General Procedure for the Synthesis of Compounds 5
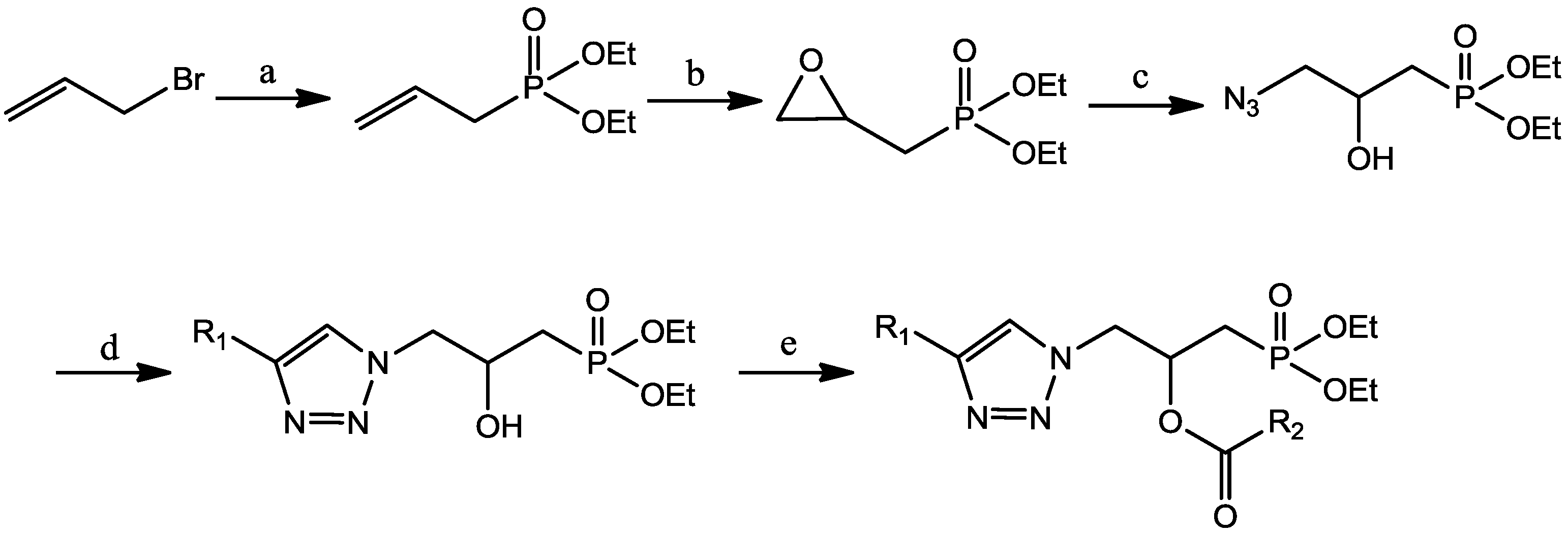
A mixture of carboxylic acid (5 mmol) and DCC (1.07 g, 5.5 mmol) was dissolved in dichloromethane (40 mL), stirred at room temperature for 1 h. Then the mixture was placed in ice-bath and DMAP (0.06 g, 0.5 mmol), compounds 4 (4 mmol) was added, stirred for 10 min. Then the mixture was stirred at room temperature overnight. The desired product was obtained by purification on a silica gel column with petroleum ether/ethyl acetate.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl benzoate (5-A1). Yield: 79%. White solid. 1H-NMR (CDCl3) δ 1.24 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H), 2.25–2.34 (m, 2H), 4.05–4.18 (m, 4H), 4.91–4.93 (m, 2H), 5.72–5.79 (m, 1H), 7.27–7.44 (m, 5H), 7.52–7.55 (m, 1H), 7.77–7.80 (m, 2H), 7.98 (s, 1H), 8.00–8.03 (m, 2H); 13C-NMR (CDCl3) δ 15.77 (d, JC-P = 6.0 Hz), 15.85 (d, JC-P = 6.0 Hz), 27.69 (d, JC-P = 140.9 Hz), 52.14 (d, JC-P = 7.9 Hz), 61.68 (d, JC-P = 1.2 Hz), 61.76 (d, JC-P = 1.7 Hz), 67.64, 120.75, 125.18, 127.65, 128.04, 128.32, 128.76, 129.23, 130.01, 133.03, 147.22, 164.74; 31P-NMR (CDCl3) δ 25.53; HR-MS: for C22H26N3O5P [M+H]+ calculated m/z 444.16, found m/z 444.1682839.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl 4-nitrobenzoate (5-A2). Yield: 80%. White solid. 1H-NMR (CDCl3) δ 1.28 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.0 Hz, 3H), 2.28 (d, J = 6.7 Hz, 1H), 2.34 (d, J = 6.8 Hz, 1H), 4.07–4.21 (m, 4H), 4.94–4.97 (m, 2H), 5.74–5.81 (m, 1H), 7.30–7.43 (m, 3H), 7.78–7.81 (m, 2H), 8.00 (S, 1H), 8.17–8.20 (m, 2H), 8.23–8.27 (m, 2H); 13C-NMR (CDCl3) δ 16.18 (d, JC-P = 3.9 Hz), 16.26 (d, JC-P = 4.1 Hz), 28.11 (d, JC-P = 141.1 Hz), 52.28 (d, JC-P = 7.2 Hz), 62.25 (d, JC-P = 6.6 Hz), 68.93, 125.54, 128.18, 128.73, 130.12, 130.82, 134.49, 147.80, 150.65, 163.44; 31P-NMR (CDCl3) δ 25.06; HR-MS: for C22H25N4O7P [M+H]+ calculated m/z 489.15, found m/z 489.1533621.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl 2-(naphthalen-1-yl)acetate (5-A3). Yield: 73%. White solid. 1H-NMR (CDCl3) δ 1.27 (t, J = 7.1 Hz, 6H), 1.98–2.10 (m, 2H), 4.01–4.11 (m, 6H), 4.55 (d, J = 4.6 Hz, 2H), 5.49–5.53 (m, 1H), 7.17 (s, 1H), 7.29–7.40 (m, 8H), 7.62–7.74 (m, 4H), 7.86–7.89 (m, 1H); 13C-NMR (CDCl3) δ 15.91 (d, JC-P = 5.7 Hz), 27.58 (d, JC-P = 141.0 Hz), 38.52, 52.04 (d, JC-P = 8.1 Hz), 61.68 (d, JC-P = 4.4 Hz), 61.77 (d, JC-P = 4.3 Hz), 67.45, 120.22, 123.09, 124.96, 125.34, 125.44, 126.09, 127.61, 127.81, 128.21, 128.28, 129.33, 129.95, 131.42, 131.56, 133.21, 147.13, 169.69; 31P-NMR (CDCl3) δ 25.49; HR-MS: for C27H30N3O5P [M+H]+ calculated m/z 508.19, found m/z 508.199584.