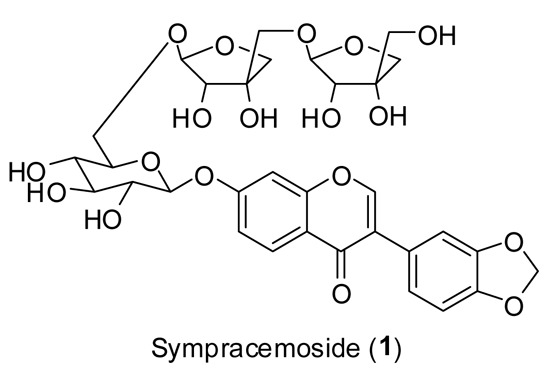
Flavonoids from Symplocos racemosa
Abstract
:1. Introduction
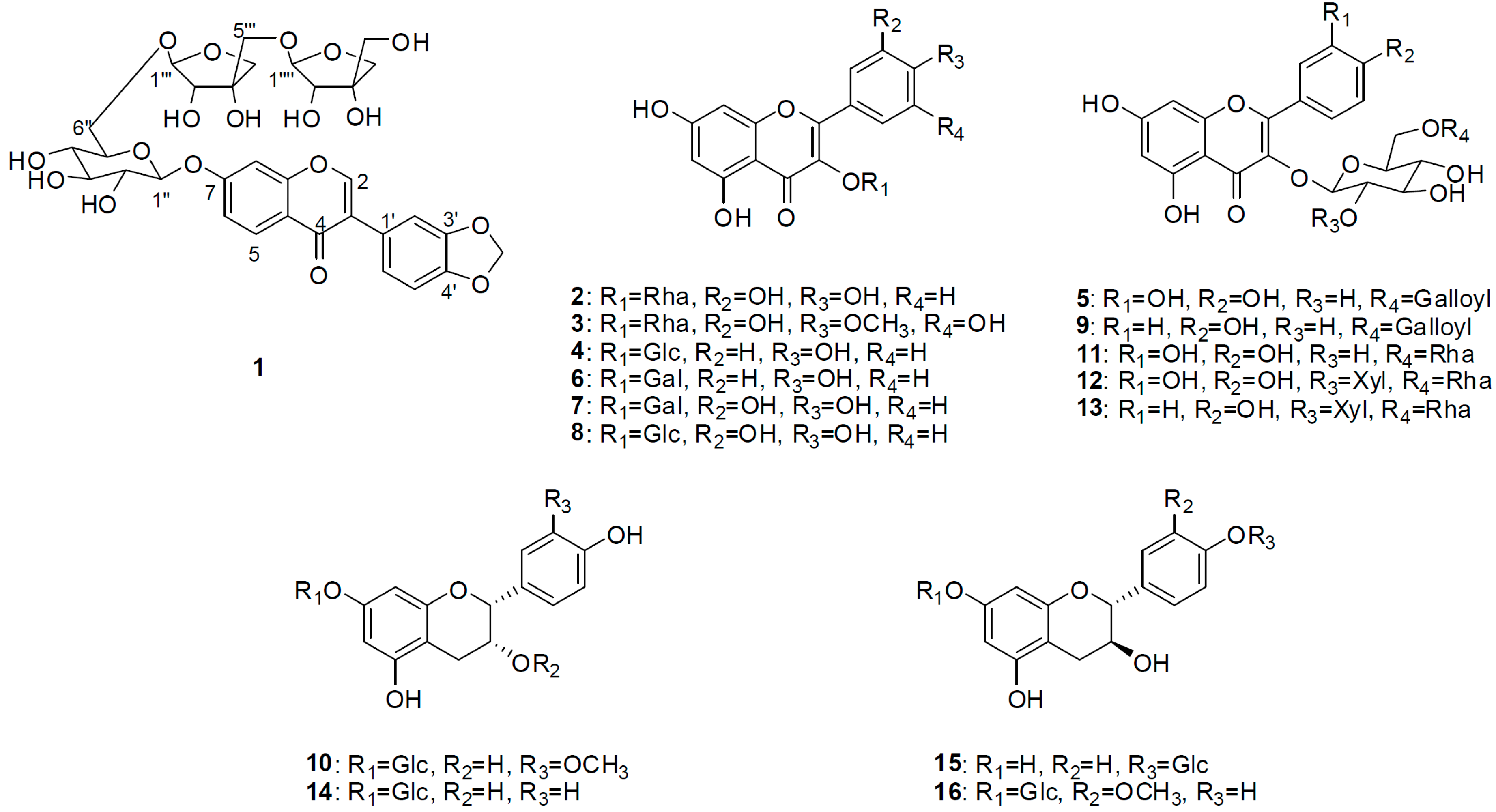
2. Results and Discussion
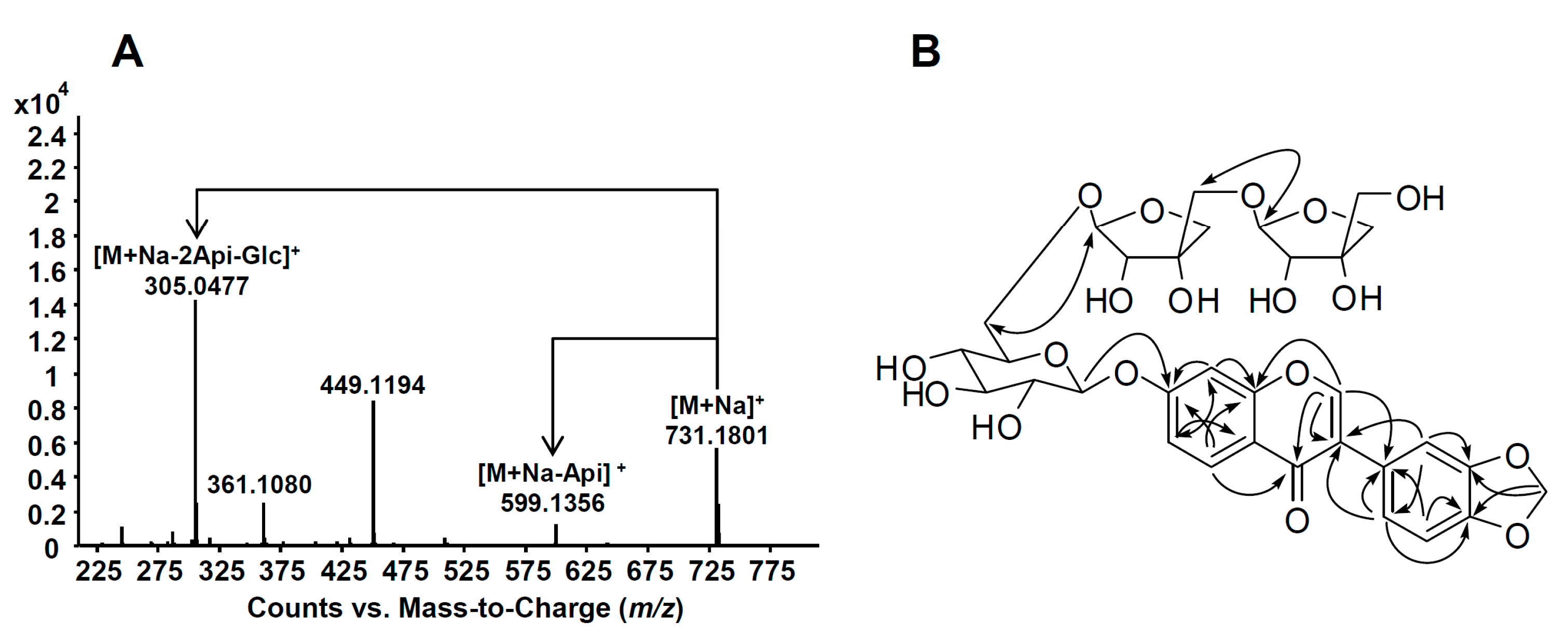
2.1. Structural Elucidation of Isolated Compounds
2.2. Inhibitory Activity against NO Production
| Compounds | IC50 (µM) | Compounds | IC50 (µM) |
|---|---|---|---|
| 1 | 100< | 9 | 42.1 |
| 2 | 100< | 10 | 100< |
| 3 | 88.2 | 11 | 100< |
| 4 | 100< | 12 | 100< |
| 5 | 100< | 13 | 100< |
| 6 | 100< | 14 | 100< |
| 7 | 100< | 15 | 100< |
| 8 | 100< | 16 | 74.3 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Measurement of NO Production
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devmurari, V.P. Phytochemical screening study and antibacterial evaluation of Symplocos racemosa Roxb. Arch. Appl. Sci. Res. 2010, 2, 354–359. [Google Scholar]
- Badoni, R.; Semwal, D.K.; Kothiyal, S.K.; Rawat, U. Chemical constituents and biological applications of the genus Symplocos. J. Asian Nat. Prod. Res. 2010, 12, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Watt, G. A Dictionary of the Economic Products of India; Periodical Experts: Delhi, India, 1972; p. 398. [Google Scholar]
