New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium
Abstract
:1. Introduction
2. Results and Discussion
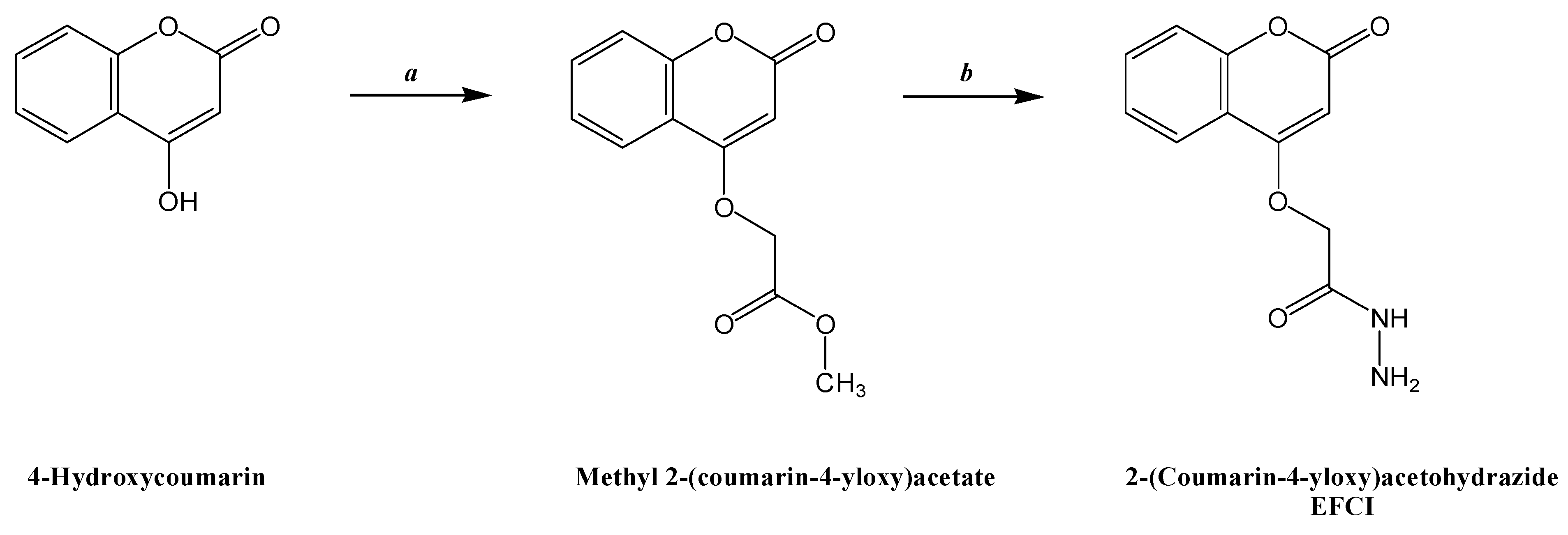
2.1. Chemistry
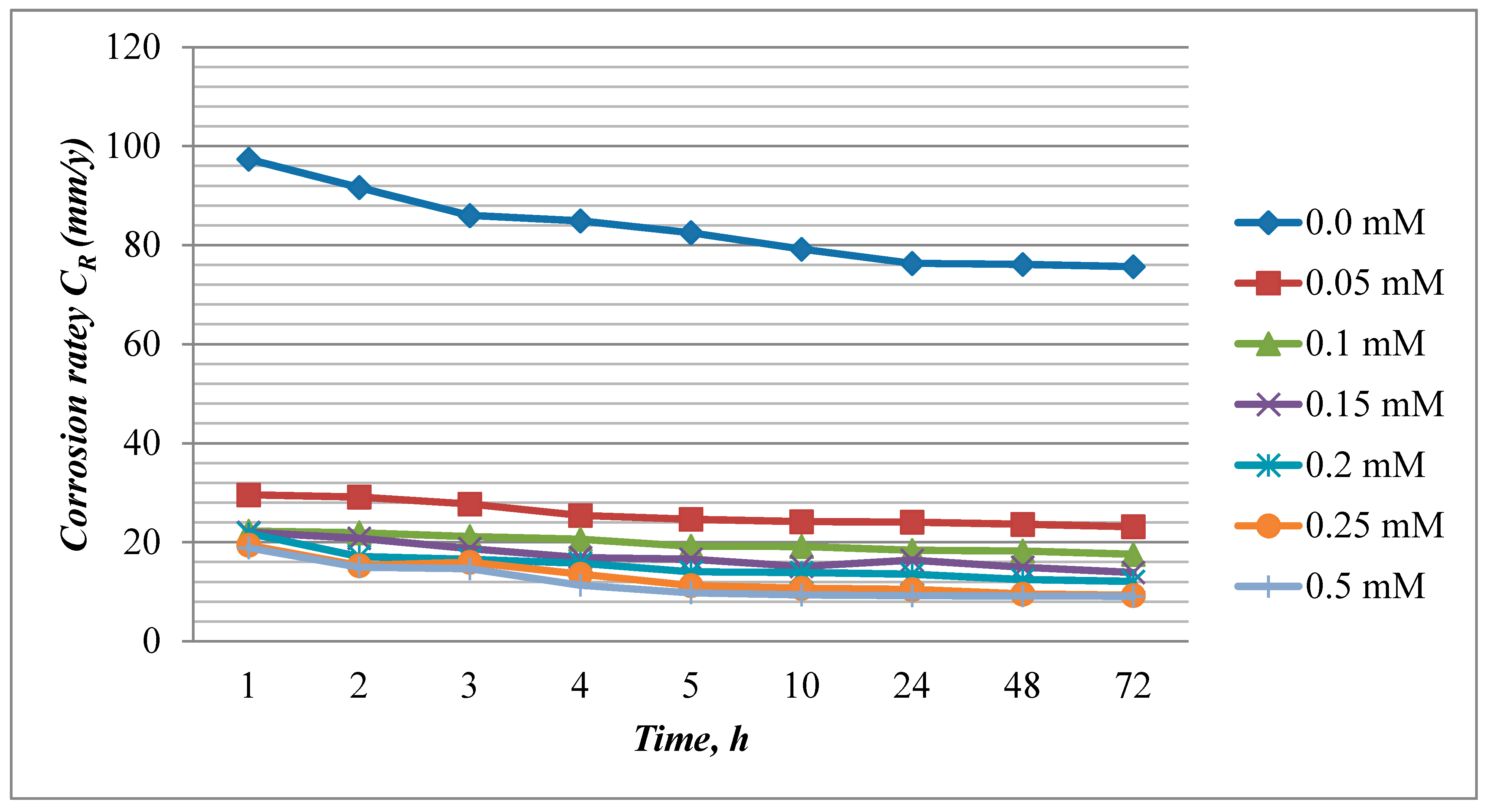
2.2. Weight Loss Method
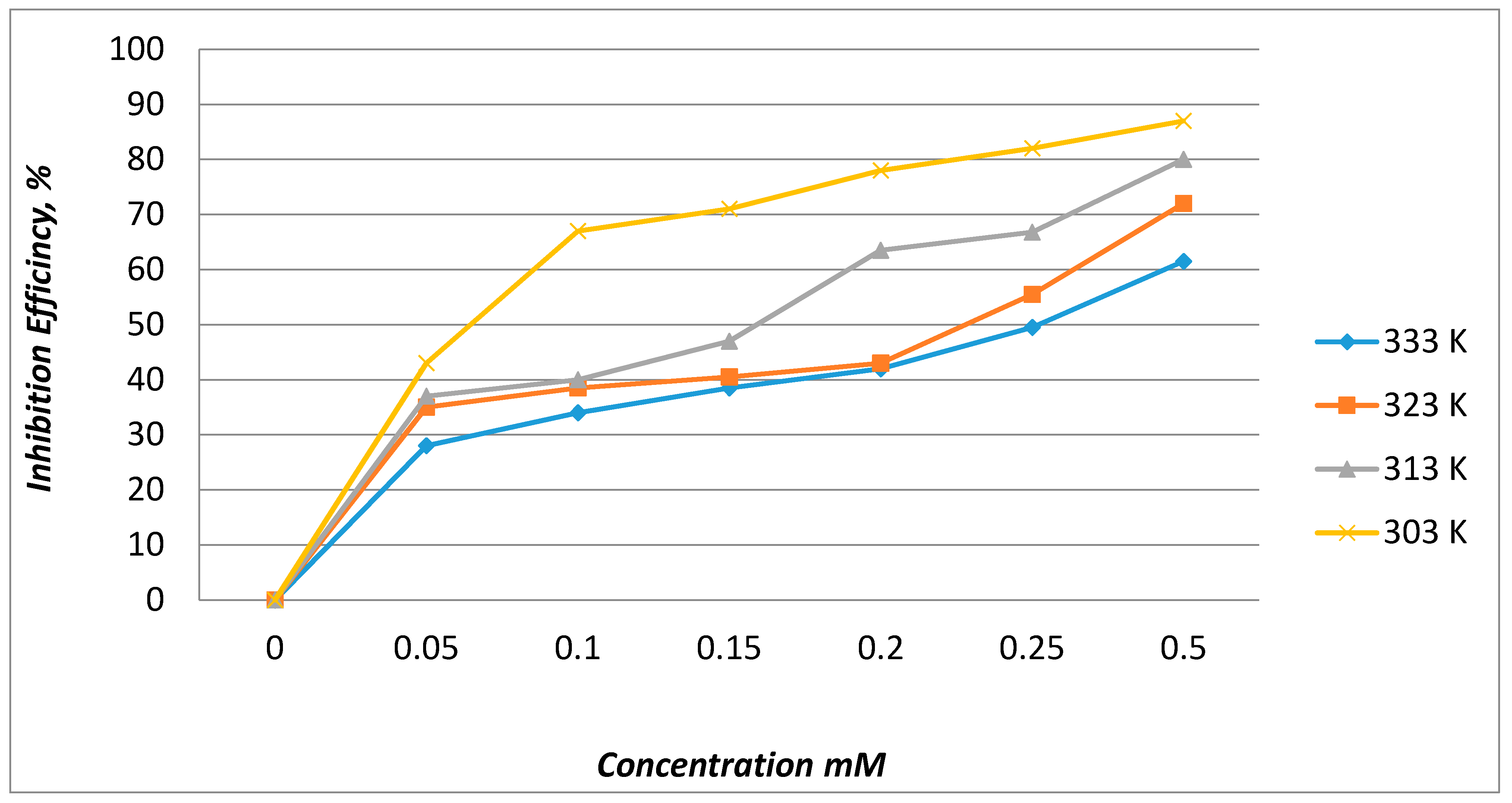
2.2.1. Effect of Concentration

2.2.2. Effect of Temperature
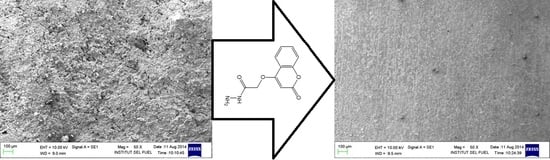
2.3. Scanning Electron Microscopy (SEM) Analysis

2.4. Adsorption Isotherm
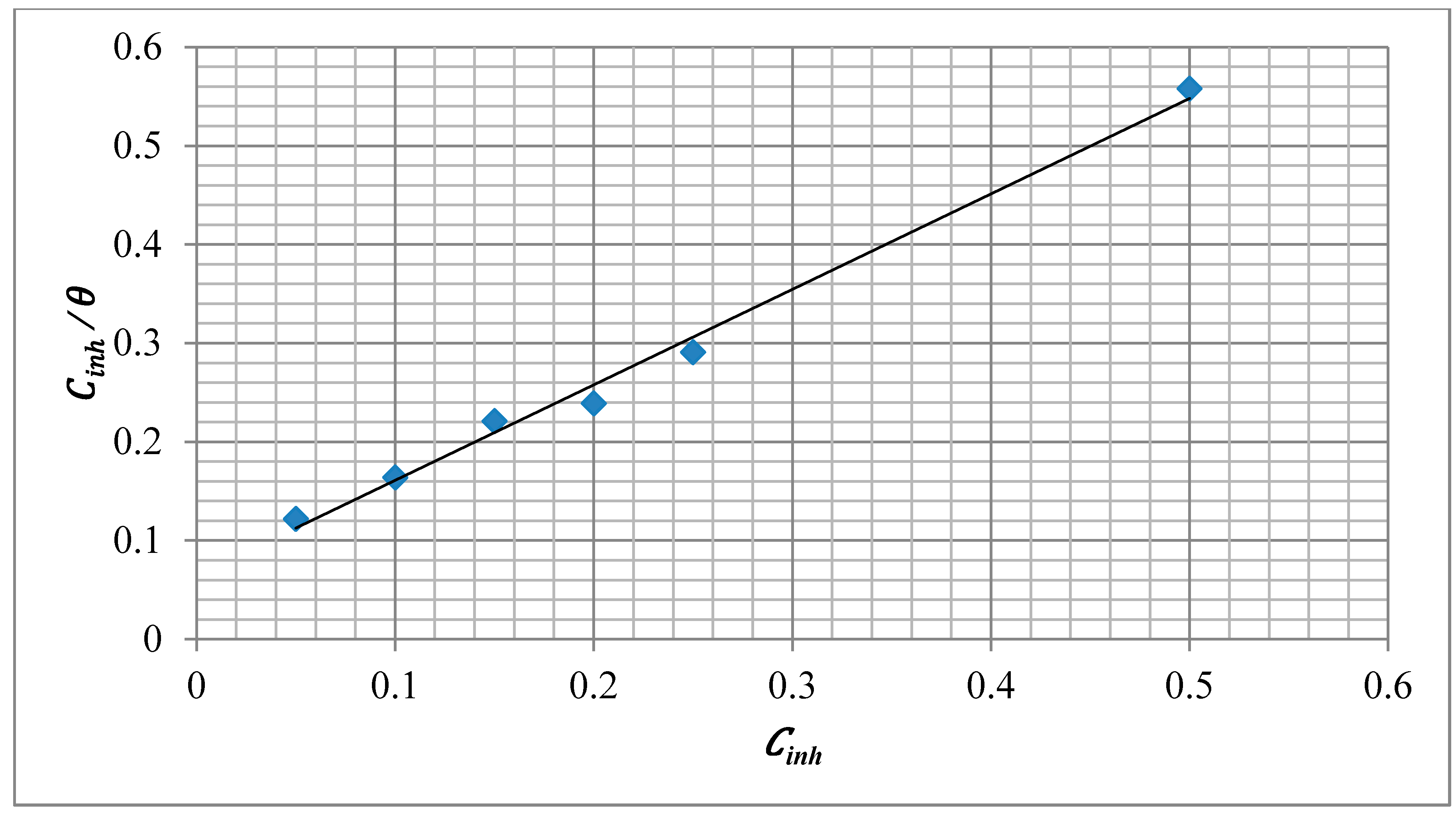
2.5. Corrosion Kinetic Parameters
| Concentration | Ea (kJ·mol−1) | ΔHa (kJ·mol−1) | ΔSa (J·mol−1·K−1) |
|---|---|---|---|
| Blank | 61.75 | 59.22 | −70.53 |
| 0.10 mM | 80.85 | 79.15 | 14.11 |
| 0.25 mM | 90.0 | 87.15 | 17.74 |
| 0.5 mM | 94.12 | 92.36 | 22.27 |


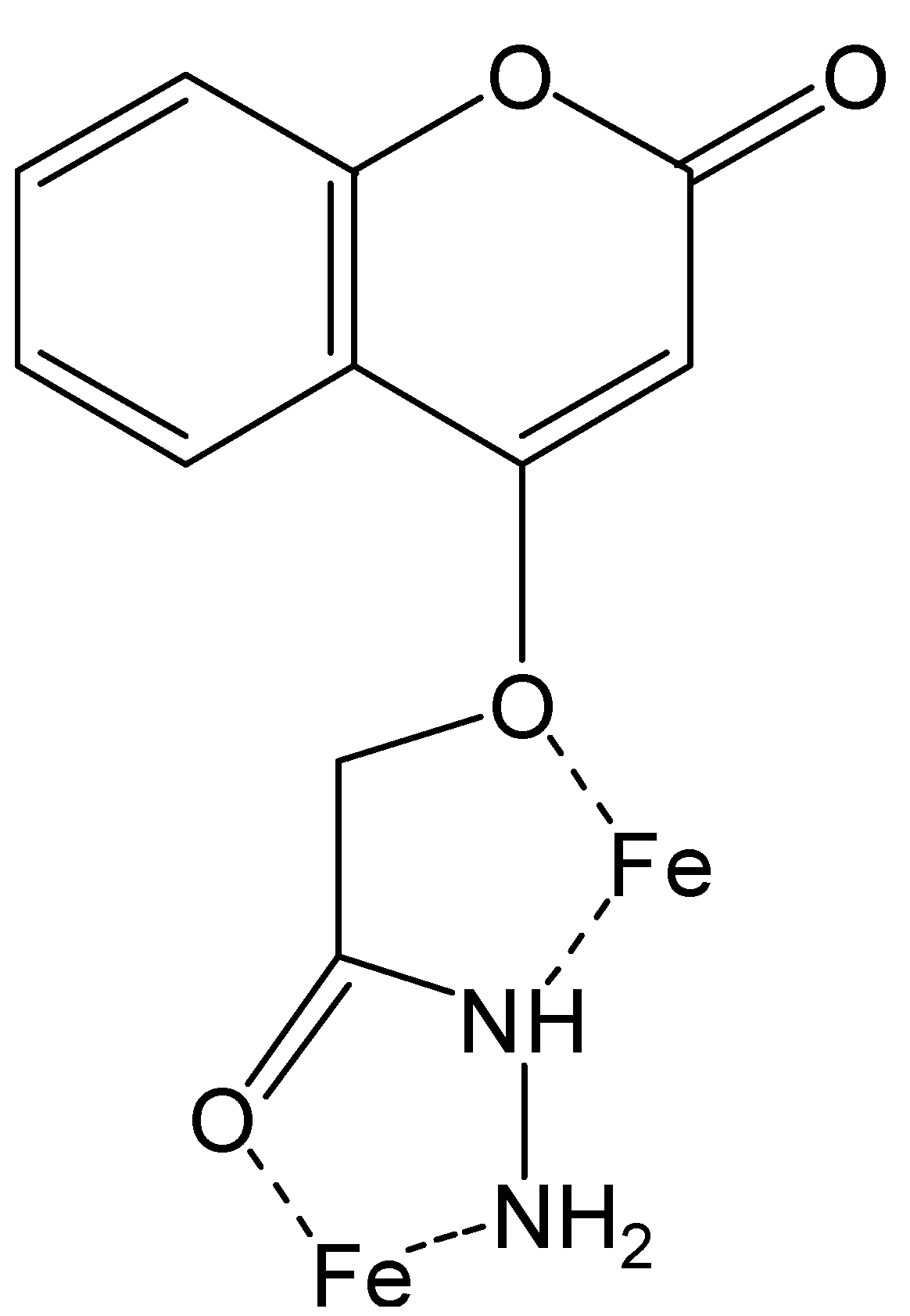
2.6. Suggested Mechanisms of Actions of New Synthesized Compound as Inhibitor
2.7. Computational Studies
2.7.1. Geometrical Isomers of the EFCI

2.7.2. Quantum Chemical Calculations

| Function | Values |
|---|---|
| EHOMO | −0.3766 Hartree |
| ELUMO | −0.1383 Hartree |
| EHOMO– ELUMO | −0.2382 Hartree |
| 0.125 | |
| 0.091 | |
| Dipole Moment | 1.394 |
2.7.3. Mulliken Charge
| Atoms | Charges | Atoms | Charges | Atoms | Charges | Atoms | Charges |
|---|---|---|---|---|---|---|---|
| C(1) | 0.3299 | O(7) | −0.2428 | C(13) | 0.0309 | H(19) | 0.0378 |
| C(2) | 0.1828 | C(8) | 0.3929 | C(14) | −0.1016 | H(20) | 0.2210 |
| O(3) | −0.3630 | O(9) | −0.3041 | C(15) | 0.0003 | H(21) | 0.0947 |
