 |
| B14 | 11 ± 0.41 | 22 ± 0.94 | 28 ± 0.00 |
| B15 | 15 ± 0.47 | 23 ± 0.47 | 22 ± 0.00 |
| B16 | 12 ± 0.00 | 23 ± 0.94 | 26 ± 0.00 |
| B17 | 17 ± 0.94 | 26 ± 0.00 | 24 ± 0.00 |
| B18 | 12 ± 0.00 | 27 ± 0.82 | 22 ± 0.00 |
| B19 | 16 ± 0.94 | 26 ± 0.00 | 21 ± 0.47 |
| B20 | 14 ± 0.00 | 22 ± 0.00 | 24 ± 0.00 |
| B21 | 17 ± 0.94 | 25 ± 0.94 | 26 ± 0.00 |
| B22 | 16 ± 0.94 | 25 ± 0.94 | 24 ± 0.00 |
| B23 | 17 ± 0.47 | 24 ± 0.00 | 26 ± 0.94 |
| B24 | 15 ± 0.82 | 26 ± 0.00 | 22 ± 0.00 |
| B25 | 13 ± 0.94 | 24 ± 0.00 | 24 ± 0.00 |
| B26 | 14 ± 0.00 | 22 ± 0.94 | 26 ± 0.00 |
| B27 | 13 ± 0.47 | 25 ± 0.82 | 23 ± 0.47 |
| B28 | 13 ± 0.94 | 26 ± 0.00 | 26 ± 0.00 |
| B29 | 11 ± 0.82 | 25 ± 0.94 | 24 ± 0.00 |
| B30 | 18 ± 0.94 | 23 ± 0.94 | 25 ± 0.47 |
| B31 | 15 ± 0.94 | 25 ± 0.94 | 26 ± 0.00 |
| B32 | 19 ± 0.94 | 22 ± 0.00 | 26 ± 0.00 |
| B33 | 17 ± 0.82 | 24 ± 0.00 | 26 ± 0.00 |
| Bacterial Code | Crude Extract (25 mg/mL) | Butanol Fraction (10 mg/mL) | Streptomycin (1 mg/mL) | Ampicillin (1 mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| B1 | 3.12 | 6.25 | 1.25 | 2.5 | 0.06 | 0.12 | 0.25 | 0.50 |
| B2 | 1.56 | 3.12 | 0.63 | 1.25 | 0.06 | 0.25 | 0.03 | 0.06 |
| B3 | 1.56 | 3.12 | 2.50 | 5.00 | 0.12 | 0.25 | 0.12 | 0.12 |
| B4 | 3.12 | 6.25 | 2.50 | 5.00 | 0.03 | 0.06 | 0.06 | 0.25 |
| B5 | 0.78 | 3.12 | 0.63 | 1.25 | 0.06 | 0.12 | 0.25 | 0.50 |
| B6 | 6.25 | 12.50 | 0.63 | 1.25 | 0.06 | 0.12 | 0.06 | 0.12 |
| B7 | 6.25 | 6.25 | 0.62 | 2.50 | 0.25 | 0.25 | 0.12 | 0.25 |
| B8 | 1.56 | 6.25 | 2.50 | 2.50 | 0.03 | 0.12 | 0.12 | 0.25 |
| B9 | 3.12 | 6.25 | 1.25 | 1.25 | 0.03 | 0.12 | 0.25 | 0.50 |
| B10 | 6.25 | 12.50 | 0.62 | 1.25 | 0.25 | 0.50 | 0.06 | 0.12 |
| B11 | 6.25 | 6.25 | 1.25 | 5.00 | 0.25 | 0.50 | 0.06 | 0.12 |
| B12 | 6.25 | 6.25 | 0.62 | 1.25 | 0.06 | 0.12 | 0.12 | 0.25 |
| B13 | 12.50 | 12.50 | 10.00 | 10.00 | 0.25 | 0.50 | 0.06 | 0.12 |
| B14 | 3.12 | 6.25 | 2.50 | 5.00 | 0.25 | 0.50 | 0.06 | 0.12 |
| B15 | 3.12 | 12.50 | 0.63 | 2.50 | 0.12 | 0.50 | 0.03 | 0.06 |
