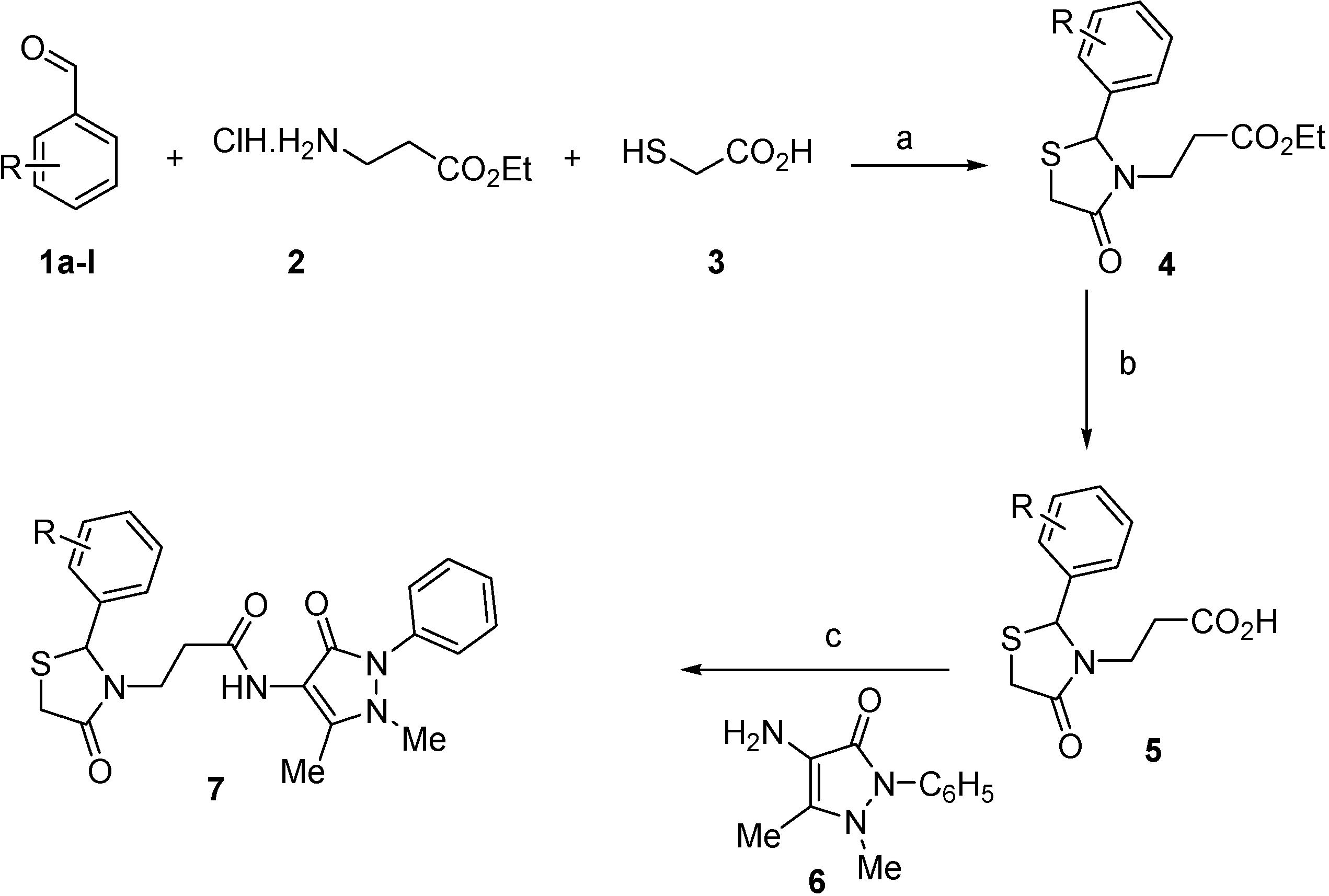
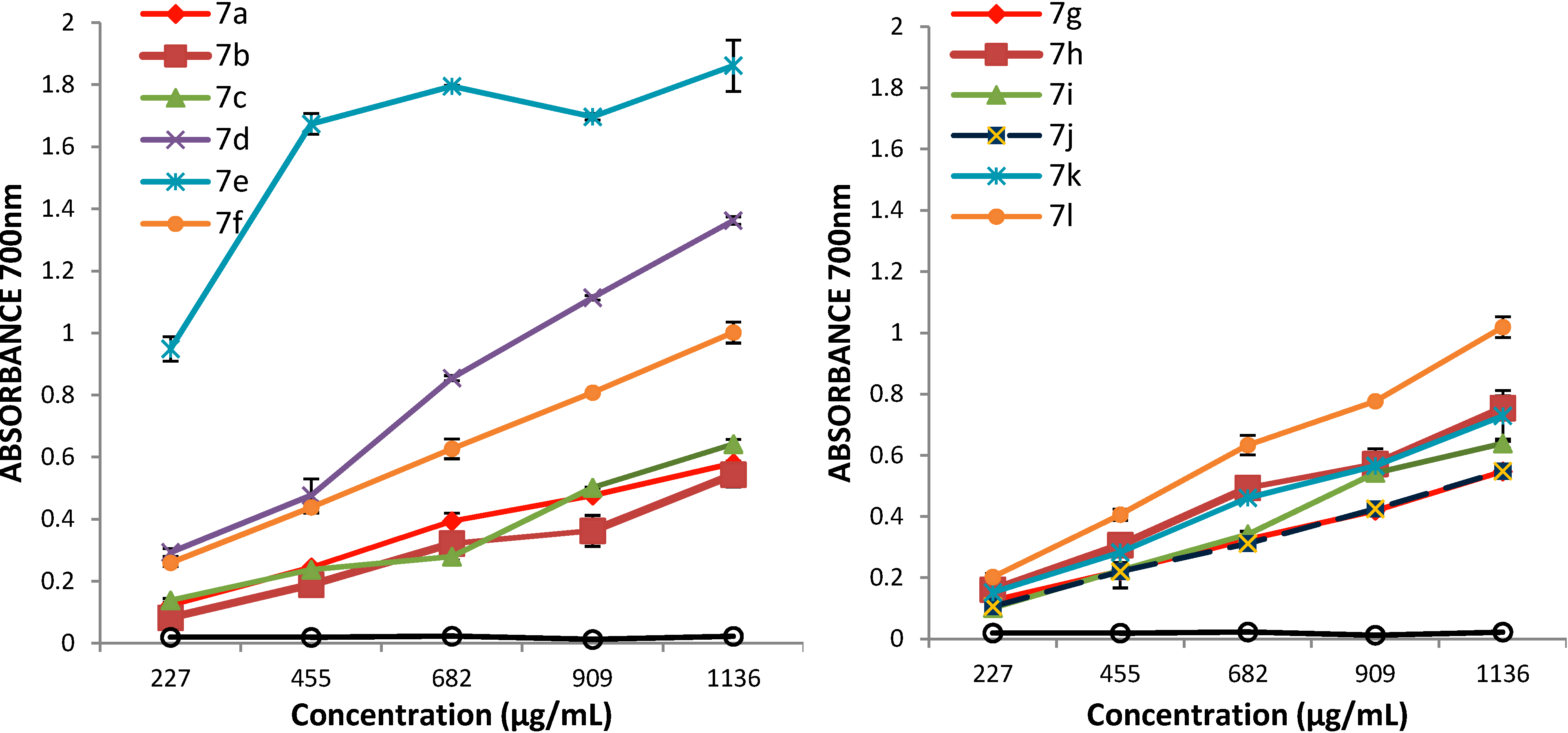
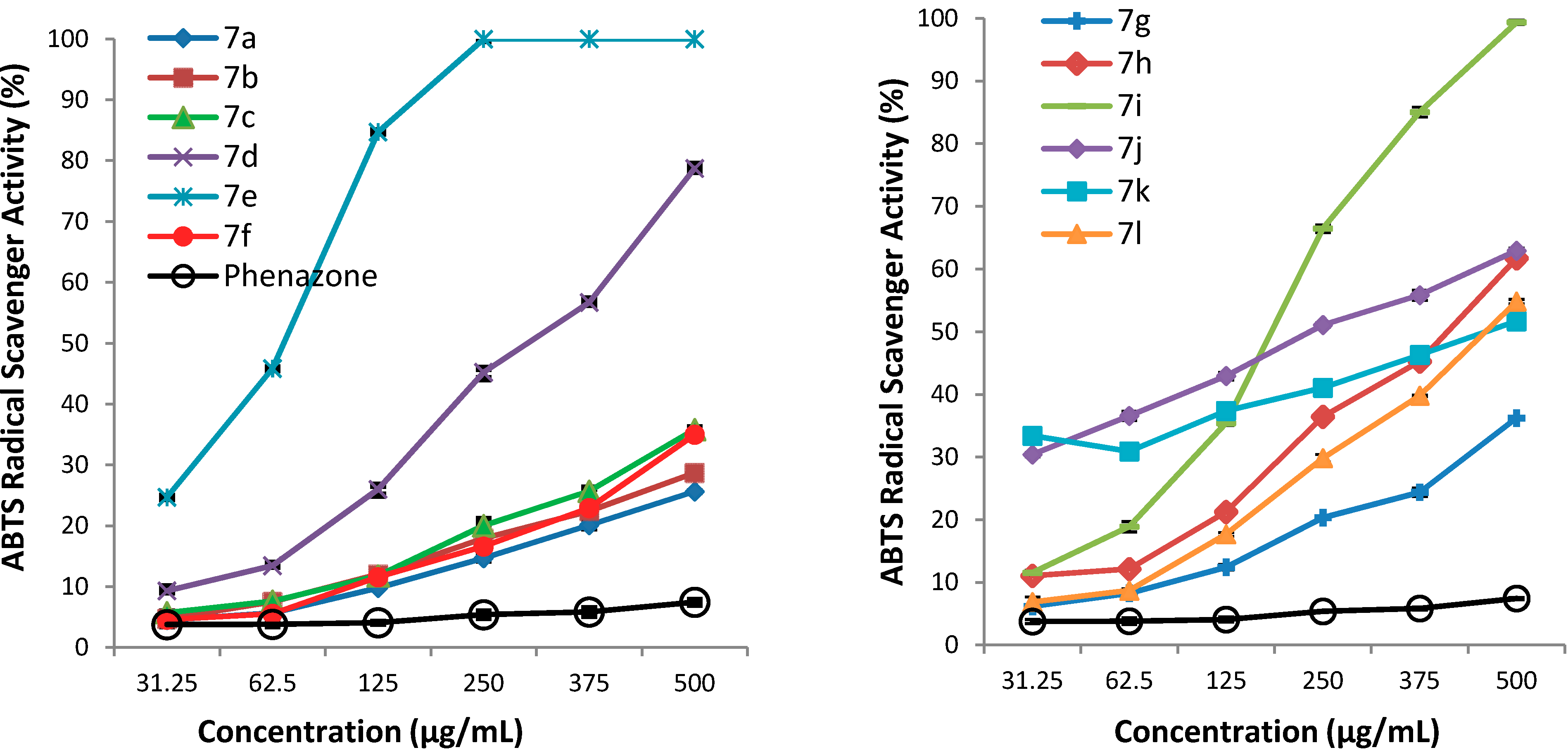
3.2. Synthetic Procedures
3.2.1. Preparation of Ethyl 3-(2-Aryl-4-oxo-thiazolidin-3-yl)-propionates 4a–l
To a solution of ethyl 3-aminopropionate hydrochloride
2 (10 mmol) in freshly distilled toluene (15 mL), aromatic aldehydes (15 mmol) were added under an inert atmosphere according to the procedure described for other compounds [
28]. The mixture was stirred for 5 min and mercaptoacetic acid
3 (20 mmol) was added. After 5 min,
N,N-diisopropylethylamine (DIPEA, 13 mmol) was added and then the mixture was heated at 110–115 °C for 36 h until completion of the reaction (TLC monitoring, using ethyl acetate/petroleum ether, 4:6, v/v, UV light at 254 nm). The mixture was neutralized with saturated solution of sodium bicarbonate and extracted with ethyl acetate (2 × 25 mL). The organic layer was separated and washed with hydrochloric acid 1M and then with saturated solution of sodium chloride. Finally, the organic layer was dried over MgSO
4 and filtered. The solvent was removed under reduced pressure and the residue was purified on a silica gel column using ethyl acetate/petroleum ether (4:6, v/v) as eluent system.
Ethyl 3-(2-phenyl-4-oxothiazolidin-3-yl)propanoate (4a). Yield: 56%, yellow liquid; IR (ATR diamond, cm−1): 2980 (CHAr), 1728 (COester), 1674 (COthiazolidine-4-one), 639 (C-S); 1H-NMR: 7.38–7.26 (m, 5H, Ar-H), 5.71 (s, 1H, CH), 4.10–4.02 (m, 2H, CH2CH3), 3.79–3.69 (m; 1H, CH2S; 1H, CH2N) 3.63 (dt, J = 15.6, 1.7Hz, 1H, CH2S), 3.05 (dtd, J = 14.1, 7.1, 1.7 Hz, 1H, CH2N), 2.66–2.56 (m, 1H, CH2CO), 2.40–2.31 (m, 1H, CH2CO), 1.2 (tt, J = 7.1, 1.7 Hz, 3H, CH3); 13C-NMR: 171.40, 171.35 (2C, CO), 139.54 (CAr), 129.19 (2C, CHAr), 129.09 (2C, CHAr), 127.00 (CHAr), 63.82 (CH), 60.75 (CH2CH3), 39.08 (CH2S), 32.63 (CH2N), 31.92 (CH2CO), 14.12 (CH3); HRMS (EI-MS): m/z Calcd for C14H17NO3S: 280.1002 [M+H]+, Found: 280.1004 [M+H]+.