| N(4) | −0.3450 | C(10) | −0.2617 | C(16) | −0.0850 | H(22) | 0.0994 |
| N(5) | −0.0837 | C(11) | 0.2436 | C(17) | 0.1646 | H(23) | 0.0994 |
| O(6) | −0.2851 | C(12) | −0.1576 | H(18) | 0.0333 | H(24) | 0.0697 |
3. Experimental Section
3.1. Chemistry
3.1.1. General Information
3.1.2. Synthesis of Methyl 2-(coumarin-4-yloxy)acetate
3.1.3. Synthesis of 2-(Coumarin-4-yloxy)acetohydrazide
3.2. Gravimetric Experiments
3.2.1. Mild Steel Specimens
3.2.2. Weight Loss Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamal, L.C.; Sethuraman, M.G. Opuntiol: An active principle of opuntia elatior as an eco-friendly inhibitor of corrosion of mild steel in acid medium. Sustain. Chem. Eng. 2014, 2, 606–613. [Google Scholar]
- Gopal, J. Priyanka dwivedi, shanthi sundaram and rajiv prakash, inhibitive effect of chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind. Eng. Chem. Res. 2013, 52, 10673–10681. [Google Scholar]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media. Mater. Lett. 2008, 62, 113–116. [Google Scholar]
- Chidiebere, M.A.; Ogukwe, C.E.; Oguzie, K.L.; Eneh, C.N.; Oguzie, E.E. Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: Experimental and theoretical studies. Ind. Eng. Chem. Res. 2012, 51, 668–677. [Google Scholar]
- Oguzie, E.E.; Oguzie, K.L.; Akalezi, C.O.; Udeze, I.O.; Ogbulie, J.N.; Njoku, V.O. Natural products for materials protection: Corrosion and microbial growth inhibition using Capsicum frutescens biomass extracts. ACS Sustain. Chem. Eng. 2013, 1, 214–225. [Google Scholar]
