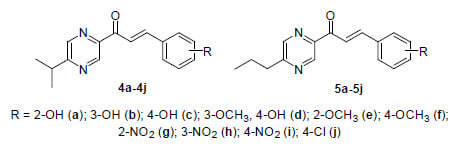
Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
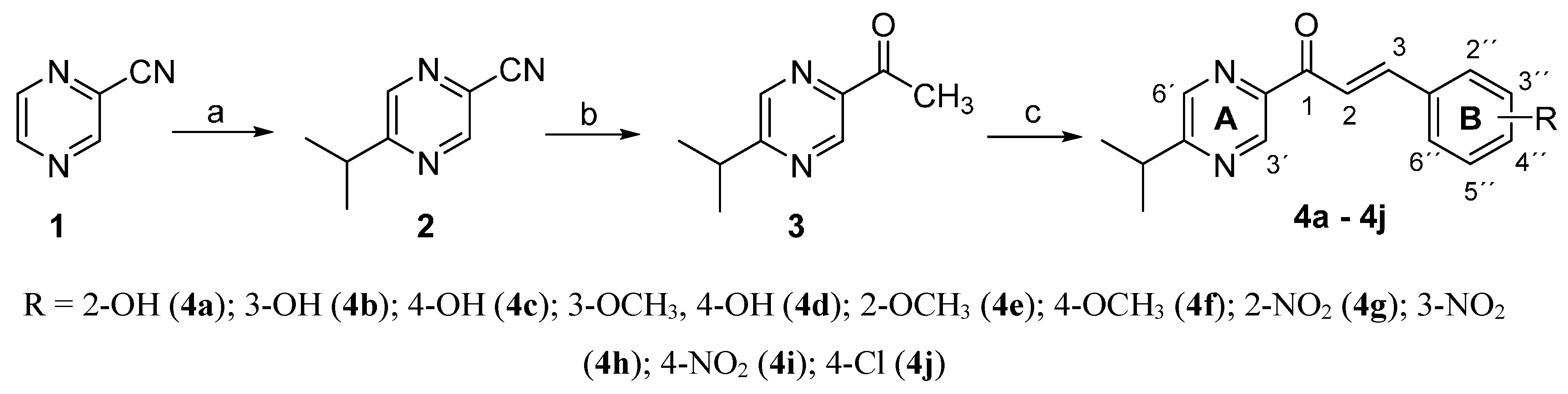
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antifungal Activity
| Compd. | MIC (μmol/L) IC80 or greater for yeasts and yeast-like organisms IC50 or greater for molds | |||||||
|---|---|---|---|---|---|---|---|---|
| CA | CT | CK | CG | TA | AF | LC | TM | |
| 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 72 h | |
| 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 120 h | |
| 4a | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 |
| ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | |
| 5a | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 500 | 500 | 7.81 |
| ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 7.81 | |
| 4b | 62.5 | 125 | ˃250 | 125 | 125 | 62.5 | 125 | 15.62 |
| 125 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | 31.25 | |
| 5b | 62.5 | 62.5 | ˃125 | 62.5 | 62.5 | 31.25 | ˃125 | 15.62 |
| 62.5 | 125 | ˃125 | 62.5 | ˃125 | ˃125 | ˃125 | 15.62 | |
| 4c | 62.5 | ˃250 | ˃250 | ˃250 | ˃250 | 62.5 | ˃250 | 62.5 |
| ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | 250 | ˃250 | 125 | |
| 5c | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 31.25 |
| ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 62.5 | |
| 4d | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 |
| ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | |
| 5d | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 |
| ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ND a | |
| 4e | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | 31.25 |
| ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | 62.5 | |
| 5e | 500 | ˃500 | ˃500 | ˃500 | ˃500 | 500 | ˃500 | 500 |
| ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 500 | |
| 4f | 250 | ˃500 | 250 | ˃500 | ˃500 | ˃500 | ˃500 | 125 |
| 250 | ˃500 | 250 | ˃500 | ˃500 | ˃500 | ˃500 | 125 | |
| 5f | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 |
| ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | |
| 4g | 32.15 | ˃250 | ˃250 | ˃250 | ˃250 | 125 | 125 | 7.81 |
| 125 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | 7.81 | |
| 5g | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 250 |
| ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | ˃500 | 250 | |
| 4h | NS b | NS b | NS b | NS b | NS b | NS b | NS b | NS b |
| NS b | NS b | NS b | NS b | NS b | NS b | NS b | NS b | |
| 5h | 15.62 | 62.5 | 32.15 | 125 | ˃250 | 62.5 | ˃250 | 7.81 |
| 62.5 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | 15.62 | |
| 4i | 7.81 | ˃250 | ˃250 | ˃250 | ˃250 | 125 | 250 | 3.90 |
| 62.5 | ˃250 | ˃250 | ˃250 | ˃250 | ˃250 | 250 | 7.81 | |
| 5i | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | 7.81 |
| ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | ˃62.5 | 15.62 | |
| 4j | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ≤62.5 |
| ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ˃125 | ≤62.5 | |
| 5j | >62.5 | >62.5 | >62.5 | >62.5 | >62.5 | >62.5 | >62.5 | 7.81 |
| >62.5 | >62.5 | >62.5 | >62.5 | >62.5 | >62.5 | >62.5 | 7.81 | |