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl acetate (5-A4). Yield: 71%. White solid. 1H-NMR (CDCl3) δ 1.34 (t, J = 7.1 Hz, 3H), 1.35 (t, J = 7.1 Hz, 3H), 2.06 (s, 3H), 2.08–2.13 (m, 1H), 2.15–2.19 (m, 1H), 4.09–4.19 (m, 4H), 4.69–4.85 (m, 2H), 5.46–5.53 (m, 1H), 7.31–7.36 (m, 1H), 7.40–7.45 (m, 2H), 7.82–7.85 (m, 2H), 7.92 (S, 1H); 13C-NMR (CDCl3) δ:15.90 (d, JC-P = 6.1 Hz), 20.37, 27.72 (d, JC-P = 141.1 Hz), 52.00 (d, JC-P = 7.1 Hz), 61.71 (d, JC-P = 1.1 Hz), 61.80 (d, JC-P = 1.1 Hz), 66.99, 120.32, 125.24, 127.75, 128.36, 129.94, 147.37, 169.18; 31P-NMR (CDCl3) δ:25.61; HR-MS: for C17H24N3O5P [M+H]+ calculated m/z 382.15, found m/z 382.1526338.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl 2-fluorobenzoate (5-A5). Yield: 73%. White solid. 1H-NMR (CDCl3) δ 1.26 (t, J = 7.2 Hz, 3H), 1.32 (t, J = 3.5 Hz, 3H), 2.24–2.34 (m, 2H), 4.06–4.19 (m, 4H), 4.93 (d, J = 5.2 Hz, 2H), 5.74–5.82 (m, 1H), 7.09–7.21 (m, 2H), 7.30–7.42 (m, 3H), 7.47–7.55 (m, 1H), 7.80–7.83 (m, 2H), 7.91–7.94 (m, 1H), 8.06 (S, 1H); 13C-NMR (CDCl3) δ:15.90 (d, JC-P = 5.8 Hz), 15.98 (d, JC-P = 5.6 Hz), 27.77 (d, JC-P = 140.7 Hz), 52.28 (d, JC-P = 7.2 Hz), 61.88 (d, JC-P = 1.4 Hz), 62.00 (d, JC-P = 1.5 Hz), 68.28, 116.65 (d, JC-F = 22.3 Hz), 117.40 (d, JC-F = 9.6 Hz), 120.89 (d, JC-F = 2.6 Hz), 123.92 (d, JC-F = 3.9 Hz), 125.34, 127.79, 128.46, 130.18, 132.00, 134.85 (d, JC-F = 9.1 Hz), 147.46, 159.86, 162.62 (d, JC-F = 3.5 Hz), 163.31; 31P-NMR (121.5MHz, CDCl3) δ:25.26; HR-MS: for C22H25FN3O5P [M+H]+ calculated m/z 462.15, found m/z 462.1588621.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl 2-chloronicotinate (5-A6). Yield: 75%. White solid. 1H-NMR (CDCl3) δ 1.30 (t, J = 6.3 Hz, 3H), 1.34 (t, J = 6.3 Hz, 3H), 2.25–2.34 (m, 2H), 4.08–4.21 (m, 4H), 4.86–5.00 (m, 2H), 5.76–5.83 (m, 1H), 7.30–7.35 (m, 2H), 7.38–7.43 (m, 2H), 7.79–7.82 (m, 2H), 8.04 (S, 1H), 8.24–8.27 (m, 1H), 8.48–8.50 (m, 1H); 13C-NMR (CDCl3) δ 16.06 (d, JC-P = 3.2 Hz), 16.14 (d, JC-P = 3.2 Hz), 27.95 (d, JC-P = 141.5 Hz), 52.21 (d, JC-P = 8.0 Hz), 62.08 (d, JC-P = 3.1 Hz), 62.17 (d, JC-P = 2.9 Hz), 68.83, 120.89, 122.13, 125.43, 125.76, 128.01, 128.59, 130.01, 140.49, 147.65, 149.52, 152.00, 162.85; 31P-NMR (CDCl3) δ 24.87; HR-MS: for C21H24ClN4O5P [M+H]+ calculated m/z 479.12, found m/z 479.1245606.