- Krishnaveni, K.; Ravichandran, J.; Selvaraj, A. Effect of Morinda tinctoria leaves extract on the corrosion inhibition of mild steel in acid medium. Acta Metall. Sin. 2013, 26, 321–327. [Google Scholar]
- Raja, P.B.; Sethuraman, M.G. Inhibition of corrosion of mild steel in sulphuric acid medium by Calotropis procera. Pigment Resin Technol. 2009, 38, 33–37. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O. An interesting and efficient green corrosion inhibitor for aluminium from extracts of Chlomolaena odorata L. in acidic solution. J. Appl. Electrochem. 2010, 40, 1977–1984. [Google Scholar]
- Badiea, A.M.; Mohana, K.N. Corrosion mechanism of lowcarbon steel in industrial water and adsorption thermodynamics in the presence of some plant extracts. J. Mater. Eng. Perform. 2009, 18, 1264–1271. [Google Scholar]
- Li, X.-H.; Deng, S.-D.; Fu, H. Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of cold rolled steel in hydrochloric acid solution. J. Appl. Electrochem. 2010, 40, 1641–1649. [Google Scholar]
- Noor, E.A. Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminum in alkaline solutions. J. Appl. Electrochem. 2009, 39, 1465–1475. [Google Scholar]
- Majjane, A.; Rair, D.; Chahine, A.; Et-tabirou, M.; Ebn Touhami, M.; Touir, R. Preparation and characterization of a new glass system inhibitor for mild steel corrosion in hydrochloric solution. Corros. Sci. 2012, 60, 98–103. [Google Scholar]