- Ahmed, V.U.; Rashid, M.A; Abbasi, M.A.; Rasool, N.; Zubair, M. New salirepin derivatives from Symplocos racemosa. J. Asian Nat. Prod. Res. 2007, 9, 209–215. [Google Scholar]
- Ahmad, V.U.; Abbasi, M.A.; Hussain, H.; Akhtar, M.N.; Farrooq, U.; Fatima, N.; Choudhary, M.I. Phenolic glycoside from Symplocos racemosa: Natural inhibitors of phosphodiestease I. Phytochemistry 2003, 63, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Abbast, M.A.; Ahmad, V.U.; Zubair, M.; Nawaz, S.A.; Lodhi, M.A.; Farooq, U.; Choudhary, M.I. Lipoxygenase inhibiting ethyl substituted glycoside from Symplocos racemosa. Nat. Prod. Res. 2005, 19, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Ahmad, V.U.; Abbasi, M.; Ali, Z.; Rasool, N.; Zubair, M.; Lodhi, M.A.; Choudhary, M.I.; Khan, I.A. α-Chymotrypsin inhibiting benzylated glycoside from Symplocos racemosa. Phytochem. Lett. 2008, 1, 54–58. [Google Scholar] [CrossRef]
- Yuldashev, M.P.; Batirov, É.K.; Vdovin, A.D.; Malikov, V.M.; Yagudaev, M.R. Chem. Nat. Comp. 1989, 25, 303–308.