Ethyl 3-[2-(4-chlorophenyl)-4-oxothiazolidin-3-yl]propanoate (
4b). Yield: 46%, colorless liquid, IR (ATR diamond, cm
−1): 2980 (CH
Ar), 1727 (CO
ester), 1674 (CO
thiazolidine-4-one), 767 (C-Cl), 622 (C-S) [
29];
1H-NMR: 7.22–7.09 (m, 4H, Ar-H), 5.59 (d,
J = 2.0 Hz, 1H, CH), 3.92 (q,
J = 7.1 Hz, 2H, C
H2CH
3), 3.67–3.52 (m; 1H, CH
2S, 1H, CH
2N), 3.48 (d,
J = 15.5 Hz, 1H, CH
2S), 2.88 (dt,
J = 14.2, 7.2 Hz, 1H, CH
2N), 2.48 (dt,
J = 16.6, 7.2 Hz, 1H, CH
2CO), 2.23(dt,
J = 16.6, 6.3 Hz, 1H, CH
2CO), 1.05 (t,
J = 7.2 Hz, 3H, CH
3);
13C-NMR: 171.15, 171.01 (2C, CO), 138.28, 134.66 (2C, C
Ar), 129.12 (2C, CH
Ar), 128.46 (CH
Ar), 62.88 (CH), 60.61 (
CH
2CH
3), 38.91 (CH
2S), 32.37 (CH
2N), 31.80 (
CH
2CO), 14.03 (CH
3); HRMS (EI-MS):
m/z Calcd for C
14H
16ClNO
3S: 314.0612 [M+H]
+, Found: 314.0614 [M+H]
+.
Ethyl 3-[2-(4-fluorophenyl)-4-oxothiazolidin-3-yl]propanoate (
4c). Yield: 42%, colorless liquid, IR (ATR diamond, cm
−1): 2981 (CH
Ar), 1727 (CO
ester), 1674 (CO
thiazolidine-4-one), 1155 (C-F), 636 (C-S) [
29];
1H-NMR: 7.33–7.25 (m, 2H, Ar-H), 7.03 (t,
J = 8.6 Hz, 2H, Ar-H), 5.71 (d,
J = 2.0 Hz, 1H, CH), 4.06 (qd,
J = 7.1, 0.9 Hz, 2H, C
H2CH
3 ), 3.77–3.59 (m; 2H, CH
2S; 1H, CH
2N), 3.01 (dt,
J = 14.3, 7.1 Hz, 1H, CH
2N), 2.60 (dt,
J = 16.4, 7.1 Hz, 1H, CH
2CO), 2.34 (dt,
J = 16.4, 6.3 Hz, 1H, CH
2CO), 1.22–1.13 (m, 3H, CH
3);
13C-NMR: 171.32, 171.17 (2C, CO), 164.14/161.67, 135.31/135.28 (2C, C
Ar), 129.05, 128.96 (2C, CH
Ar), 116.11, 115.90 (2C, CH
Ar), 63.10 (CH), 60.72 (
CH
2CH
3), 38.90 (CH
2S), 32.50 (CH
2N), 31.84 (
CH
2CO), 14.03 (CH
3); HRMS (EI-MS):
m/z Calcd for C
14H
16FNO
3S: 298.0900 [M+H]
+, Found: 298.0909 [M+H]
+.