- Singh, A.K.; Shukla, S.K.; Quraishi, M.A.; Ebenso, E.E. Investigation of adsorption characteristics of N,N'-[(methylimino)-dimethylidyne]di-2,4-xylidine as corrosion inhibitor at mild steel/sulphuric acid interface. J. Taiwan Inst. Chem. Eng. 2012, 43, 463–472. [Google Scholar]
- Zhao, J.; Chen, G. The synergistic inhibition effect of oleic-based imidazoline and sodium benzoate on mild steel corrosion in a CO2-saturated brine solution. Electrochim. Acta 2012, 69, 247–255. [Google Scholar]
- Shibli, S.M.A.; Saji, V.S. Co-inhibition characteristics of sodium tungstate with potassium iodate on mild steel corrosion. Corros. Sci. 2005, 47, 2213–2224. [Google Scholar]
- Vracar, L.M.; Drazic, D.M. Adsorption and corrosion inhibitive properties of some organic molecules on iron electrode in sulfuric acid. Corros. Sci. 2002, 44, 1669–1680. [Google Scholar]
- Gopal, J.; Shukla, S.K.; Dwived, P.; Sundaram, S.; Prakash, R. Inhibitive Effect of Argemone mexicana Plant Extract on Acid Corrosion of Mild Steel. Ind. Eng. Chem. Res. 2011, 50, 11954–11959. [Google Scholar]
- Zhang, Q.B.; Hua, Y.X. Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim. Acta 2009, 54, 1881–1887. [Google Scholar]
- Khaled, K.F. Understanding corrosion inhibition of mild steel in acid medium by some furan derivatives: A comprehensive overview. J. Electrochem. Soc. 2010, 157, C116–C124. [Google Scholar]