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl 4-methoxybenzoate (5-A7). Yield: 65%. White solid. 1H-NMR (CDCl3) δ 1.25 (t, J = 7.1 Hz, 3H), 1.31 (t, J = 7.1 Hz, 3H), 2.23–2.32 (m, 2H), 3.81 (s, 3H), 4.05–4.18 (m, 4H), 4.89–4.91 (m, 2H), 5.67–5.75 (m, 1H), 6.87–6.90 (m, 2H), 7.27–7.41 (m, 3H), 7.78–7.80 (m, 2H), 7.95–7.98 (m, 3H); 13C-NMR (CDCl3) δ:16.00 (d, JC-P = 5.9 Hz), 16.08 (d, JC-P = 5.6 Hz), 27.95 (d, JC-P = 140.6 Hz), 52.36 (d, JC-P = 7.2 Hz), 55.16, 61.88 (d, JC-P = 1.6 Hz), 61.98 (d, JC-P = 1.7 Hz), 67.51, 113.55, 120.80, 121.22, 125.41, 127.86, 128.52, 130.21, 131.59, 147.48, 163.58, 164.70; 31P-NMR (CDCl3) δ 25.72; HR-MS: for C23H28N3O6P [M+H]+ calculated m/z 474.17, found m/z 474.1788486.
1-(Diethoxyphosphoryl)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)propan-2-yl 3,5-dinitrobenzoate (5-A8). Yield: 77%. White solid. 1H-NMR (CDCl3) δ 1.32 (t, J = 6.9 Hz, 3H), 1.34 (t, J = 6.9Hz, 3H), 2.36 (d, J = 6.7 Hz, 1H), 2.42 (d, J = 6.7 Hz, 1H), 4.11–4.21 (m, 4H), 4.92–5.05 (m, 2H), 5.84–5.91 (m, 1H), 7.22–7.35 (m, 3H), 7.66–7.69 (m, 2H), 8.11 (S, 1H), 8.99–9.04 (m, 3H); 13C-NMR (CDCl3) δ:16.06 (d, JC-P = 5.9 Hz), 27.95 (d, JC-P = 141.8 Hz), 52.12 (d, JC-P = 8.9 Hz), 62.12 (d, JC-P = 6.6 Hz), 62.20 (d, JC-P = 6.5 Hz), 69.72, 120.88, 122.30, 125.14, 127.98, 128.48, 129.12, 129.73, 132.57, 147.43, 148.16, 161.22; 31P-NMR (CDCl3) δ 24.82; HR-MS: for C22H24N5O9P [M+H]+ m/z calculated 534.13, found m/z 534.1384403.
Ethyl 1-(2-(benzoyloxy)-3-(diethoxyphosphoryl)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B1). Yield: 78%. White solid. 1H-NMR (CDCl3) δ 1.21–1.41 (m, 9H), 2.23–2.29 (m, 2H), 4.06–4.20 (m, 4H), 4.40 (q, J = 7.1 Hz, 2H), 4.97 (d, J = 4.9 Hz, 2H), 5.67–5.75 (m, 1H), 7.44 (t, J = 7.6 Hz, 2H), 7.56–7.61 (m, 1H), 7.98–8.01 (m, 2H), 8.29 (s, 1H); 13C-NMR (CDCl3) δ:14.08, 16.09 (d, JC-P = 6.3 Hz), 16.17 (d, JC-P = 6.3 Hz), 28.01 (d, JC-P = 140.5 Hz), 52.60 (d, JC-P = 6.3 Hz), 61.07, 62.05 (d, JC-P = 10.2 Hz), 62.16 (d, JC-P = 6.5 Hz), 67.72, 128.40, 128.61, 128.84, 129.60, 133.48, 140.20, 160.37, 165.04; 31P-NMR (CDCl3) δ 25.19; HR-MS: for C19H26N3O7P [M+H]+ calculated m/z 440.15, found m/z 440.1581131.