Ethyl 3-[2-(4-bromophenyl)-4-oxothiazolidin-3-yl]propanoate (
4d). Yield: 76%, light yellow liquid; IR (ATR diamond, cm
−1): 2979 (CH
Ar), 1727 (CO
ester), 1674 (CO
thiazolidine-4-one), 625 (C-S) [
29];
1H-NMR: 7.39 (dd,
J = 8.5, 1.9 Hz, 2H, Ar-H), 7.11 (dd,
J = 8.5, 1.9 Hz, 2H, Ar-H), 5.62 (d,
J = 1.9 Hz, 1H, CH), 4.01–3.94 (m, 2H, C
H2CH
3), 3.68–3.58 (m;1H, CH
2S; 1H, CH
2N), 3.57–3.50 (m, 1H, CH
2S), 2.93 (m, 1H, CH
2N), 2.58–2.49 (m, 1H, CH
2CO), 2.34–2.23 (m, 1H, CH
2CO), 1.11 (td,
J = 7.1, 1.9 Hz, 3H, CH
3);
13C-NMR: 171.38, 171.27 (2C, CO), 138.90, 123.14 (2C, C
Ar), 132.29 (2C, CH
Ar), 128.88 (2C, CH
Ar), 63.20 (CH), 60.85 (
CH
2CH
3), 39.11 (CH
2N), 32.59 (CH
2S), 32.01 (
CH
2CO), 14.23 (CH
3); HRMS (EI-MS):
m/z Calcd for C
14H
16BrNO
3S: 358.0107 [M+H]
+, Found: 358.0106 [M+H]
+.
Ethyl 3-[2-(2-methoxyphenyl)-4-oxothiazolidin-3-yl]propanoate (
4e). Yield: 82%, yellow liquid; IR (ATR diamond, cm
−1): 2979 (CH
Ar), 1727 (CO
ester), 1674 (CO
thiazolidine-4-one), 644 (C-S) [
29];
1H-NMR (400 MHz, CDCl
3, δ ppm): 7.19 (ddd,
J = 8.3, 7.5, 1.7 Hz, 1H, Ar-H), 7.01 (dd,
J = 7.5, 1.7 Hz, 2H, Ar-H), 6.87–6.79 (m, 1H, Ar-H), 5.94 (d,
J = 2.0 Hz, 1H, CH), 4.02–3.95 (m, 2H, C
H2CH
3), 3.80–3.71 (m; 3H, OCH
3; 1H, CH
2N), 3.59 (dd,
J = 15.4, 2.0 Hz, 1H, CH
2S), 3.44 (d,
J = 15.4 Hz, 1H, CH
2S), 2.97 (dt,
J = 14.2, 7.4 Hz, 1H, CH
2N), 2.54 (dt,
J = 16.5, 7.4 Hz, 1H, CH
2CO), 2.34 (ddd,
J = 16.4, 7.4, 6.0 Hz, 1H, CH
2CO), 1.11 (t,
J = 7.4 Hz, CH
3);
13C-NMR: 171.72, 170.93 (2C, CO), 156.63, 127.37 (2C, C
Ar), 129.64, 126.33, 120.53, 110.85 (4C, CH
Ar), 60.35 (
CH
2CH,
3), 58.26 (CH), 55.30 (CH
3O), 39.00 (CH
2S), 32.11 (CH
2N), 31.77 (
CH
2CO), 13.84 (CH
3); HRMS (EI-MS):
m/z Calcd for C
15H
19NO
4S: 310.1107 [M+H]
+, Found: 310.1111 [M+H]
+.
Ethyl 3-[2-(3-methoxyphenyl)-4-oxothiazolidin-3-yl]propanoate (4f). Yield: 72%, slightly yellow liquid; IR (ATR diamond, cm−1): 2979 (CHAr), 1727 (COester), 1674 (COthiazolidine-4-one), 645 (C-S); 1H-NMR: 7.29 (t, J = 7.9 Hz, 1H, Ar-H), 6.88 (ddd, J = 7.9, 3.0, 1.6 Hz, 2H, Ar-H), 6.84 (t, J = 2.0 Hz, 1H, Ar-H), 5.71 (d, J = 2.0 Hz, 1H, CH), 4.10 (qd, J = 7.2, 1.0 Hz, 2H, CH2CH3), 3.82–3.75 (m; 3H, OCH3; 1H, CH2-N; 1H, CH2S), 3.67 (d, J = 15.6 Hz, 1H, CH2S), 3.11 (dt, J = 14.2, 7.2 Hz, 1H, CH2N), 2.64 (dt, J = 16.2, 7.2 Hz, 1H, CH2CO), 2.41 (dt, J = 16.2, 6.4 Hz, 1H, CH2CO), 1.23 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR: 171.55, 171.38 (2C, CO), 160.16, 141.12 (2C, CAr), 130.21, 119.13, 114.61, 112.43 (4C, CHAr), 63.82 (CH), 60.80 (CH2CH3), 55.33 (CH3O), 39.18 (CH2S), 32.64 (CH2N), 31.95 (CH2CO), 14.13 (CH3); HRMS (EI-MS): m/z Calcd for C15H19NO4S: 310.1108 [M+H]+, Found: 310.1110 [M+H]+.
Ethyl 3-[2-(4-methoxyphenyl)-4-oxothiazolidin-3-yl]propanoate (
4g). Yield: 71%, light yellow liquid; IR (ATR diamond, cm
−1): 2979 (CH
Ar), 1728 (CO
ester), 1673 (CO
thiazolidine-4-one), 628 (C-S) [
29];
1H-NMR: 7.09–7.05 (m, 2H, Ar-H), 6.70–6.66 (m, 2H, Ar-H), 5.50 (d,
J = 1.9 Hz, 1H, CH), 3.89–3.83 (m, 2H, C
H2CH
3), 3.57–3.55 (m, 3H, OCH
3), 3.53–3.47 (m; 1H, CH
2S; 1H, CH
2N), 3.42 (dd,
J = 15.6, 2.8 Hz, 1H, CH
2S), 2.91–2.82 (m, 1H, CH
2N), 2.40 (m, 1H, CH
2CO), 2.19–2.10 (m, 1H, CH
2CO), 1.02–0.97 (m, 3H, CH
3);
13C-NMR: 171.67, 170.99 (2C, CO), 160.06, 130.67 (2C, C
Ar), 128.10 (2C, CH
Ar), 113.81 (2C, CH
Ar), 62.86 (CH), 60.04 (
CH
2CH
3), 54.70 (CH
3O), 38.35 (CH
2S), 32.09 (CH
2N), 31.29 (
CH
2CO), 13.57 (CH
3); HRMS (EI-MS):
m/z Calcd for C
15H
19NO
4S: 310.1108 [M+H]
+, Found: 310.1110 [M+H]
+.
Ethyl 3-[2-(2-nitrophenyl)-4-oxothiazolidin-3-yl]propanoate (4h). Yield: 95%, yellow liquid; IR (ATR diamond, cm−1): 2981 (CHAr), 1726 (COester), 1676 (COthiazolidine-4-one), 641 (C-S), 1524 (sim NO2), 1343 (asim NO2); 1H-NMR: 7.92 (dt, J = 8.0, 2.4 Hz, 1H, Ar-H), 7.61–7.54 (m, 1H, Ar-H), 7.40–7.33 (m, 1H, Ar-H), 7.19 (dq, J = 8.0, 1.5 Hz, 1H, Ar-H), 6.17 (t, J = 1.9 Hz, 1H, CH), 3.88 (dd, J = 7.2, 3.8 Hz, 2H, CH2CH3), 3.83–3.73 (m, 1H, CH2N), 3.55–3.47 (m, 1H, CH2S), 3.38 (dd, J = 15.8, 3.8 Hz, 1H, CH2S), 2.93–2.83 (m, 1H, CH2N), 2.56–2.45 (m, 1H, CH2CO), 2.43–2.32 (m, 1H, CH2CO), 1.05–0.98 (m, 3H, CH3); 13C-NMR: 171.81, 170.79 (2C, CO), 146.62, 136.28 (2C, CAr), 134.18, 128.81, 125.65, 125.26 (4C, CHAr), 60.31 (CH2CH3), 58.42 (CH), 38.99 (CH2S), 31.59 (CH2CO), 30.82 (CH2N), 13.58 (CH3); HRMS (EI-MS): m/z Calcd for C14H16N2O5S: 325.0853 [M+H]+, Found: 325.0854 [M+H]+.