- Farag, S.F.; Ahmed, A.S.; Terashima, K.; Takaya, Y.; Niwa, M. Isoflavnoid glycoside form Dalbergia sissoo. Phytochemistry 2001, 57, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Azimova, S.S.; Vinogradova, V.I. Natural Compounds, 1st ed.; Springer: New York, NY, USA, 2013; pp. 187–188. [Google Scholar]
- Seidel, V.; Bailleul, F.; Waterman, P.G. (Rel)-1β,2α-di-(2,4-dihydroxy–6- methoxybenzoyl)-3β,4α-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii. Phytochemistry 2000, 55, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.K.; Nassar, M.I.; Gaara, A.H.; EI-Kashak, W.A.; Brouard, I.; EI-Toumy, S.A. Secondary metabolites and bioactivities of Albizia anthelmintica. Pharmacogn. Res. 2013, 5, 80–85. [Google Scholar] [CrossRef]
- Foo, L.Y.; Karchesy, J.J. Polyphenolic glycosides from Douglas fir inner bark. Phytochemistry 1989, 28, 1237–1240. [Google Scholar] [CrossRef]
- Nishida, R.; Ohsugi, T.; Fukami, H.; Nakajima, S. Oviposition deterrent of a Rutaceae-feeding swallowtail butterfly, Papilio xuthus, from a non-host rutaceous plant, Orixa japonica. Agric. Biol. Chem. 1990, 54, 1265–1270. [Google Scholar] [CrossRef]
- Sayed, H.M.; Mohamed, M.H.; Farag, S.F.; Mohamed, G.A.; Ebel, R.; Omobuwajo, O.R.M.; Proksch, P. Phenolics of Cyperus alopecuroides rottb. Inflorescences and their biological activities. Bull. Pharm. Sci. 2006, 29, 9–32. [Google Scholar]
- Jin, C.; Michetich, R.G.; Daneshtalab, M. Flavonoids from Stellera chaaejasme. Phytochemistry 1999, 50, 505–508. [Google Scholar] [CrossRef]
- Morimoto, S.; Nonaka, G.I.; Chen, R.F.; Nishioka, I. Tannin and related compounds. LXI. Isolation and structures of novel bi- and triflavanoids from the leaves of Cassia fistula L. Chem. Pharm. Bull. 1988, 36, 39–47. [Google Scholar]
- Baek, N.I; Kennelly, E.J.; Kardono, L.B.S.; Tsauri, S.; Padmawinata, K.; Soejarto, D.D.; Kinghorn, A.D. Flavonoids and pronathocyanidin from rhizomes of Selliguea feei. Phytochemistry 1994, 36, 513–518. [Google Scholar]
- Sample Availability: Samples of the compounds 1–16 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.; Choi, J.; Chae, H.-S.; Cho, J.Y.; Kim, Y.-D.; Htwe, K.M.; Lee, W.-S.; Chin, Y.-W.; Kim, J.; Yoon, K.D. Flavonoids from Symplocos racemosa. Molecules 2015, 20, 358-365. https://doi.org/10.3390/molecules20010358
Jung M, Choi J, Chae H-S, Cho JY, Kim Y-D, Htwe KM, Lee W-S, Chin Y-W, Kim J, Yoon KD. Flavonoids from Symplocos racemosa. Molecules. 2015; 20(1):358-365. https://doi.org/10.3390/molecules20010358
Chicago/Turabian StyleJung, Mila, Janggyoo Choi, Hee-Sung Chae, Jae Youl Cho, Young-Dong Kim, Khin Myo Htwe, Woo-Shin Lee, Young-Won Chin, Jinwoong Kim, and Kee Dong Yoon. 2015. "Flavonoids from Symplocos racemosa" Molecules 20, no. 1: 358-365. https://doi.org/10.3390/molecules20010358
APA StyleJung, M., Choi, J., Chae, H.-S., Cho, J. Y., Kim, Y.-D., Htwe, K. M., Lee, W.-S., Chin, Y.-W., Kim, J., & Yoon, K. D. (2015). Flavonoids from Symplocos racemosa. Molecules, 20(1), 358-365. https://doi.org/10.3390/molecules20010358