Ethyl 1-(3-(diethoxyphosphoryl)-2-((4-nitrobenzoyl)oxy)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B2). Yield: 73%. yellow solid. 1H-NMR (CDCl3) δ 1.28–1.44 (m, 9H), 2.23–2.34 (m, 2H), 4.10–4.24 (m, 4H), 4.43 (q, J = 7.1 Hz, 2H), 5.00–5.03 (m, 2H), 5.73–5.81 (m, 1H), 8.18–8.22 (m, 2H), 8.29–8.34 (m, 3H); 13C-NMR (CDCl3) δ 13.99, 16.00 (d, JC-P = 6.3 Hz), 16.08 (d, JC-P = 6.3 Hz), 27.90 (d, JC-P = 140.6 Hz), 52.53 (d, JC-P = 6.6 Hz), 60.95, 61.95 (d, JC-P = 9.2 Hz), 62.05 (d, JC-P = 6.7 Hz), 67.63, 128.30, 128.58, 128.76, 129.49, 133.38, 140.06, 160.28, 164.93; 31P-NMR (CDCl3) δ 24.63; HR-MS: for C19H25N4O9P [M+H]+ calculated m/z 485.14, found m/z 485.1431913.
Ethyl 1-(3-(diethoxyphosphoryl)-2-(2-(naphthalen-1-yl)acetoxy)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B3). Yield: 68%. White solid. 1H-NMR (CDCl3) δ 1.31 (t, J = 7.1 Hz, 6H), 1.42 (t, J = 7.1 Hz, 3H), 2.01 (d, J = 6.7 Hz, 1H), 2.08 (d, J = 6.7 Hz, 1H), 4.05–4.16 (m, 6H), 4.43 (q, J = 7.1 Hz, 2H), 4.66 (d, J = 4.8 Hz, 2H), 5.43–5.51 (m, 1H), 7.34 (d, J = 6.1 Hz, 1H), 7.40–7.55 (m, 3H), 7.71 (s, 1H), 7.79–7.88 (m, 3H); 13C-NMR (CDCl3) δ 14.19, 16.22 (d, JC-P = 5.9 Hz), 27.95 (d, JC-P = 140.6 Hz), 38.81, 52.43 (d, JC-P = 6.3 Hz), 61.07, 62.16 (d, JC-P = 6.2 Hz), 62.24 (d, JC-P = 6.0 Hz), 67.52, 123.31, 125.31, 125.75, 126.43, 127.95, 128.27, 128.31, 128.69, 129.40, 131.69, 133.62, 140.05, 160.28, 170.03; 31P-NMR (CDCl3) δ 24.39; HR-MS: for C24H30N3O7P [M+H]+ calculated m/z 504.18, found m/z 504.1894132.
Ethyl 1-(2-acetoxy-3-(diethoxyphosphoryl)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B4). Yield: 74%. White solid. 1H-NMR (CDCl3) δ: 1.27–1.44 (m, 9H), 2.04 (s, 3H), 2.05–2.20 (m, 2H), 4.09–4.21 (m, 4H), 4.42 (q, J = 7.1 Hz, 2H), 4.76–4.93 (m, 2H), 5.45–5.54 (m, 1H), 8.33 (s, 1H); 13C-NMR (CDCl3) δ:13.83, 15.93 (d, JC-P = 6.0 Hz), 20.33, 27.71 (d, JC-P = 141.1 Hz), 52.33 (d, JC-P = 7.7 Hz), 60.77, 61.77 (d, JC-P = 3.0 Hz), 61.86 (d, JC-P = 2.8 Hz), 66.77, 128.37, 139.79, 160.14, 169.07; 31P-NMR (CDCl3) δ: 25.13; HR-MS: for C14H24N3O7P [M+H]+ calculated m/z 378.14, found m/z 378.1424631.
Ethyl 1-(3-(diethoxyphosphoryl)-2-((2-fluorobenzoyl)oxy)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B5). Yield: 78%. yellow solid. 1H-NMR (CDCl3) δ 1.26–1.41 (m, 9H), 2.26 (d, J = 6.6 Hz, 1H), 2.33 (d, J = 6.5 Hz, 1H), 4.08–4.21 (m, 4H), 4.40 (q, J = 7.1 Hz, 2H), 5.00–5.01 (m, 2H), 5.72–5.80 (m, 1H), 7.12–7.25 (m, 2H), 7.54–7.61 (m, 1H), 7.90–7.95 (m, 1H), 8.40 (s, 1H); 13C-NMR (CDCl3) δ 13.85, 15.85 (d, JC-P = 5.8 Hz), 15.93 (d, JC-P = 5.9 Hz), 27.67 (d, JC-P = 140.5 Hz), 52.41 (d, JC-P = 6.7 Hz), 60.78, 61.96 (d, JC-P = 6.6 Hz), 67.96, 116.64 (d, JC-F = 22.2 Hz), 117.14 (d, JC-F = 9.4 Hz), 123.89 (d, JC-F = 3.9 Hz), 128.67 (d, JC-F = 2.1 Hz), 131.92, 134.93 (d, JC-F = 9.3 Hz), 139.88, 160.00 (d, JC-F = 27.0 Hz), 162.46 (d, JC-F = 3.5 Hz), 163.27; 31P-NMR (CDCl3) δ:24.83; HR-MS: for C19H25FN3O7P [M+H]+ calculated m/z 458.14, found m/z 458.1486913.