| B16 | 1.56 | 3.12 | 5.00 | 5.00 | 0.06 | 0.25 | 0.12 | 0.25 |
| B17 | 1.56 | 3.12 | 1.25 | 2.50 | 0.12 | 0.25 | 0.12 | 0.25 |
| B18 | 6.25 | 6.25 | 1.25 | 2.50 | 0.12 | 0.25 | 0.12 | 0.25 |
| B19 | 6.25 | 12.50 | 10.00 | 10.00 | 0.06 | 0.12 | 0.06 | 0.12 |
| B20 | 1.56 | 3.12 | 1.25 | 2.50 | 0.12 | 0.25 | 0.12 | 0.25 |
| B21 | 6.25 | 6.25 | 1.25 | 2.50 | 0.12 | 0.50 | 0.12 | 0.12 |
| B22 | 6.25 | 12.50 | 2.50 | 5.00 | 0.12 | 0.25 | 0.06 | 0.25 |
| B23 | 1.56 | 3.12 | 0.63 | 1.25 | 0.31 | 0.12 | 0.12 | 0.25 |
| B24 | 3.12 | 6.25 | 5.00 | 10.00 | 0.25 | 0.50 | 0.06 | 0.12 |
| B25 | 12.5 | 12.50 | 1.25 | 2.50 | 0.06 | 0.25 | 0.25 | 0.50 |
| B26 | 6.25 | 12.50 | 10.00 | 10.00 | 0.25 | 0.25 | 0.06 | 0.25 |
| B27 | 3.12 | 6.25 | 1.25 | 2.50 | 0.12 | 0.25 | 0.25 | 0.25 |
| B28 | 0.78 | 3.12 | 1.25 | 5.00 | 0.12 | 0.50 | 0.03 | 0.12 |
| B29 | 6.25 | 6.25 | 0.63 | 1.25 | 0.06 | 0.12 | 0.06 | 0.12 |
| B30 | 1.56 | 6.25 | 2.50 | 2.50 | 0.25 | 0.25 | 0.06 | 0.12 |
| B31 | 6.25 | 6.25 | 1.25 | 2.50 | 0.12 | 0.12 | 0.03 | 0.12 |
| B32 | 3.12 | 6.25 | 2.50 | 2.50 | 0.25 | 0.50 | 0.12 | 0.12 |
| B33 | 6.25 | 12.50 | 1.25 | 5.00 | 0.25 | 0.50 | 0.06 | 0.12 |
| Chemical Test | Result |
|---|---|
| Alkaloids | Positive |
| Tannins | Positive |
| Saponins | Positive |
| Flavonoid | Positive |
| Reducing sugar | Positive |
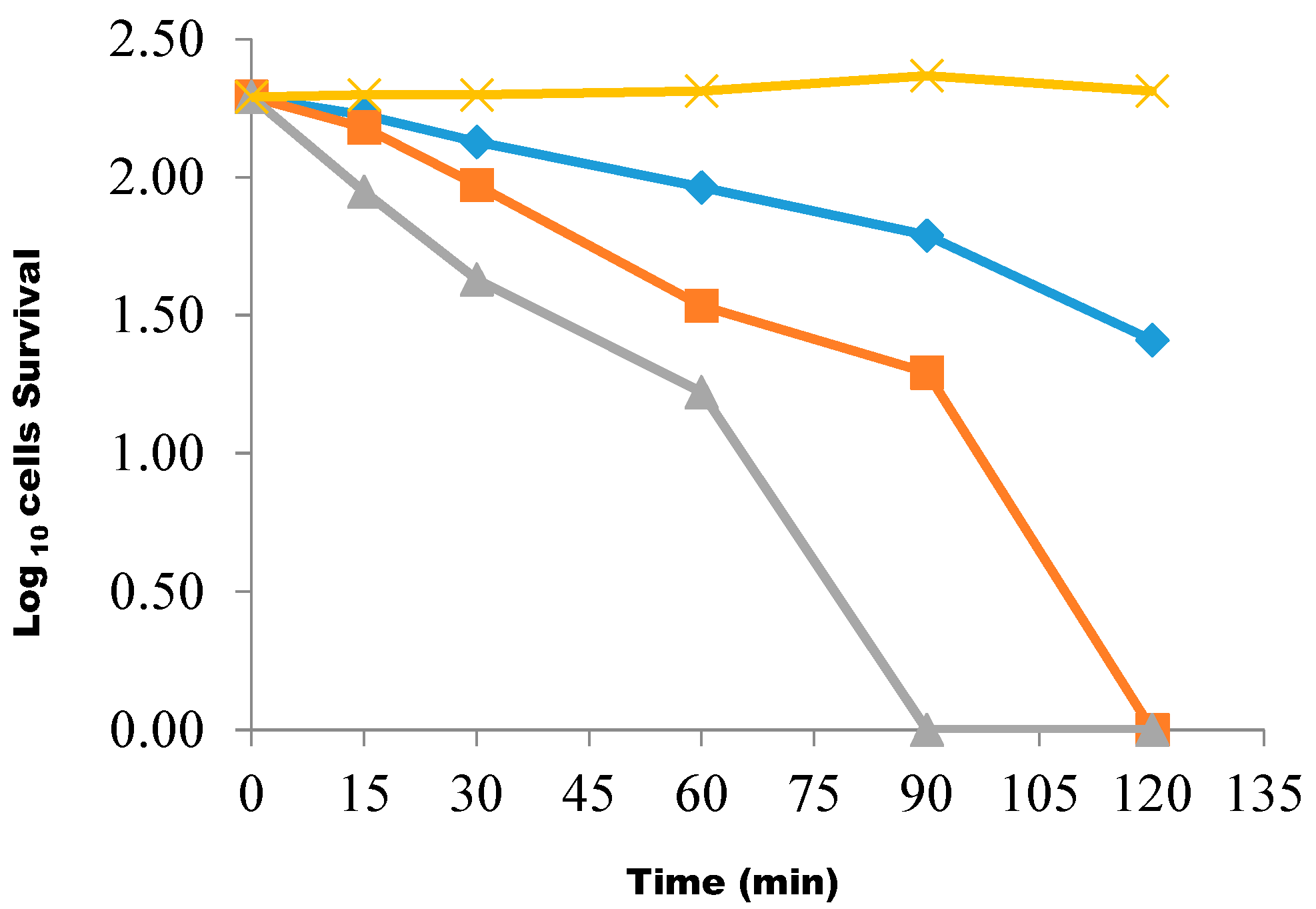
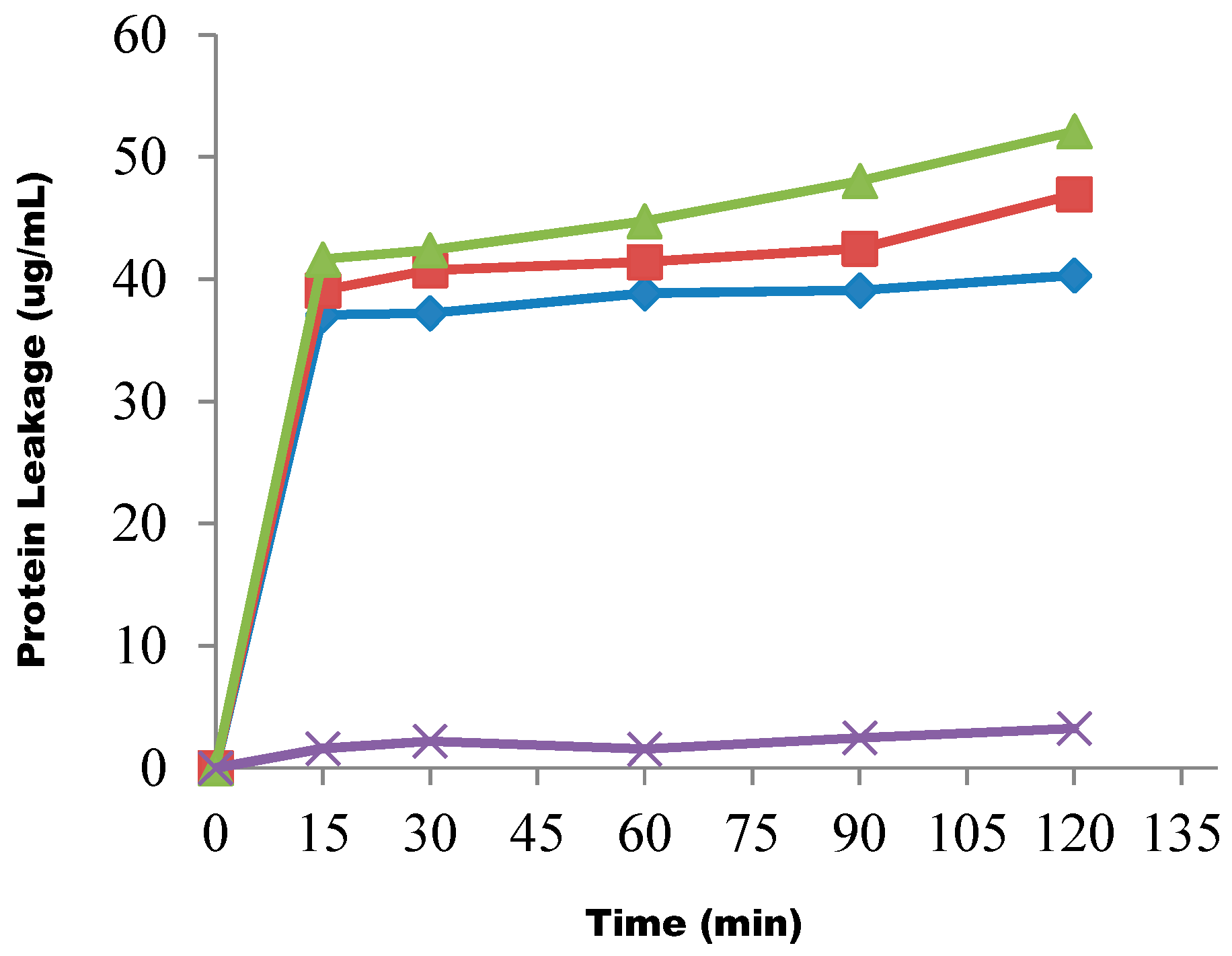

3. Discussion
4. Experimental Section
4.1. Plant Materials
4.2. Preparation of the Plant Extract
4.3. Preparation of Test Bacterial Isolates
4.4. Culture Media Used
4.5. Phytochemical Assays
4.6. Fractionation of Crude Extract of the Plant Sample
4.7. The Antibiograms of P. americana Crude Stem Bark Extract, Fractions and the Standard Antibiotics—Streptomycin and Ampicillin—Against Test Isolates
4.8. Determination of Minimum Inhibitory Concentrations (MICs) of the Extract and Fractions of P. americana
4.9. Determination of Minimum Bactericidal Concentrations of the Extract and Fractions of P. americana