Ethyl 3-[2-(3-nitrophenyl)-4-oxothiazolidin-3-yl]propanoate (4i). Yield: 36%, white solid, m.p. 84–85 °C; IR (ATR diamond, cm−1): 2982 (CHAr), 1727 (COester), 1676 (COthiazolidine-4-one), 1528 (sym. NO2), 1349 (asym. NO2), 639 (C-S); 1H-NMR: 8.15–8.04 (m, 2H, Ar-H), 7.63 (dt, J = 8.0, 1.4 Hz, 1H, Ar-H), 7.52 (t, J = 7.8 Hz, 1H, Ar-H), 5.83 (d , J = 2.0 Hz, 1H, CH), 3.99 (d, J = 7.2 Hz, 2H, CH2CH3), 3.79–3.66 (m; 1H, CH2S; 1H, CH2N), 3.59 (d, J = 15.6 Hz,1H, CH2S), 3.01–2.89 (m, 1H, CH2N), 2.64–2.53 (m, 1H, CH2CO), 2.35 (m, 1H, CH2CO), 1.11 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR: 171.12, 170.98 (2C, CO), 148.31, 142.16 (2C, CAr), 132.85, 130.05, 123.73, 121.82 (4C, CHAr), 62.43 (CH), 60.59 (CH2CH3), 38.85 (CH2S), 32.14 (CH2N), 31.64 (CH2CO), 13.83 (CH3); HRMS (EI-MS): m/z Calcd for C14H16N2O5S: 325.0852 [M+H]+, Found: 325.0853 [M+H]+.
Ethyl 3-{2-[(3-hydroxy-4-methoxy)phenyl]-4-oxothiazolidin-3-yl}propanoate (4j). Yield: 56%, white solid, m.p. 74–75 °C; IR (ATR diamond, cm−1): 3422 (OH), 2930 (CHAr), 1712 (COester), 1654 (COthiazolidine-4-one), 648 (C-S); 1H-NMR: 6.83 (d, J = 1.5 Hz, 1H, Ar-H), 6.76–6.72 (m, 2H, Ar-H; s, 1H, OH), 5.58 (d, J = 1.5 Hz, 1H, CH), 4.05–3.99 (m, 2H, CH2CH3), 3.79 (s, 3H, OCH3), 3.73–3.63 (m; 1H CH2S; 1H, CH2N), 3.58 (d, J = 15.6 Hz, 1H, CH2S), 3.04 (dt, J = 14.2, 7.2 Hz, 1H, CH2N), 2.58–2.48 (m, 1H, CH2CO), 2.32 (dt, J = 16.3, 6.5 Hz, 1H, CH2CO), 1.15 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR: 171.35, 171.26 (2C, CO), 147.59, 146.33, 131.85 (3C, CAr), 118.77, 113.17, 110.79 (3C, CHAr), 63.59 (CH), 60.61 (CH2CH3), 55.82 (OCH3), 38.83 (CH2S), 32.49 (CH2N), 31.67 (CH2CO), 13.90 (CH3); HRMS (EI-MS): m/z Calcd for C15H19NO5S: 326.10577 [M+H]+, Found: 326.1059 [M+H]+.
Ethyl 3-{2-[(3-methoxy-4-hydroxy)phenyl]-4-oxothiazolidin-3-yl}propanoate (4k). Yield: 34%, white solid, m.p. 134–135 °C; IR (ATR diamond, cm−1): 3222 (OH), 2999 (CHAr), 1719 (COester), 1657 (COthiazolidine-4-one), 643 (C-S); 1H-NMR: 6.92–6.83 (m, 3H, Ar-H), 6.00 (s, 1H, OH), 5.71–5.68 (m, 1H, CH), 4.15–4.08 (m, 2H, CH2CH3), 3.90 (s, 3H, OCH3), 3.81–3.67 (m; 2H, CH2S; 1H, CH2N), 3.12 (dt, J = 14.2, 7.1 Hz, 1H, CH2N), 2.63 (dt, J = 16.3, 7.2 Hz, 1H, CH2CO), 2.39 (ddd, J = 16.3, 6.9, 6.0 Hz, 1H, CH2CO), 1.25 (t, J = 7.1 Hz, 3H, CH3); 13C-NMR: 171.40, 171.36 (2C, CO), 147.27, 146.69, 130.64 (3C, CAr), 120.79, 114.53, 109.22 (3C, CHAr), 64.17 (CH), 60.82 (CH2CH3), 56.06 (OCH3), 39.00 (CH2S), 32.85 (CH2N), 31.91 (CH2CO), 14.13 (CH3); HRMS (EI-MS): m/z Calcd for C15H19NO5S: 326.1057 [M+H]+, Found: 326.1059 [M+H]+.
Ethyl 3-[2-(4-methylphenyl)-4-oxothiazolidin-3-yl]propanoate (4l). Yield: 77%, colorless liquid; IR (ATR diamond, cm−1): 2980 (CHAr), 1728 (COester), 1674 (COthiazolidine-4-one), 632 (S-C); 1H-NMR: 7.19 (q, J = 8.2 Hz, 4H, Ar-H), 5.71 (d, J = 2.0 Hz, 1H, CH), 4.09 (qd, J =7.1, 1.3 Hz, 2H, CH2CH3), 3.80–3.71 (m; 1H, CH2S; 1H, CH2N), 3.64 (d, J = 15.5 Hz, 1H, CH2S), 3.08 (dt, J = 14.2, 7.1 Hz, 1H, CH2N), 2.62 (dt, J = 16.3, 7.2 Hz, 1H, CH2CO), 2.41–2.36 (m, 1H, CH2CO), 2.34 (s, 3H, CH3), 1.22 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR: 171.12, 171.10 (2C, CO), 138.94, 136.27 (2C, CAr), 129.55 (2C, CHAr), 126.84 (2C, CHAr), 63.47 (CH), 60.51 (CH2CH3), 38.82 (CH2S), 32.47 (CH2N), 31.72 (CH2CO), 21.00, 13.94 (2C, CH3); HRMS (EI-MS): m/z Calcd for C15H19NO3S: 294.1158 [M+H]+, Found: 294.1161 [M+H]+.
3.2.2. Preparation of 3-(2-Aryl-4-oxothiazolidin-3-yl)-propanoic Acids 5a–l
To a solution of ethyl 3-(2-aryl-4-oxothiazolidin-3-yl)-propanate
4a–
l (13.2 mmol) in a mixture of EtOH and THF (1:1, 25 mL:25 mL), potassium hydroxide 1 M (26 mmol) was added according to the procedure for alkaline hydrolysis of esters [
30]. The mixture of reaction was stirred for 6–10 h at room temperature until completion of the reaction (TLC monitoring, using ethyl acetate/petroleum ether, 4:6, v/v, UV light at 254 nm). After that, the mixture was neutralized with hydrochloric acid 1 M to pH 2, stirred again for another 20 min and finally extracted with ethyl acetate (2 × 25 mL). The organic layer was dried over MgSO
4 and filtered. The solvent was removed under reduced pressure and the residue was triturated with ethyl ether.