1-(Diethoxyphosphoryl)-3-(4-(ethoxycarbonyl)-1H-1,2,3-triazol-1-yl)propan-2-yl 2-chloronicotinate (5-B6). Yield: 76%. yellow solid. 1H-NMR (CDCl3) δ 1.29–1.42 (m, 9H), 2.25–2.34 (m, 2H), 4.10–4.23 (m, 4H), 4.41 (q, J = 7.1 Hz, 2H), 4.93–5.08 (m, 2H), 5.75–5.82 (m, 1H), 7.36–7.40 (m, 1H), 8.25–8.29 (m, 1H), 8.40 (s, 1H), 8.53–8.55 (m, 1H); 13C-NMR (CDCl3) δ: 13.90, 15.95 (d, JC-P = 3.5 Hz), 16.03 (d, JC-P = 3.5 Hz), 27.76 (d, JC-P = 141.3 Hz), 52.30 (d, JC-P = 7.6 Hz), 60.95, 62.09 (d, JC-P = 4.5 Hz), 62.18 (d, JC-P = 4.4 Hz), 68.49, 122.08, 125.45, 128.64, 139.97, 140.42, 149.46, 152.01, 160.10, 162.64; 31P-NMR (CDCl3) δ: 24.53; HR-MS: for C18H24ClN4O7P [M+H]+ calculated m/z 475.11, found m/z 475.1143897.
Ethyl 1-(3-(diethoxyphosphoryl)-2-((4-methoxybenzoyl)oxy)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B7). Yield: 80%. White solid. 1H-NMR (CDCl3) δ 1.20–1.41 (m, 9H), 2.20–2.29 (m, 2H), 3.86 (s, 3H), 4.07–4.20 (m, 4H), 4.40 (q, J = 7.1 Hz, 2H), 4.96 (d, J = 4.8 Hz, 2H), 5.66–5.70 (m, 1H), 6.89–6.94 (m, 2H), 7.92–7.96 (m, 2H), 8.29 (s, 1H); 13C-NMR (CDCl3) δ:13.98, 16.00 (d, JC-P = 5.7 Hz), 16.07 (d, JC-P = 6.0 Hz), 27.89 (d, JC-P = 140.5 Hz), 52.55 (d, JC-P = 6.3 Hz), 55.20, 60.97, 62.08 (d, JC-P = 6.5 Hz), 67.24, 113.58, 120.96, 128.52, 131.62, 140.05, 160.30, 163.66, 164.63; 31P-NMR (CDCl3) δ 25.16; HR-MS: for C20H28N3O8P [M+H]+ calculated m/z 470.16, found m/z 470.1686778.
Ethyl 1-(3-(diethoxyphosphoryl)-2-((3,5-dinitrobenzoyl)oxy)propyl)-1H-1,2,3-triazole-4-carboxylate (5-B8). Yield: 76%. White solid. 1H-NMR (CDCl3) δ 1.31–1.40 (m, 9H), 2.37 (d, J = 6.7 Hz, 1H), 2.44 (d, J = 6.7 Hz, 1H), 4.13–4.23 (m, 4H), 4.37 (q, J = 7.1 Hz, 2H), 5.01–5.14 (m, 2H), 5.84–5.92 (m, 1H), 8.45 (s, 1H), 9.09 (d, J = 2.1 Hz, 2H), 9.20–9.22 (m, 1H); 13C-NMR (CDCl3) δ 13.87, 16.05 (d, JC-P = 6.0 Hz), 27.93 (d, JC-P = 141.6 Hz), 52.35 (d, JC-P = 8.8 Hz), 60.99, 62.18 (d, JC-P = 7.2 Hz), 62.28 (d, JC-P = 6.9 Hz), 69.54, 122.50, 128.64, 129.29, 132.55, 139.97, 148.35, 160.07, 161.20; 31P-NMR (CDCl3) δ 24.38; HR-MS: for C19H24N5O11P [M+H]+ calculated m/z 530.12, found m/z 530.1282694.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl benzoate (5-C1). Yield: 68%. White solid. 1H-NMR (CDCl3) δ: 1.23–1.35 (m, 15H), 2.20–2.33 (m, 2H), 4.06–4.19 (m, 4H), 4.76–4.87 (m, 2H), 5.65–5.73 (m, 1H), 7.36–7.47 (m, 3H), 7.56–7.61 (m, 1H), 7.99–8.03 (m, 2H); 13C-NMR (CDCl3) δ: 16.02 (d, JC-P = 6.3 Hz), 16.10 (d, JC-P = 6.3 Hz), 28.01 (d, JC-P = 140.7 Hz), 30.03, 30.41, 52.04 (d, JC-P = 7.6 Hz), 61.87 (d, JC-P = 1.4 Hz), 61.95 (d, JC-P = 1.4 Hz), 67.95, 119.79, 128.25, 129.03, 129.45, 133.27, 157.44, 164.95; 31P-NMR (CDCl3) δ: 25.58; HR-MS: for C20H30N3O5P [M+H]+ calculated m/z 424.19, found m/z 424.199584.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl4-nitrobenzoate (5-C2). Yield: 76%. White solid. 