| FLU | 0.24 | ˃500 | 125 | 41.64 | 250 | ˃500 | ˃500 | 6.51 |
| 0.24 | ˃500 | 250 | 250 | 500 | ˃500 | ˃500 | 104 | |
| VOR | 0.005 | 125 | 0.65 | 83.58 | 3.26 | 0.49 | 208 | 0.08 |
| 0.007 | 250 | 1.95 | 250 | 14.32 | 1.3 | 250 | 0.12 | |
| TER | ˃6.86 c | ˃6.86 c | ˃6.86 c | ˃6.86 c | NA d | NA d | NA d | 0.01–1.72 c |
2.2.2. Antimycobacterial Activity
| Compd. | R | % Inhibition at 6.25 μg/mL | MIC90 (μg/mL) | CC50 (μg/mL) | SI |
|---|---|---|---|---|---|
| 4a | 2-OH | 76 | ND | ND | ND |
| 5a | 2-OH | 50 | ND | ND | ND |
| 4b | 3-OH | 0 | ND | ND | ND |
| 5b | 3-OH | 0 | ND | ND | ND |
| 4c | 4-OH | 59 | ND | ND | ND |
| 5c | 4-OH | 35 | ND | ND | ND |
| 4d | 3-OCH3, 4-OH | ND | ND | ND | ND |
| 5d | 3-OCH3, 4-OH | 20 | ND | ND | ND |
| 4e | 2-OCH3 | ND | ND | ND | ND |
| 5e | 2-OCH3 | 71 | ND | ND | ND |
| 4f | 4-OCH3 | ND | ND | ND | ND |
| 5f | 4-OCH3 | ND | ND | ND | ND |
| 4g | 2-NO2 | 97 | 6.25 | 0.84 | 0.13 |
| 5g | 2-NO2 | 57 | ND | ND | ND |
| 4h | 3-NO2 | 0 | ND | ND | ND |
| 5h | 3-NO2 | 0 | ND | ND | ND |
| 4i | 4-NO2 | 91 | 6.25 | 1.14 | 0.18 |
| 5i | 4-NO2 | 100 | >6,25 | ND | ND |
| 4j | 4-Cl | 0 | ND | ND | ND |
| 5j | 4-Cl | 12 | ND | ND | ND |
| isoniazid | ND | 0.025–0.05 | >1000 | >40,000 | |
| rifampicin | 98 | 0.015–0.125 | >100 | >800 | |
3. Experimental Section
3.1. Chemistry
3.1.1. Materials and Methods
3.1.2. Synthesis of (E)-1-(5-isopropylpyrazin-2-yl)-3-phenylprop-2-en-1-ones 4a–4j
3.2. Biological Evaluation
3.2.1. Evaluation of in Vitro Antifungal Activity
3.2.2. Evaluation of in Vitro Antimycobacterial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lonnroth, K.; Castro, K.G.; Chakaya, J.M.; Chauhan, L.S.; Floyd, K.; Glaziou, P.; Raviglione, M.C. Tuberculosis control and elimination 2010–50: Cure, care, and social development. Lancet 2010, 375, 1814–1829. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Zumla, A.I. Tuberculosis. Lancet 2011, 378, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Raviglione, M.; Marais, B.; Floyd, K.; Lonnroth, K.; Getahun, H.; Migliori, G.B.; Harries, A.D.; Nunn, P.; Lienhardt, C.; Graham, S.; et al. Scaling up interventions to achieve global tuberculosis control: Progress and new developments. Lancet 2012, 379, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.R.; Nunn, P.; Dheda, K.; Schaaf, H.S.; Zignol, M.; van Soolingen, D.; Jensen, P.; Bayona, J. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet 2010, 375, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Albanna, A.S.; Menzies, D. Drug-resistant tuberculosis: What are the treatment options? Drugs 2011, 71, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Abubakar, I.; Alffenaar, J.W.; Bothamley, G.; Caminero, J.A.; Carvalho, A.C.; Chang, K.C.; Codecasa, L.; Correia, A.; Crudu, V.; et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in europe: A TBNET consensus statement. Eur. Respir. J. 2014, 44, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Maurer, E.; Lass-Florl, C. Mucormycosis: From the pathogens to the disease. Clin. Microbiol. Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Drogari-Apiranthitou, M. Epidemiology of mucormycosis in Europe. Clin. Microbiol. Infect. 