- Aytac, A.U.; Ozmen, M.; Kabasakaloğlu, M. Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater. Chem. Phys. 2005, 89, 176–181. [Google Scholar]
- Dandia, A.; Gupta, L.; Singh, P.; Quraishi, M.A. Ultrasound-assisted synthesis of pyrazolo[3,4-b]pyridines as potential corrosion inhibitors for mild steel in 1.0 M HCl. ACS Sustain. Chem. Eng. 2013, 1, 1303–1310. [Google Scholar]
- Migahed, M.A.; Mohammed, H.M.; Al-Sabagh, A.M. Corrosion inhibition of H-11 type carbon steel in 1 M hydrochloric acid solution by N-propyl amino lauryl amide and its ethoxylated derivatives. Mater. Chem. Phys. 2003, 80, 169–175. [Google Scholar]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar]
- Boudalia, M.; Guenbour, A.; Bellaouchou, A.; Laqhaili, A.; Mousaddak, M.; Hakiki, A.; Hammouti, B.; Ebenso, E.E. Corrosion inhibition of organic oil extract of leaves of Lanvandula stoekas on stainless steel in concentrated phosphoric acid solution. Int. J. Electrochem. Sci. 2013, 8, 7414–7424. [Google Scholar]
- Musa, A.Y.; Mohamad, A.B.; Kadhum, A.A.H.; Takriff, M.S.; Lim, T.T. Synergistic effect of potassium iodide with phthalazone on the corrosion inhibition of mild steel in 1.0 M HCl. Corros. Sci. 2011, 53, 3672–3677. [Google Scholar]
- Hermas, A.A.; Morad, M.S. A comparative study on the corrosion behaviour of 304 austenitic stainless steel in sulfamic and sulfuric acid solutions. Corros. Sci. 2008, 50, 2710–2717. [Google Scholar]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Shikara, M.; Mohamad, A. Synthesis, structure elucidation and DFT studies of new thiadiazoles. Int. J. Phys. Sci. 2011, 6, 6692–6697. [Google Scholar]