4.10. Determination of the Rate of Killing of the Test Isolate Using the Butanolic Fraction
4.11. Determination of Protein Leakage from the Test Organisms
4.12. Determination of Potassium Ion Leakage from the Test Organisms
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edeoga, J.H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Pelczar, M.J.; Chan, E.C.; Kruz, N.R. Microbiology,, 5th ed.; Tata McGraw-Hill Publ. Co. Ltd.: New Delhi, India, 2006; pp. 826–829. [Google Scholar]
- Schlundt, J.; Toyofuku, H.; Jansen, J.; Herbst, S.A. Emerging foodborne zoonoses. Rev. Sci. Technol. 2004, 23, 513–515. [Google Scholar]
- Eglezos, S.; Huang, B.; Dykes, E.A.; Fegan, N. The prevalence and concentration of Bacillus cereus in retail food products in Brisbane, Australia. Foodborne Pathog. Dis. 2010, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, P.C.B.; Sirianni, N.M.; Le Bron, C.I.; Samaan, M.N.; Sutton, F.N.; Reyes, A.E.; Peruski, L.F. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringensis and Bacillus mycoides from a range of clinical and environmental sources as determined by the E test. J. Clin. Microbiol. 2004, 42, 3626–3634. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Sogn, C.; Samake, F.B.; Paulsen, B.S.; Michaelsen, T.F.; Keita, A. Wound healing plants in Mali, the Bamako region; an ethnobotanical survey and complement fixation of water extracts from selected plants. Pharm. Biol. 2002, 40, 117–128. [Google Scholar] [CrossRef]
- Navel, E.; Nerman, M.J.; Sabo, E.; Neeman, E. Defatted Avocado pulp reduced body weight and total hepatic fat but increased plasma cholesterol in male rats fed diets with cholesterol. J. Nutr. 2002, 131, 2015–2018. [Google Scholar]
- Anaka, O.N.; Ozohua, R.I.; Okpo, S.O. Effect of the aqueous seed extract of Persea americana Mill. (Lauraceae) on the blood pressure of sprague dawley rats. Afr. J. Pharm. Pharmacol. 2009, 3, 485–490. [Google Scholar]