3-(2-Phenyl-4-oxothiazolidin-3-yl)propanoic acid (5a). Yield: 56%, white solid, m.p. 124 °C; IR (ATR diamond, cm−1): 3063 (OH), 1741 (COOH), 1725 (COthiazolidine-4-one), 699 (C-S); 1H-NMR: 11.00–10.88 (m, 1H, COOH), 7.42–7.29 (m, 5H, Ar-H), 5.74 (d, 1H, CH), 3.81(dd, J = 15.7, 2.0 Hz, 1H, CH2S), 3.78–3.68 (m; 1H, CH2S; 1H, CH2N), 3.10 (dt, J = 14.2, 7.1 Hz, 1H, CH2N), 2.69 (dt, J = 16.9, 7.2 Hz, 1H, CH2CO), 2.43 (dt, J = 16.9, 6.4 Hz, 1H, CH2CO); 13C-NMR: 175.99, 171.10 (2C, CO), 139.15 (CAr), 129.41 (2C, CHAr), 129.20 (2C, CHAr), 127.14 (CHAr), 64.90 (CH), 39.05 (CH2S), 32.76 (CH2N), 31.63 (CH2CO); HRMS (EI-MS): m/z Calcd for C12H14NO3S: 252.0691 [M+H]+, Found: 252.0691 [M+H]+.
3-[2-(4-Chlorophenyl)-4-oxothiazolidin-3-yl]propanoic acid (5b). Yield: 63%, white solid, m.p. 125–126 °C; IR (ATR diamond, cm−1): 3088 (OH), 1742 (COOH), 1724 (COthiazolidine-4-one), 768 (C-Cl), 622 (C-S); 1H-NMR: 10.17 (s, 1H, COOH), 7.57–7.11 (m, 4H, Ar-H), 5.75 (s, 1H, CH), 3.88–3.65 (m; 2H, CH2S; 1H, CH2N), 3.18–3.03 (m, 1H, CH2N), 2.75–2.64 (m, 1H, CH2CO), 2.52–2.30 (s, 1H, CH2CO); 13C-NMR: 176.11, 172.08 (2C, CO), 138.87, 135.36 (2C, CAr), 129.54 (2C, CHAr), 128.68 (2C, CHAr), 63.62 (CH), 39.13 (CH2S), 32.77 (CH2N), 31.76 (CH2CO); HRMS (EI-MS): m/z Calcd for C12H13ClNO3S: 286.0299 [M+H]+, Found: 286.0300 [M+H]+.
3-[2-(4-Fluorophenyl)-4-oxothiazolidin-3-yl]propanoic acid (5c). Yield: 70%, white solid, m.p. 94–95 °C; IR (ATR diamond, cm−1): 3076 (OH), 1743 (COOH), 1724 (COthiazolidine-4-one), 1223 (C-F), 623 (C-S); 1H-NMR: 10.69 (s, 1H, COOH), 7.34 (dd, J = 7.5, 4.0 Hz, 2H, Ar-H), 7.15–7.06 (m, 2H, Ar-H), 5.77 (s, 1H, CH), 3.86–3.69 (m; 2H, CH2S; 1H CH2N), 3.11 (dd, J = 14.4, 7.1 Hz, 1H, CH2N), 2.77–2.65 (m, 1H, CH2CO), 2.46 (dd, J = 14.4, 7.8 Hz, 1H, CH2CO); 13C-NMR: 176.06, 172.10 (2C, CO), 164.44/161.96, 134.98/134.96 (2C, CAr), 129.35, 129.29 (2C, CHAr), 116.42, 116.40 (2C, CHAr), 63.65 (CH), 39.06 (CH2S), 32.81 (CH2N), 31.73 (CH2CO); HRMS (EI-MS): m/z Calcd for C12H13FNO3S: 270.0595 [M+H]+, Found: 270.0596 [M+H]+.
3-[2-(4-Bromophenyl)-4-oxothiazolidin-3-yl]propanoic acid (5d). Yield: 75%, white solid, m.p. 116–117 °C; IR (ATR diamond, cm−1): 3083 (OH), 1741 (COOH), 1724 (COthiazolidine-4-one), 653 (C-Br), 621 (C-S); 1H-NMR: 10.12 (s, 1H, COOH), 7.52 (d, J = 8.1 Hz, 2H, Ar-H), 7.24–7.14 (m, 2H, Ar-H), 5.71 (d, J = 2.1 Hz, 1H, CH), 3.84–3.65 (m; 2H CH2S; 1H, CH2N), 3.07 (dt, J = 14.1, 7.0 Hz, 1H, CH2N), 2.69 (dt, J = 17.1, 7.0 Hz, 1H, CH2CO), 2.45 (dt, J = 16.9, 6.2 Hz, 1H, CH2CO); 13C-NMR: 176.10, 171.99 (2C, CO), 138.32, 132.40 (2C, CAr), 128.85 (2C, CHAr), 123.42 (2C, CHAr), 63.55 (CH), 39.04 (CH2S), 32.67 (CH2N), 31.71 (CH2CO); HRMS (EI-MS): m/z Calcd for C12H13BrNO3S: 329.9794 [M+H]+, Found: 329.9795 [M+H]+.
3-[2-(2-Methoxyphenyl)-4-oxothiazolidin-3-yl]propanoic acid (5e). Yield: 79%, brown sticky product; IR (ATR diamond, cm−1) 2940 (OH), 1724 (COOH), 1634 (COthiazolidine-4-one), 608 (C-S); 1H-NMR: 10.18 (s, 1H, COOH), 7.34–7.28 (m, 1H, Ar-H), 7.16–7.12 (m, 1H, Ar-H), 6.98–6.90 (m, 2H, Ar-H), 6.11–6.04 (m, 1H, CH), 3.85 (s, 3H, OCH3), 3.83–3.74 (m; 1H, CH2S, 1H, CH2N), 3.63 (d, J = 15.6 Hz, 1H, CH2S), 3.11 (dt, J = 14.3, 7.3 Hz, 1H, CH2N), 2.69 (dt, J = 16.9, 7.3 Hz, 1H, CH2CO), 2.55–2.46 (m, 1H, CH2CO); 13C-NMR: 175.48, 172.87 (2C, CO), 156.97, 127.13 (2C, CAr), 130.15, 126.98, 120.88, 111.17 (4C, CHAr), 59.07 (CH), 55.61 (OCH3), 39.20 (CH2S), 32.63 (CH2N), 31.70 (CH2CO); HRMS (EI-MS): m/z Calcd for C13H16NO4S: 282.0794 [M+H]+, Found: 282.0795 [M+H]+.
3-[2-(3-Methoxyphenyl)-4-oxothiazolidin-3-yl]propanoic acid (5f). Yield: 73%, white solid, m.p. 156 °C; IR (ATR diamond, cm−1): 2946 (OH), 1723 (COOH), 1627 (COthiazolidine-4-one), 645 (C-S); 1H-NMR (DMSO-d6): 12.34 (s, 1H, COOH), 7.36–7.30 (m, 1H, Ar-H), 6.93 (dt, J = 7.8, 1.6 Hz, 3H, Ar-H), 5.82 (d, J = 1.9 Hz, 1H, CH), 3.84 (dd, J = 15.6, 1.9 Hz, 1H, CH2S), 3.76 (s, 3H, OCH3), 3.68–3.59 (m; 1H, CH2S, 1H, CH2N), 2.85 (ddd, J = 14.4, 8.7, 6.2 Hz, 1H, CH2N), 2.57–2.50 (m, 1H, CH2CO), 2.28 (ddd, J = 16.4, 8.7, 5.9 Hz, 1H, CH2CO); 13C-NMR: 172.45, 170.72 (2C, CO), 159.60, 141.95 (2C, CAr), 130.16, 118.87, 114.19, 112.51 (4C, CHAr), 62.03 (CH), 55.19 (OCH3), 38.70 (CH2S), 31.78 (CH2N), 31.40 (CH2CO); HRMS (EI-MS): m/z Calcd for C13H16NO4S: 282.0794 [M+H]+, Found: 282.0797 [M+H]+.
3-[2-(4-Methoxyphenyl)-4-oxothiazolidin-3-yl]propanoic acid (5g). Yield: 83%; white solid, m.p. 108–110 °C; IR (ATR diamond, cm−1): 2929 (OH), 1730 (COOH), 1640 (COthiazolidine-4-one), 624 (C-S); 1H-NMR (DMSO-d6):12.35 (s, 1H, COOH), 7.34 (d, J =8.2 Hz, 2H, Ar-H), 6.96 (d, J = 8.2 Hz, 2H, Ar-H), 5.81 (s, 1H, CH), 3.79 (d, J = 14.4 Hz; 3H, OCH3; 1H, CH2S), 3.69–3.55 (m; 1H, CH2S; 1H, CH2N), 2.89–2.77 (m, 1H, CH2N), 2.47 (d, J = 7.6 Hz, 1H, CH2CO), 2.25 (dt, J = 14.4, 7.6 Hz, 1H, CH2CO); 13C-NMR: 172.88, 170.91 (2C, CO), 160.06, 132.15 (2C, CAr), 129.05 (2C, CHAr), 114.69 (2C, CHAr), 62.33 (CH), 55.65 (OCH3), 38.91 (CH2S), 32.37 (CH2N), 31.77 (CH2CO); HRMS (EI-MS): m/z Calcd for C13H16NO4S: 282.079455 [M+H]+, Found: 282.0796 [M+H]+.