2014, 20, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, T.R.; Chin-Hong, P.V. Endemic mycoses in immunocompromised hosts. Curr. Infect. Dis. Rep. 2013, 15, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 310–333. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3',4',5'-tetramethoxychalcone and its analogues as potent nuclear factor κb inhibitors and their anticancer activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Balsera, B.; Mulet, J.; Fernandez-Carvajal, A.; de la Torre-Martinez, R.; Ferrer-Montiel, A.; Hernandez-Jimenez, J.G.; Estevez-Herrera, J.; Borges, R.; Freitas, A.E.; Lopez, M.G.; et al. Chalcones as positive allosteric modulators of α7 nicotinic acetylcholine receptors: A new target for a privileged structure. Eur. J. Med. Chem. 2014, 86, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Hadjipavlou-Litina, D. Recent progress in therapeutic applications of chalcones. Exp. Opin. Ther. Pat. 2011, 21, 1575–1596. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Jasamai, M.; Jantan, I. Synthesis and biological evaluation of chalcone derivatives (mini review). Mini Rev. Med. Chem. 2012, 12, 1394–1403. [Google Scholar] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Jiang, S.M.; Chen, Z.H.; Ye, B.J.; Piao, H.R. Synthesis and anti-bacterial activity of some heterocyclic chalcone derivatives bearing thiofuran, furan, and quinoline moieties. Arch. Pharm. (Weinheim) 2011, 344, 689–695. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, T.T.N.; Do, T.H.; Huynh, T.N.P.; Tran, C.D.; Thai, K.M. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Raj, C.G.D.; Sarojini, B.K.; Hegde, S.; Sreenivasa, S.; Ravikumar, Y.S.; Bhanuprakash, V.; Revanaiah, Y.; Ragavendra, R. In vitro biological activities of new heterocyclic chalcone derivatives. Med. Chem. Res. 2013, 22, 2079–2087. [Google Scholar] [CrossRef]
- Kumar, C.S.; Loh, W.S.; Ooi, C.W.; Quah, C.K.; Fun, H.K. Structural correlation of some heterocyclic chalcone analogues and evaluation of their antioxidant potential. Molecules 2013, 18, 11996–12011. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.T.; Bulakowska, A.; Polak, J.; Pirska, D.; Konieczny, W.; Gryn, P.; Skladanowski, A.; Sabisz, M.; Lemka, K.; Pieczykolan, A.; et al. Structural factors affecting cytotoxic activity of (E)-1-(benzo[d][1,3]oxathiol-6-yl)-3-phenylprop-2-en-1-one derivatives. Chem. Biol. Drug Des. 2014, 84, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Vontor, T.; Palat, K.; Odlerova, Z. Functional derivatives of 3-alkyl-2-pyrazinecarboxylic acid. Cesk. Farm. 1986, 35, 162–167. [Google Scholar]
- Dlabal, K.; Dolezal, M.; Machacek, M. Preparation of some 6-substituted N-pyrazinyl-2-pyrazinecarboxamides. Collect. Czechoslov. Chem. Commun. 1993, 58, 452–454. [Google Scholar] [CrossRef]