- Lu, Q.Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.I.; Hober, D. Inhibition of prostate cancer growth by an avocado extract: Role of lipid—Soluble bioactive substances. J. Nutr. Biochem. 2005, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pamplona-Roger, G.D. Foods that Heal; Review and Herald Publishing Association: Hagerstown, MD, USA, 2004; pp. 11–16. [Google Scholar]
- Adeyemi, O.O.; Okpo, S.O.; Oguti, O.O. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill. (Lauraceae). Fitoterapia 2002, 2, 375–380. [Google Scholar] [CrossRef]
- Oelrichs, P.B.; Ng, J.C.; Seawright, A.A.; Ward, A.; Schaffeler, L.; MacLeod, J.K. Isolation and identification of a compound from avocado leaves which causes necrosis of the acinar epithelium of lactating mammary gland and the myocardium. Natl. Toxins 1995, 3, 344–349. [Google Scholar] [CrossRef]
- Owolabi, M.A.; Jaja, S.I.; Coker, H.A. Vasorelaxant action of aqueous extract of the leaves of Persea americana on isolated thoracic rat aorta. Fitoterapia 2005, 76, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Trease, G.E.; Evans, W.C. Textbook of Pharmacognosy; Balliere: Tindall, London, UK, 2002; pp. 57–59, 343–383. [Google Scholar]
- Aherne, S.A.; Kerry, J.P.; O’Brien, N.M. Effects of plant extracts on antioxidant induced stress in Caco-2 cells. Br. J. Nutr. 2007, 97, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic against bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.T.; Wang, R.; Helmann, J.D. Antibiotic that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol. Microbiol. 2002, 45, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Sanati, H.; Belanger, P.R.; Fratt, R.; Ghannoun, M. A new triazole, voriconazole (UK-109, 496) blocks sterol biosynthesis in Candida albicans and Candiada krussi. Antimicrob. Agents Chemother. 1997, 41, 2492–2496. [Google Scholar] [PubMed]
- Harborne, J.B. Phytochemical Methods-Guide to Modern Techniques of Plant Analysisl; Chapman & Hall: London, UK, 1998; pp. 60–66. [Google Scholar]