- Kandemirli, F.; Sagdinc, S. Theoretical study of corrosion inhibition of amides and thiosemicarbazones. Corros. Sci. 2007, 49, 2118–2130. [Google Scholar]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar]
- Ebenso, E.E.; Isabirye, D.A.; Eddy, N.O. Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int. J. Mol. Sci. 2010, 11, 2473–2498. [Google Scholar]
- Ashassi-Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Effect of some pyrimidinic Schiff bases on the corrosion of mild steel in HCl solution. Electrochim. Acta 2005, 50, 3446–3452. [Google Scholar]
- Khaleda, K.F.; Fadl-Allahb, S.A.; Hammoutic, B. Some benzotriazole derivatives as corrosion inhibitors for copper in acidic medium: Experimental and quantum chemical molecular dynamics approach. Mater. Chem. Phys. 2009, 117, 148–155. [Google Scholar]
- Bahrami, M.J.; Hosseini, S.M.A.; Pilvar, P. Experimental and theoretical investigation of organic compounds as inhibitors for mild steel corrosion in sulfuric acid medium. Corros. Sci. 2010, 52, 2793–2803. [Google Scholar]
- Cruz, J.; Pandiyan, T.; Garcıa-Ochoa, E. A new inhibitor for mild carbon steel: Electrochemical and DFT studies. J. Electroanal. Chem. 2005, 583, 8–16. [Google Scholar]
- Costa, J.M.; Lluch, J.M. The use of quantum mechanics calculations for the study of corrosion inhibitors. Corros. Sci. 1984, 24, 924–933. [Google Scholar]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta 2003, 48, 2635–2640. [Google Scholar]