1H-NMR (CDCl3) δ: 1.26–1.38 (m, 15H), 2.27 (d, J = 6.6 Hz, 1H), 2.33 (d, J = 6.6 Hz, 1H), 4.08–4.21 (m, 4H), 4.83–4.86 (m, 2H), 5.69–5.76 (m, 1H), 7.42 (s, 1H), 8.17–8.21 (m, 2H), 8.27–8.31 (m, 2H); 13C-NMR (CDCl3) δ: 16.18 (d, JC-P = 4.0 Hz), 16.26 (d, JC-P = 3.6 Hz), 28.16 (d, JC-P = 141.4 Hz), 30.15, 30.56, 52.05 (d, JC-P = 7.6 Hz), 62.21 (d, JC-P = 6.5 Hz), 68.96, 119.81, 123.48, 130.78, 134.58, 150.65, 157.75, 163.36; 31P-NMR (CDCl3) δ: 24.99; HR-MS: for C20H29N4O7P [M+H]+ calculated m/z 469.18, found m/z 469.1846622.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 2-(naphthalen-1-yl)acetate (5-C3). Yield: 61%. White solid. 1H-NMR (CDCl3) δ: 1.21–1.33 (m, 15H), 1.91-2.13 (m, 2H), 4.03–4.15 (m, 6H), 4.55–4.56 (m, 2H), 5.42–5.51 (m, 1H), 6.89 (s, 1H), 7.36–7.56 (m, 4H), 7.79–7.87 (m, 2H), 7.94–7.97 (m, 1H); 13C-NMR (CDCl3) δ: 16.31 (d, JC-P = 6.3 Hz), 27.98 (d, JC-P = 141.2 Hz), 30.23, 30.53, 38.99, 52.26 (d, JC-P = 8.0 Hz), 62.09 (d, JC-P = 3.8 Hz), 62.18 (d, JC-P = 3.9 Hz), 68.02, 119.39, 123.54, 125.40, 125.87, 126.53, 128.03, 128.28, 128.75, 129.73, 131.90, 133.74, 157.71, 170.22; 31P-NMR (CDCl3) δ: 25.39; HR-MS: for C25H34N3O5P [M+H]+ calculated m/z 488.22, found m/z 488.2308841.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl acetate (5-C4). Yield: 71%. White solid. 1H-NMR (CDCl3) δ 1.26–1.37 (m, 15H), 2.03–2.21 (m, 5H), 4.08–4.19 (m, 4H), 4.58–4.76 (m, 2H), 5.41–5.47 (m, 1H), 7.37 (s, 1H); 13C-NMR (CDCl3) δ 16.16 (d, JC-P = 6.1 Hz), 20.60, 28.02 (d, JC-P = 141.2 Hz), 30.11, 30.47, 52.02 (d, JC-P = 7.9 Hz), 61.92 (d, JC-P = 6.3 Hz), 67.34, 119.48, 157.53, 169.36; 31P-NMR (CDCl3) δ 26.22; HR-MS: for C15H28N3O5P [M+H]+ calculated m/z 362.18, found m/z 362.183934.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 2-fluorobenzoate (5-C5). Yield: 66%. yellow solid. 1H-NMR (CDCl3) δ: 1.25–1.38 (m, 15H), 2.18–2.33 (m, 2H), 4.08–4.20 (m, 4H), 4.82–4.84 (m, 2H), 5.70–5.74 (m, 1H), 7.11–7.25 (m, 2H), 7.49 (s, 1H), 7.54–7.57 (m, 1H), 7.90–7.95 (m, 1H); 13C-NMR (CDCl3) δ: 15.99 (d, JC-P = 5.9 Hz), 16.07 (d, JC-P = 5.9 Hz), 27.92 (d, JC-P = 141.0 Hz), 30.01, 30.41, 52.11 (d, JC-P = 7.6 Hz), 61.99 (d, JC-P = 1.2 Hz), 62.07 (d, JC-P = 1.4 Hz), 68.42, 116.72 (d, JC-F = 22.3 Hz), 117.55 (d, JC-F = 9.5 Hz), 119.91 (d, JC-F = 2.3 Hz), 123.96 (d, JC-F = 3.9 Hz), 132.07, 134.90 (d, JC-F = 9.2 Hz), 157.45, 162.67 (d, JC-F = 3.5 Hz), 163.40; 31P-NMR (CDCl3) δ:24.90; HR-MS: for C20H29FN3O5P [M+H]+ calculated m/z 442.18, found m/z 442.1901622.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 4-methoxybenzoate (5-C6). Yield: 43%. yellow solid. 1H-NMR (CDCl3) δ 1.21–1.37 (m, 15H), 2.19–2.29 (m, 2H), 3.87 (s, 3H), 4.05–4.18 (m, 4H), 4.78–4.80 (m, 2H), 5.58–5.67 (m, 1H), 6.89–6.94 (m, 2H), 7.35 (s, 1H), 7.93–7.98 (m, 2H); 13C-NMR (CDCl3) δ: 16.23 (d, JC-P = 6.0 Hz), 16.30 (d, JC-P = 5.9 Hz), 28.27 (d, JC-P = 140.7 Hz), 30.24, 30.62, 52.28 (d, JC-P = 7.4 Hz), 55.41, 62.05 (d, JC-P = 1.9 Hz), 62.13 (d, JC-P = 2.0 Hz), 67.82, 113.73, 119.89, 121.55, 131.78, 157.68, 163.79, 164.91; 31P-NMR (CDCl3) δ 25.68; HR-MS: for C21H32N3O6P [M+H]+ calculated m/z 454.20, found m/z 454.2101487.