- Russell, A.D.; Furr, J.R. The antibacterial activity of a new chloroxylenol preparation containing ethylenediamine tetra-acetic acid. J. Appl. Bacteriol. 1977, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Irobi, O.N.; Moo-Young, M.; Anderson, W.A.; Daramola, S.O. Antimicrobial activity of the bark of Bridelia ferruginea (Euphobiaceae). Int. J. Pharmacol. 1994, 34, 87–90. [Google Scholar] [CrossRef]
- Akinpelu, D.A.; Kolawole, D.O. Phytochemical and antimicrobial activity of leaf extract of Piliostigma thonningii (Schum.). Sci. Focus J. 2004, 7, 64–70. [Google Scholar]
- Olorundare, E.E.; Emudianugbe, T.S.; Khaar, G.S.; Kuteyi, S.A.; Irobi, D.N. Antibacterial Properties of Leaf Extract of Cassia alata. Biol. Res. Commun. 1992, 4, 113–117. [Google Scholar]
- Odenholt, I.; Lowdin, E.; Cars, O. Pharmacodynamics of telithromycin in vitro against respiratory tract pathogens. Antimicrob. Agents Chemother. 2001, 45, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of protein-dye binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extract and the fraction are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinpelu, D.A.; Aiyegoro, O.A.; Akinpelu, O.F.; Okoh, A.I. Stem Bark Extract and Fraction of Persea americana (Mill.) Exhibits Bactericidal Activities against Strains of Bacillus cereus Associated with Food Poisoning. Molecules 2015, 20, 416-429. https://doi.org/10.3390/molecules20010416
Akinpelu DA, Aiyegoro OA, Akinpelu OF, Okoh AI. Stem Bark Extract and Fraction of Persea americana (Mill.) Exhibits Bactericidal Activities against Strains of Bacillus cereus Associated with Food Poisoning. Molecules. 2015; 20(1):416-429. https://doi.org/10.3390/molecules20010416
Chicago/Turabian StyleAkinpelu, David A., Olayinka A. Aiyegoro, Oluseun F. Akinpelu, and Anthony I. Okoh. 2015. "Stem Bark Extract and Fraction of Persea americana (Mill.) Exhibits Bactericidal Activities against Strains of Bacillus cereus Associated with Food Poisoning" Molecules 20, no. 1: 416-429. https://doi.org/10.3390/molecules20010416