3-[2-(2-Nitrophenyl)-4-oxothiazolidin-3-yl]propanoic acid (5h). 50%; white solid, m.p. 268 °C; IR (ATR diamond, cm−1): 2913 (OH), 1724 (COOH), 1629 (COthiazolidine-4-one), 1515 (sym. NO2), 1344 (asym. NO2), 674 (C-S); 1H-NMR (DMSO-d6): 12.34 (s, 1H, COOH), 8.12 (dd, J = 8.4, 1.3 Hz, 1H, Ar-H), 7.82 (td, J = 7.4, 1.3 Hz, 1H, Ar-H), 7.62 (ddd, J = 8.4, 7.4, 1.3 Hz, 1H, Ar-H), 7.37 (dd, J = 7.8, 1.3 Hz, 1H, Ar-H), 6.26 (d, J = 1.7 Hz, 1H, CH), 3.79–3.67 (m; 1H, CH2S; 1H, CH2N), 3.59 (J = 15.7 Hz, 1H, CH2S), 2.86 (dt, J = 14.2, 7.4 Hz, 1H, CH2N), 2.58 (ddd, J = 16.7, 7.8, 6.8 Hz, 1H, CH2CO), 2.42 (ddd, J = 16.7, 7.8, 5.6 Hz, 1H, CH2CO); 13C-NMR: 172.83, 171.75 (2C, CO), 146.96, 136.64 (2C, CAr), 134.99, 129.38, 126.21, 125.44 (4C, CHAr), 58.04 (CH), 38.89 (CH2S), 31.69 (CH2N), 30.58 (CH2CO); HRMS (EI-MS): m/z Calcd for C12H13N2O5S: 295.0539 [M+H]+, Found: 297.0540 [M+H]+.
3-[2-(3-Nitrophenyl)-4-oxothiazolidin-3-yl]propanoic acid (5i). Yield: 55%, slight brown solid; m.p. 160–162 °C; IR (ATR diamond, cm−1): 2917 (OH), 1725 (COOH), 1625 (COthiazolidine-4-one), 1525 (sym. NO2), 1350 (asym. NO2), 677 (C-S); 1H-NMR (DMSO-d6): 12.33 (s, 1H, COOH), 8.31–8.13 (m, 2H, Ar-H), 7.87 (dt, J = 7.9, 1.4 Hz, 1H, Ar-H), 7.71 (t, J = 7.9 Hz, 1H, Ar-H), 6.06 (d, J = 1.9 Hz, 1H, CH), 3.92 (dd, J = 15.4, 1.9 Hz, 1H, CH2S), 3.73–3.61 (m; 1H, CH2S; 1H, CH2N), 2.85 (ddd, J = 14.3, 8.4, 6.3 Hz, 1H, CH2N), 2.61–2.49 (m, 1H, CH2CO), 2.32 (ddd, J = 16.6, 8.4, 6.3 Hz, 1H, CH2CO); 13C-NMR: 172.85, 171.26 (2C, CO), 148.47, 143.44 (2C, CAr), 133.93, 131.14, 124.13, 122.25 (4C, CHAr), 61.42 (CH), 39.13 (CH2S), 32.12 (CH2N), 31.87 (CH2CO); HRMS (EI-MS): m/z Calcd for C12H13N2O5S: 295.0540 [M+H]+, Found: 297.0541 [M+H]+.
3-{2-[(3-Hydroxy-4-methoxy)phenyl]-4-oxothiazolidin-3-yl}propanoic acid (5j). Yield 75%; white solid, m.p. 172 °C; IR (ATR diamond, cm−1): 3208 (OH), 1733 (COOH), 1611 (COthiazolidine-4-one), 617 (C-S); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 12.29 (s, 1H, COOH), 9.16 (s, 1H, OH), 6.93–6.87 (m, 1H, Ar-H), 6.80–6.74 (m, 2H, Ar-H), 5.72 (d, J = 1.8 Hz, 1H, CH), 3.80–3.74 (m; 3H, OCH3, 1H, CH2S), 3.64 (d, J = 15.4 Hz, 1H, CH2S), 3.60–3.52 (m, 1H, CH2N), 2.88–2.79 (m, 1H, CH2N), 2.47–2.45 (m, 1H, CH2CO), 2.25 (m, 1H, CH2CO); 13C-NMR: 172.44, 170.44 (2C, CO), 148.21, 146.92, 132.12 (3C, CAr), 118.24, 113.75, 111.98 (3C, CHAr), 62.10 (CH), 55.62 (OCH3), 38.51 (CH2S), 31.91 (CH2N), 31.33 (CH2CO); HRMS (EI-MS): m/z Calcd for C13H16NO5S: 298.07430 [M+H]+, Found: 298.0744 [M+H]+.
3-{2-[(3-Methoxy-4-hydroxy)phenyl]-4-oxothiazolidin-3-yl}propanoic acid (5k). Yield 60%, white solid, m.p. 136–137 °C; IR (ATR diamond, cm−1): 2946 (OH), 1726 (COOH), 1626 (COthiazolidine-4-one), 615 (C-S); 1H-NMR (DMSO-d6): 12.30 (s, 1H, COOH), 9.22 (s, 1H, OH), 6.93 (s, 1H, Ar-H), 6.87–6.74 (m, 2H, Ar-H), 5.75 (s, 1H, CH), 3.79 (d, J = 9.2 Hz; 3H OCH3, 1H, CH2S), 3.67–3.52 (m; 1H, CH2S, 1H, CH2N), 2.93–2.82 (m, 1H, CH2N), 2.47 (dd, J = 9.2, 5.9 Hz, 1H, CH2CO), 2.30–2.17 (m, 1H, CH2CO); 13C-NMR: 172.90, 170.90 (2C, CO), 148.32, 147.63, 130.76 (3C, CAr), 120.43, 115.88, 111.45 (3C, CHAr), 62.91 (CH), 56.14 (OCH3), 38.97 (CH2S), 32.42 (CH2N), 31.80 (CH2CO); HRMS (EI-MS): m/z Calcd for C13H16NO5S: 298.0743 [M+H]+, Found: 298.0744 [M+H]+.
3-[2-(4-Methylphenyl)-4-oxothiazolidin-3-yl]propanoic acid (5l). Yield 71%, white solid, m.p. 119–121 °C; IR (ATR diamond, cm−1): 3307 (OH), 1662 (COOH), 1557 (COtiazolidine-4-one), 632 (S-C); 1H-NMR (DMSO-d6): 12.30 (s, 1H, COOH), 7.23 (d, J = 7.9 Hz, 2H, Ar-H), 7.15 (d, J = 7.9 Hz, 2H, Ar-H), 5.83 (d, J = 2.0 Hz, 1H, CH), 3.78 (dd, J = 15.4, 2.0 Hz, 1H, CH2S), 3.56 (d, 1H, CH2S), 3.51 (ddd, J = 14.0, 8.9, 5.4 Hz, 1H, CH2N), 2.74 (ddd, J = 14.0, 8.9, 6.9 Hz, 1H, CH2N), 2.31–2.21 (m; 3H, CH3; 1H, CH2CO), 2.06–1.97 (m, 1H, CH2CO); 13C-NMR: 176.84, 170.28 (2C, CO), 137.95, 137.75 (2C, CAr), 129.32 (2C, CHAr), 126.98 (2C, CHAr), 62.07 (CH) 38.87 (CH2S), 34.25 (CH2CO), 31.97 (CH2N), 20.80 (CH3); HRMS (EI-MS): m/z Calcd for C13H16NO3S: 266.0845 [M+H]+, Found: 266.0848 [M+H]+.