1-(4-(tert-Butyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 3,5-dinitrobenzoate (5-C7). Yield: 63%. White solid. 1H-NMR (CDCl3) δ: 1.24–1.37 (m, 15H), 2.27–2.35 (m, 2H), 4.10–4.21 (m, 4H), 4.77–4.89 (m, 2H), 5.76–5.84 (m, 1H), 7.42 (s, 1H), 9.09–9.10 (m, 2H), 9.23–9.24 (m, 1H); 13C-NMR (CDCl3) δ: 16.33 (d, JC-P = 5.8 Hz), 28.39 (d, JC-P = 141.9 Hz), 30.19, 30.66, 52.06 (d, JC-P = 8.6 Hz), 62.34 (d, JC-P = 6.8 Hz), 62.43 (d, JC-P = 6.8 Hz), 69.96, 119.69, 122.65, 129.43, 133.06, 148.65, 158.11, 161.37 ; 31P-NMR (CDCl3) δ:24.65; HR-MS: for C20H28N5O9P [M+H]+ calculated m/z 514.16, found m/z 514.1697404.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl benzoate (5-D1). Yield: 76%. White solid. 1H-NMR (CDCl3) δ 1.20–1.35 (m, 6H), 2.22 (d, J = 6.5 Hz, 1H), 2.28 (d, J = 6.5 Hz, 1H), 4.06–4.19 (m, 4H), 4.69 (s, 2H), 4.88–4.90 (m, 2H), 5.65–5.73 (m, 1H), 7.45 (t, J = 7.6 Hz, 2H), 7.56–7.61 (m, 1H), 7.78 (s, 1H), 7.98–8.01 (m, 2H); 13C-NMR (CDCl3) δ: 16.12 (d, JC-P = 6.3 Hz), 16.20 (d, JC-P = 6.3 Hz), 28.04 (d, JC-P = 140.7 Hz), 35.90, 52.45 (d, JC-P = 6.8 Hz), 62.13 (d, JC-P = 6.6 Hz), 67.86, 123.99, 128.41, 128.95, 129.61, 133.46, 144.68, 165.09; 31P-NMR (CDCl3) δ: 25.14; HR-MS: for C17H23ClN3O5P [M+H]+ calculated m/z 416.11, found m/z 416.1136615.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 4-nitrobenzoate (5-D2). Yield: 78%. White solid. 1H-NMR (CDCl3) δ: 1.29 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H), 2.26 (d, J = 6.9 Hz, 1H), 2.32 (d, J = 7.1 Hz, 1H), 4.08–4.21 (m, 4H), 4.70 (s, 2H), 4.91–4.94 (m, 2H), 5.72–5.76 (m, 1H), 7.83 (s, 1H), 8.17 (d, J = 2.0 Hz, 1H), 8.20 (d, J = 1.9 Hz, 1H), 8.27 (d, J = 2.0 Hz, 1H), 8.29 (d, J = 1.7 Hz, 1H); 13C-NMR (CDCl3) δ: 16.15 (d, JC-P = 3.8 Hz), 16.23 (d, JC-P = 3.7 Hz), 28.04 (d, JC-P = 141.1 Hz), 35.85, 52.28 (d, JC-P = 7.0 Hz), 62.20 (d, JC-P = 1.7 Hz), 62.28 (d, JC-P = 1.5 Hz), 68.77, 123.49, 124.00, 130.79, 134.39, 144.75, 150.64, 163.32; 31P-NMR (CDCl3) δ:24.70; HR-MS: for C17H22ClN4O7P [M+H]+ calculated m/z 461.09, found m/z 461.0987397.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 2-(naphthalen-1-yl)acetate (5-D3). Yield: 80%. White solid. 1H-NMR (CDCl3) δ: 1.26–1.35 (m, 6H), 1.94–2.04 (m, 2H), 4.06–4.16 (m, 6H), 4.41–4.46 (m, 2H), 4.51–4.53 (m, 2H), 5.41–5.44 (m, 1H), 6.59 (s, 1H), 7.37–7.57 (m, 4H), 7.84–7.96 (m, 3H); 13C-NMR (CDCl3) δ: 16.28 (d, JC-P = 5.9 Hz), 27.90 (d, JC-P = 141.0 Hz), 35.67, 39.11, 52.39 (d, JC-P = 7.4 Hz), 62.16 (d, JC-P = 6.6 Hz), 62.25 (d, JC-P = 6.5 Hz), 67.72, 123.50, 125.48, 126.01, 126.63, 128.22, 128.27, 128.75, 129.70, 131.80, 133.67, 144.29, 169.87; 31P-NMR (CDCl3) δ: 25.14; HR-MS: for C22H27ClN3O5P [M+H]+ calculated m/z 480.14, found m/z 480.1449616.