- Al-Amiery, A.A. Antimicrobial and antioxidant activities of new metal complexes derived from (E)-3-((5-phenyl-1,3,4-oxadiazol-2-ylimino)methyl)naphthalen-2-ol. Med. Chem. Res. 2012, 21, 3204–3213. [Google Scholar]
- Xia, S.; Qiu, M.; Yu, L.; Liu, F.; Zhao, H. Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance. Corros. Sci. 2008, 50, 2021–2029. [Google Scholar]
- Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B.; Rahoma, A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct. 2010, 969, 233–327. [Google Scholar]
- Obot, I.B.; Ebenso, E.E.; Akpan, I.A.; Gasem, Z.M.; Afolabi Ayo, S. Thermodynamic and density functional theory investigation of sulphathiazole as green corrosion inhibitor at mild steel/hydrochloric acid interface. Int. J. Electrochem. Sci. 2012, 7, 1978–1996. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O. Anti-corrosive properties of xanthone on mild steel corrosion in sulphuric acid: Experimental and theoretical investigations. Curr. Appl. Phys. 2011, 11, 382–392. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O.; Eseola, A.O. Anticorrosion potential of 2-Mesityl-1H-imidazo[4,5-f][1,10]phenanthroline on mild steel in sulfuric acid solution: Experimental and theoretical study. Ind. Eng. Chem. Res. 2011, 50, 2098–2110. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar]