- Krinkova, J.; Dolezal, M.; Hartl, J.; Buchta, V.; Pour, M. Synthesis and biological activity of 5-alkyl-6-(alkylsulfanyl)- or 5-alkyl-6-(arylsulfanyl)pyrazine-2-carboxamides and corresponding thioamides. Farmaco 2002, 57, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, M.; Palek, L.; Vinsova, J.; Buchta, V.; Jampilek, J.; Kralova, K. Substituted pyrazinecarboxamides: Synthesis and biological evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Kalinowski, D.S.; Vejsova, M.; Kunes, J.; Pour, M.; Jampilek, J.; Buchta, V.; Richardson, D.R. Identification and characterization of thiosemicarbazones with antifungal and antitumor effects: Cellular iron chelation mediating cytotoxic activity. Chem. Res. Toxicol. 2008, 21, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, M.; Zitko, J.; Kesetovicova, D.; Kunes, J.; Svobodova, M. Substituted N-phenylpyrazine-2-carboxamides: Synthesis and antimycobacterial evaluation. Molecules 2009, 14, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, M.; Zitko, J.; Osicka, Z.; Kunes, J.; Vejsova, M.; Buchta, V.; Dohnal, J.; Jampilek, J.; Kralova, K. Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides. Molecules 2010, 15, 8567–8581. [Google Scholar] [CrossRef] [PubMed]
- Jandourek, O.; Dolezal, M.; Paterova, P.; Kubicek, V.; Pesko, M.; Kunes, J.; Coffey, A.; Guo, J.H.; Kralova, K. N-substituted 5-amino-6-methylpyrazine-2,3-dicarbonitriles: Microwave-assisted synthesis and biological properties. Molecules 2014, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Hartl, J.; Patel, A.; Palat, K.; Buchta, V. Ring substituted 3-phenyl-1-(2-pyrazinyl)-2-propen-1-ones as potential photosynthesis-inhibiting, antifungal and antimycobacterial agents. Farmaco 2002, 57, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Chlupacova, M.; Opletalova, V.; Kunes, J.; Silva, L.; Buchta, V.; Duskova, L.; Kralova, K. Synthesis and biological evaluation of some ring-substituted (E)-3-aryl-1-pyrazin-2-ylprop-2-en-1-ones. Folia Pharm. Univ. Carol. 2005, 33, 31–43. [Google Scholar]
- Opletalova, V.; Pour, M.; Kunes, J.; Buchta, V.; Silva, L.; Kralova, K.; Chlupacova, M.; Meltrova, D.; Peterka, M.; Poslednikova, M. Synthesis and biological evaluation of (E)-3-(nitrophenyl)-1-(pyrazin-2-yl)prop-2-en-1-ones. Collect. Czechoslov. Chem. Commun. 2006, 71, 44–58. [Google Scholar] [CrossRef]
- Minisci, F.; Bernardi, R.; Bertini, F.; Galli, R.; Perchinu, M. Nucleophilic character of alkyl radicals. VI. New convenient selective alkylation of heteroaromatic bases. Tetrahedron 1971, 27, 3575–3580. [Google Scholar] [CrossRef]
- Fontana, F.; Minisci, F.; Barbosa, M.C.N.; Vismara, E. Homolytic alkylation of heteroaromatic bases: The problem of monoalkylation. Tetrahedron 1990, 46, 2525–2538. [Google Scholar] [CrossRef]
- Tauber, J.; Imbri, D.; Opatz, T. Radical addition to iminium ions and cationic heterocycles. Molecules 2014, 19, 16190–16222. [Google Scholar] [CrossRef] [PubMed]
- Punta, C.; Minisci, F. Minisci reaction: A Friedel-Crafts type process with opposite reactivity and selectivity: Selective homolytic alkylation, acylation carboxylation and carbamoylation of heterocyclic aromatic bases. Trends Heterocycl. Chem. 2008, 13, 1–68. [Google Scholar]
- Opletalova, V.; Patel, A.; Boulton, M.; Dundrova, A.; Lacinova, E.; Prevorova, M.; Appeltauerova, M.; Coufalova, M. 5-alkyl-2-pyrazinecarboxamides, 5-alkyl-2-pyrazinecarbonitriles and 5-alkyl-2-acetylpyrazines as synthetic intermediates for antiinflammatory agents. Collect. Czechoslov. Chem. Commun. 1996, 61, 1093–1101. [Google Scholar] [CrossRef]