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl acetate (5-D4). Yield: 54%. White solid. 1H-NMR (CDCl3) δ 1.31–1.38 (m, 6H), 2.05–2.15 (m, 2H), 2.06 (s, 3H), 4.09–4.20 (m, 4H), 4.65–4.82 (m, 4H), 5.41–5.48 (m, 1H), 7.74 (s, 1H); 13C-NMR (CDCl3) δ 16.31 (d, JC-P = 6.0 Hz), 20.76, 28.14 (d, JC-P = 141.0 Hz), 36.00, 52.44 (d, JC-P = 6.9 Hz), 62.17 (d, JC-P = 3.3 Hz), 62.26 (d, JC-P = 3.2 Hz), 67.31, 123.85, 144.81, 169.54; 31P-NMR (CDCl3) δ 25.19; HR-MS: for C12H21ClN3O5P [M+H]+ calculated m/z 354.09, found m/z 354.0980115.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 2-fluorobenzoate (5-D5). Yield: 52%. White solid. 1H-NMR (CDCl3) δ 1.25–1.38 (m, 6H), 2.23–2.31 (m, 2H), 4.08–4.20 (m, 4H), 4.70 (s, 2H), 4.89–4.91 (m, 2H), 5.70–5.74 (m, 1H), 7.12–7.25 (m, 2H), 7.55–7.58 (m, 1H), 7.85 (s, 1H), 7.89–7.95 (m, 1H); 13C-NMR (CDCl3) δ 16.05 (d, JC-P = 5.9 Hz), 16.13 (d, JC-P = 5.9 Hz), 27.93 (d, JC-P = 140.8 Hz), 35.86, 52.46 (d, JC-P = 6.8 Hz), 62.18 (d, JC-P = 6.5 Hz), 68.29, 116.84 (d, JC-F = 22.3 Hz), 124.06 (d, JC-F = 2.1 Hz), 124.09 (d, JC-F = 1.9 Hz), 132.13, 135.05 (d, JC-F = 9.1 Hz), 144.58, 160.04, 162.71 (d, JC-F = 3.5 Hz), 163.48; 31P-NMR (CDCl3) δ 25.02; HR-MS: for C17H22ClFN3O5P [M+H]+ calculated m/z 434.10, found m/z 434.1042397.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 4-methoxybenzoate (5-D6). Yield: 34%. White solid. 1H-NMR (CDCl3) δ: 1.24–1.38 (m, 6H), 2.19 (d, J = 6.6 Hz, 1H), 2.25 (d, J = 6.6 Hz, 1H), 3.87 (s, 3H), 4.06–4.19 (m, 4H), 4.69 (s, 2H), 4.86–4.88 (m, 2H), 5.62–5.66 (m, 1H), 6.90–6.95 (m, 2H), 7.74 (s, 1H), 7.93–7.98 (m, 2H); 13C-NMR (CDCl3) δ: 16.26 (d, JC-P = 6.0 Hz), 16.33 (d, JC-P = 5.7 Hz), 28.20 (d, JC-P = 140.5 Hz), 36.04, 52.62 (d, JC-P = 6.4 Hz), 55.45, 62.24 (d, JC-P = 6.6 Hz), 67.63, 113.83, 121.35, 124.03, 131.87, 144.83, 163.90, 164.93; 31P-NMR (CDCl3) δ:25.39; HR-MS: for C18H25ClN3O6P [M+H]+ calculated m/z 446.12, found m/z 446.1242262.
1-(4-(Chloromethyl)-1H-1,2,3-triazol-1-yl)-3-(diethoxyphosphoryl)propan-2-yl 3,5-dinitrobenzoate (5-D7). Yield: 76%. White solid. 1H-NMR (CDCl3) δ 1.26–1.38 (m, 6H), 2.32 (d, J = 6.8 Hz, 1H), 2.39 (d, J = 7.0 Hz, 1H), 4.12–4.23 (m, 4H), 4.68 (s, 2H), 4.88–5.02 (m, 2H), 5.79–5.87 (m, 1H), 7.87 (s, 1H), 9.09–9.10 (m, 2H), 9.21–9.22 (m, 1H); 13C-NMR (CDCl3) δ 16.18 (d, JC-P = 5.9 Hz), 28.13 (d, JC-P = 141.8 Hz), 35.76, 52.24 (d, JC-P = 8.4 Hz), 62.30 (d, JC-P = 6.8 Hz), 62.40 (d, JC-P = 6.6 Hz), 69.71, 122.60, 124.00, 129.38, 132.73, 144.74, 148.49, 161.27; 31P-NMR (CDCl3) δ 24.38; HR-MS: for C17H21ClN5O9P [M+H]+ calculated m/z 506.08, found m/z 506.0838179.