3.2.3. Preparation of the 3-(2-Aryl-4-oxo-thiazolidin-3-yl)-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) Propionamide Derivatives 7a–l
4-Aminophenazone (
6, 3.3 mmol),
N-(3-dimethylaminopropyl)-
N′-ethylcarbodiimide hydrochloride (EDCI·HCl) (3.3 mmol) and 1-hydroxybenzotriazole (HOBt) (3.3 mmol) were added to a cold solution of 3-(2-aryl-4-oxo-thiazolidin-3-yl)-propanoic acid
5a–
l (3 mmol) in dichloromethane (10–20 mL) under inert atmosphere according to the procedure for amide bond formation [
31]. The mixture was stirred for 24 h at room temperature until completion of the reaction (TLC monitoring, using dichloromethane-methanol, 10:0.5–0.8, v/v, UV light at 254). After that, the mixture was washed successively with hydrochloric acid 1M, sodium bicarbonate solution 10% and saturated solution of sodium chloride. The organic layer was collected, dried using anhydrous magnesium sulphate and concentrated by rotary evaporator. The residue was purified on a silica gel column using dichloromethane-methanol, 10:0.5–0.8, v/v as eluent system, and finally the product was triturated with cold ethyl ether.
3-(2-Phenyl-4-oxothiazolidin-3-yl)-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7a). Yield: 52%, white solid, m.p. 173–175 °C; IR (ATR diamond, cm−1): 3183 (NH), 3031 (CH) 1660 (CONH), 1652 (COthiazolidine-4-one), 1651 (COpyrazolin-5-one), 637 (C-S); 1H-NMR: 9.17 (s, 1H, NH), 7.47–7.41 (m, 2H, Ar-H), 7.40–7.36 (m, 2H, Ar-H), 7.34–7.27 (m, 4H, Ar-H), 7.25–7.20 (m, 2H, Ar-H), 5.82 (d, J = 1.9 Hz, 1H, CH), 3.88 (dt, J = 13.6, 6.5 Hz, 1H, CH2N), 3.77 (dd, J = 15.4, 1.9 Hz, 1H, CH2S), 3.66 (d, J = 15.4 Hz, 1H, CH2S), 3.08 (s, 3H, CH3N), 2.95–2.87 (m,1H, CH2N), 2.53 (dt, J = 15.4, 6.5 Hz, 1H, CH2CO), 2.40 (dt, J = 15.4, 6.5 Hz, 1H, CH2CO), 2.18 (s, 3H, CH3); 13C-NMR: 171.50, 170.29, 162.16 (3C, CO), 139.91, 134.50 (2C, CAr), 150.60, 108.28 (2C, Cpyrazoline), 129.40, (2C, CHAr), 129.04 (2C, CHAr), 127.30 (2C, CHAr), 127.09 (2C, CHAr), 124.73, 129.11 (2C, CHAr), 63.44 (CH), 39.53 (CH2N), 35.91, 12.16 (2C, CH3) 33.12 (CH2CO), 32.87 (CH2S); HRMS (EI-MS): m/z Calcd for C23H25N4O3S: 437.1642 [M+H]+, Found: 437.1644 [M+H]+.
3-[2-(4-Chlorophenyl)-4-oxothiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7b). Yield: 74%, white solid, m.p. 182–183 °C; IR (ATR diamond, cm−1): 3172 (NH), 3027 (CH), 1682 (CONH), 1647 (COthiazolidine-4-one), 1616 (COpyrazolin-5-one), 756 (C-Cl), 639 (C-S); 1H-NMR: 9.27–9.17 (m, 1H, NH), 7.48–7.43 (m, 2H, Ar-H), 7.39–7.35 (m, 2H, Ar-H), 7.32–7.25 (m, 3H, Ar-H), 7.17 (d, J = 8.5 Hz, 2H, Ar-H), 5.81 (d, J = 1.8 Hz, 1H, CH), 3.89–3.81 (m, 1H, CH2N), 3.78–3.63 (m, 2H, CH2S), 3.09 (s, 3H, CH3N), 2.86 (dt, J = 14.2, 6.5 Hz, 1H, CH2N), 2.53 (dt, J = 15.3, 6.5 Hz, 1H, CH2CO), 2.40 (dt, J = 15.3, 6.5 Hz, 1H, CH2CO), 2.18 (s, 3H, CH3); 13C-NMR: 171.26, 170.21, 162.04 (3C, CO), 138.41, 134.71, 134.33 (3C, CAr), 129.34 (2C, CHA), 129.19 (2C, CHA), 128.51 (2C, CHA), 127.31 (2C, CHA), 150.47, 108.03 (2C, Cpyrazoline), 124.71 (CHAr), 62.62 (CH), 39.33 (CH2N), 35.76, 12.02 (2C, CH3), 33.01 (CH2CO), 32.69 (CH2S); HRMS (EI-MS): m/z Calcd for C23H24ClN4O3S: 471.1252 [M+H]+, Found: 471.1251 [M+H]+.
3-[2-(4-Fluorophenyl)-4-oxothiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7c). Yield: 57%, light yellow solid; m.p. 86–88 °C; IR (ATR diamond, cm−1): 3245 (NH), 3013 (CH), 1652 (CONH), 1648 (COthiazolidine-4-one), 1620 (COpyrazolin-5-one), 1150 (C-F), 623 (C-S); 1H-NMR: 9.37 (d, J = 46.9 Hz, 1H, NH), 7.47–7.41 (m, 2H, Ar-H), 7.38–7.33 (m, 2H, Ar-H), 7.29 (dd, J = 7.2, 1.6 Hz, 1H, Ar-H), 7.25–7.18 (m, 2H, Ar-H), 7.01–6.93 (m, 2H, Ar-H), 5.81 (d, J = 1.8 Hz, 1H, CH), 3.85 (dd, J = 7.7, 6.6 Hz, 1H, CH2N), 3.76–3.62 (m, 2H, CH2S), 3.08 (s, 3H, CH3N), 2.85 (dd, J = 13.6, 6.6 Hz, 1H, CH2N), 2.56–2.47 (m, 1H, CH2CO), 2.44–2.35 (m, 1H, CH2CO), 2.16 (s, 3H, CH3); 13C-NMR: 171.31/171.28, 170.40/170.38, 162.15 (3C, CO), 164.14/161.67, 135.61/135.58 134.35/134.28 (3C, CAr), 129.40 (CHAr), 129.17, 129.08 (2C, CHAr), 127.41 (2C, CHAr), 124.83 (2C, CHAr), 116.00, 115.90 (2C, CHAr), 150.63, 108.03 (2C, Cpyrazoline), 62.67 (CH), 39.34 (CH2N), 35.77, 12.03 (2C, CH3), 32.99 (CH2CO), 32.80 (CH2S); HRMS (EI-MS): m/z Calcd for C23H24FN4O3S: 455.1542 [M+H]+, Found: 455.1543 [M+H]+.
3-[2-(4-Bromophenyl)-4-oxothiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7d). Yield: 92%, yellow solid; m.p. 110–112 °C; IR (ATR diamond, cm−1): 3196 (NH), 2984 (CH), 1664 (CONH), 1655 (COthiazolidine-4-one), 1621 (COpyrazolin-5-one), 643 (C-S), 668 (C-Br); 1H-NMR: 9.67 (s, 1H, NH), 7.40 (ddd, J = 13.8, 11.9, 7.6 Hz, 6H, Ar-H), 7.29 (d, J = 5.8 Hz, 1H, Ar-H), 7.09 (d, J = 8.3 Hz, 2H, Ar-H), 5.80 (s, 1H, CH), 3.90–3.82 (m, 1H, CH2N), 3.68 (dd, J = 37.7, 15.5 Hz, 2H, CH2S), 3.08 (s, 3H, CH3N), 2.83–2.75 (m, 1H, CH2N), 2.49 (dt, J = 12.7, 6.3 Hz, 1H, CH2CO), 2.43–2.35 (m, 1H, CH2CO), 2.16 (s, 3H, CH3); 13C-NMR: 171.16, 170.36, 162.11 (3C, CO), 138.99, 134.27, 132.09 (3C, CAr), 129.33 (2C, CHAr), 128.80 (2C, CHAr), 127.33 (2C, CHAr), 124.75 (2C, CHAr), 122.79 (CHAr), 150.65, 107.96 (2C, Cpyrazoline), 62.49 (CH), 39.29 (CH2N), 35.69, 11.96 (2C, CH3), 32.84 (CH2CO), 32.68 (CH2S); HRMS (EI-MS): m/z Calcd for C23H24BrN4O3S: 515.0747 [M+H]+, Found: 515.0745 [M+H]+.