- Kucerova-Chlupacova, M.; Opletalova, V.; Jampilek, J.; Dolezel, J.; Dohnal, J.; Pour, M.; Kunes, J.; Vorisek, V. New hydrophobicity constants of substituents in pyrazine rings derived from RP-HPLC study. Collect. Czechoslov. Chem. Commun. 2008, 73, 1–18. [Google Scholar] [CrossRef]
- Chlupacova, M. Chalcones and Their Analogues as Potential Drugs. Ph.D. Thesis, Charles University in Prague, Hradec Kralove, Czech Republic, 2006. [Google Scholar]
- Tchernev, G.; Penev, P.K.; Nenoff, P.; Zisova, L.G.; Cardoso, J.C.; Taneva, T.; Ginter-Hanselmayer, G.; Ananiev, J.; Gulubova, M.; Hristova, R.; et al. Onychomycosis: Modern diagnostic and treatment approaches. Wien. Med. Wochenschr. 2013, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Drummond-Main, C.; Paquet, M. Evidence-based optimal fluconazole dosing regimen for onychomycosis treatment. J. Dermatol. Treat. 2013, 24, 75–80. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H. Terbinafin-Topika: Ultimative Verkurzung der Therapiedauer bei Tinea pedis. Hautarzt 2008, 59, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Czaika, V.; Nenoff, P.; Glockner, A.; Becker, K.; Fegeler, W.; Schmalreck, A.F. Detection of azole susceptibility patterns in clinical yeast strains isolated from 1998 to 2008. New Microbiol. 2014, 37, 465–494. [Google Scholar] [PubMed]
- Scott, L.J.; Simpson, D. Voriconazole: A review of its use in the management of invasive fungal infections. Drugs 2007, 67, 269–298. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Novelli, A.; Aversa, F.; Cesaro, S.; de Rosa, F.G.; Girmenia, C.; Micozzi, A.; Sanguinetti, M.; Viscoli, C. Voriconazole in clinical practice. J. Chemother. 2012, 24, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Dolezel, J.; Buchta, V.; Vejsova, M.; Paterova, P. Antifungal effects of (5Z)-5-arylmethylidenerhodanines with a special view to members of Mucorales. Folia Pharm. Univ. Carol. 2014, 42, 2–13. [Google Scholar]
- Gupta, A.K.; Kohli, Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br. J. Dermatol. 2003, 149, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved StandardCLSI document M27-A3, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved StandardCLSI Document M38-A2, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Collins, L.; Franzblau, S.G. Microplate alamar blue assay versus Bactec 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [PubMed]
- Sample Availability: Samples of all compounds, except for 4q and 5d are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucerova-Chlupacova, M.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity. Molecules 2015, 20, 1104-1117. https://doi.org/10.3390/molecules20011104
Kucerova-Chlupacova M, Kunes J, Buchta V, Vejsova M, Opletalova V. Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity. Molecules. 2015; 20(1):1104-1117. https://doi.org/10.3390/molecules20011104
Chicago/Turabian StyleKucerova-Chlupacova, Marta, Jiri Kunes, Vladimir Buchta, Marcela Vejsova, and Veronika Opletalova. 2015. "Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity" Molecules 20, no. 1: 1104-1117. https://doi.org/10.3390/molecules20011104