- Junaedi, S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B.; Takriff, M.S. Synthesis and characterization of novel corrosion inhibitor derived from oleic acid: 2-Amino 5-Oleyl-1,3,4-Thiadiazol (AOT). Int. J. Electrochem. Sci. 2012, 7, 3543–3554. [Google Scholar]
- Deng, Q.; Shi, H.W.; Ding, N.N.; Chen, B.Q.; He, X.P.; Liu, G.; Tang, Y.; Long, Y.T.; Chen, G.R. Novel triazolylbis-amino acidderivatives readily synthesized via click chemistry as potentialcorrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 57, 220–227. [Google Scholar]
- Tao, Z.; Hea, W.; Wang, S.; Zhang, S.; Zhou, G. A study of differential polarization curves and thermodynamic properties for mild steel in acidic solution with nitrophenyltriazole derivative. Corros. Sci. 2012, 60, 205–213. [Google Scholar]
- Sample Availability: Samples of the synthesized inhibitor available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B. New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium. Molecules 2015, 20, 366-383. https://doi.org/10.3390/molecules20010366
Al-Amiery AA, Al-Majedy YK, Kadhum AAH, Mohamad AB. New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium. Molecules. 2015; 20(1):366-383. https://doi.org/10.3390/molecules20010366
Chicago/Turabian StyleAl-Amiery, Ahmed A., Yasameen K. Al-Majedy, Abdul Amir H. Kadhum, and Abu Bakar Mohamad. 2015. "New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium" Molecules 20, no. 1: 366-383. https://doi.org/10.3390/molecules20010366