3-[2-(2-Methoxyphenyl)-4-oxo-thiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7e). Yield: 60%, white solid; m.p.162 °C; IR (ATR diamond, cm−1): 3243 (NH), 2942 (CH), 1675 (CONH), 1647 (COthiazolidine-4-one), 1620 (COpyrazolin-5-one) 617 (C-S); 1H-NMR: 9.01 (d, J = 6.1 Hz, 1H, NH), 7.41 (dd, J = 20.5, 7.8 Hz, 4H, Ar-H), 7.28 (t, J = 7.8 Hz, 2H, Ar-H), 7.08 (d, J = 7.3 Hz, 1H, Ar-H), 6.90 (d, J = 8.5 Hz, 2H, Ar-H), 6.10 (s, 1H, CH), 3.93 (dd, J = 13.9, 6.8 Hz, 1H, CH2N), 3.87–3.81 (m, 3H, OCH3), 3.72 (d, J = 15.3 Hz, 1H, CH2S), 3.58 (d, J = 15.3 Hz, 1H, CH2S), 3.08 (s, 3H, CH3N), 3.01 (dd, J = 13.9, 6.8 Hz, 1H, CH2N), 2.59 (dt, J = 14.8, 6.8 Hz, 1H, CH2CO), 2.48 (dt, J = 14.8, 6.8 Hz, 1H, CH2CO), 2.20 (s, 3H, CH3); 13C-NMR: 172.14, 170.09, 162.00 (3C, CO), 156.99, 134.48, 127.79 (3C, CAr), 129.80 (CHAr), 129.26 (2C, CHAr), 127.04 (2C, CHAr), 126.81, 124.49, 120.77, 111.08 (4C, CHAr), 150.45, 108.38 (2C, Cpyrazoline), 58.81 (CH), 55.59 (OCH3), 39.75 (CH2N), 35.90, 12.12 (2C, CH3), 33.36 (CH2CO), 32.63 (CH2S); HRMS(EI-MS): m/z Calcd for C24H27N4O4S: 467.1747 [M+H]+, Found:467.1748 [M+H]+.
3-[2-(3-Methoxyphenyl)-4-oxo-thiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7f). Yield: 75%, yellow solid; m.p. 74–76 °C; IR (ATR diamond, cm−1): 3247 (NH), 2930 (CH), 1667 (CONH), 1659 (COthiazolidine-4-one), 1640 (COpyrazolin-5-one), 641 (C-S); 1H-NMR: 9.33 (d, J = 17.4 Hz, 1H, NH), 7.45 (t, J = 7.7 Hz, 2H, Ar-H), 7.38 (d, J = 7.7 Hz, 2H, Ar-H), 7.29 (t, J = 7.2 Hz, 1H, Ar-H), 7.23 (dd, J = 11.5, 4.6 Hz, 1H, Ar-H), 6.86–6.78 (m, 3H, Ar-H), 5.82 (s, 1H, CH), 3.95–3.86 (m, 1H, CH2N), 3.82–3.74 (m; 3H, OCH3; 1H, CH2S), 3.66 (d, J = 15.4 Hz, 1H, CH2S), 3.09 (s, 3H, CH3N), 2.99–2.89 (m, 1H, CH2N), 2.59–2.50 (m, 1H, CH2CO), 2.47–2.38 (m, 1H, CH2CO), 2.18 (s, 3H, CH3); 13C-NMR: 171.38, 170.28, 162.09 (3C, CO), 160.10, 141.45, 134.37 (3C, CAr), 130.06, 124.65, 119.20, 114.48, 112.24 (5C, CHAr), 129.29 (2C, CHAr), 127.19 (2C, CHAr), 150.56, 108.17 (2C, Cpyrazoline), 63.24 (CH), 55.31 (OCH3), 39.47 (CH2N), 35.78, 12.03 (2C, CH3), 32.95 (CH2CO), 32.75 (CH2S); HRMS (EI-MS): m/z Calcd for C24H27N4O4S: 467.1748 [M+H]+, Found: 467.1746 [M+H]+.
3-[2-(4-Methoxyphenyl)-4-oxothiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7g). Yield: 86%; white solid; m.p. 120 °C; IR (ATR diamond, cm−1): 3247 (NH), 2929 (CH), 1656 (CONH), 1651 (COthiazolidine-4-one), 1610 (COpyrazolin-5-one), 623 (C-S); 1H-NMR: 9.21 (s, 1H, NH), 7.45 (t, J = 7.7 Hz, 2H, Ar-H), 7.38 (d, J = 7.7 Hz, 2H, Ar-H), 7.31–7.27 (m, 1H, Ar-H), 7.19 (d, J = 8.6 Hz, 2H, Ar-H), 6.83 (d, J = 8.6 Hz, 2H, Ar-H), 5.79 (s, 1H, CH), 3.87 (dt, J = 13.7, 6.6 Hz, 1H, CH2N), 3.76 (d, J = 19.3 Hz; 3H, OCH3; 1H, CH2S), 3.67 (d, J = 15.5 Hz, 1H, CH2S), 3.09 (s, 3H, CH3N), 2.96–2.88 (m, 1H, CH2N), 2.53 (dt, J = 13.1, 6.6 Hz, 1H, CH2CO), 2.45-–2.36 (m, 1H, CH2CO), 2.18 (s, 3H, CH3); 13C-NMR: 171.30, 170.32, 162.14 (3C, CO), 160.12, 134.45, 131.46 (3C, CAr), 129.37 (2C, CHAr), 128.65 (2C, CHAr), 127.28 (2C, CHAr), 124.72, 119.20, 114.41 (3C, CHAr), 150.61, 108.20 (2C, Cpyrazoline), 63.14 (CH), 55.41 (OCH3), 39.33 (CH2N), 35.86, 12.09 (2C, CH3), 33.05 (CH2CO), 32.95 (CH2S); HRMS (EI-MS): m/z Calcd for C24H27N4O4S: 467.1747 [M+H]+, Found: 467.1748 [M+H]+.
3-[2-(2-Nitrophenyl)-4-oxothiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7h). Yield: 62%; light yellow solid; m.p. 166 °C; IR (ATR diamond, cm−1): 3244 (NH), 3016 (CH), 1686 (CONH), 1647 (COthiazolidine-4-one), 1622 (COpyrazolin-5-one), 1522 (sym. NO2), 1339 (asym. NO2), 677 (C-S); 1H-NMR: 9.22 (s, 1H, NH), 8.11 (d, J = 8.0 Hz, 1H, Ar-H), 7.64 (t, J = 7.4 Hz, 1H, Ar-H), 7.47 (dd, J = 10.7, 4.5 Hz, 3H, Ar-H), 7.39–7.35 (m, 2H, Ar-H), 7.31 (t, J = 7.4 Hz, 1H, Ar-H), 7.23–7.18 (m, 1H, Ar-H), 6.35 (s, 1H, CH), 3.91–3.83 (m, 1H, CH2N), 3.62 (d, J = 15.7 Hz, 1H, CH2S), 3.54 (d, J = 15.7 Hz, 1H, CH2S), 3.11 (s, 3H, CH3N), 2.91 (dt, J = 13.8, 6.9 Hz, CH2N), 2.62 (dt, J = 8.0, 7.1 Hz, 1H, CH2CO), 2.46 (dd, J = 13.8, 8.0 Hz, 1H, CH2CO), 2.18 (s, 3H, CH3); 13C-NMR: 172.44, 170.06, 161.94 (3C, CO), 147.16, 137.34, 136.60 (3C, CAr), 134.36, 125.95, 125.79 (3C, CHAr), 124.81 (2C, CHAr), 129.34, 128.93 (2C, CHAr), 127.29 (2C, CHAr), 150.47, 107.87 (2C, Cpyrazoline), 59.25 (CH), 39.53 (CH2N), 35.82, 12.14 (2C, CH3), 33.31 (CH2CO), 31.40 (CH2S); HRMS (EI-MS): m/z Calcd for C23H24N5O5S: 482.1492 [M+H]+, Found: 482.1492 [M+H]+.
3-[2-(3-Nitrophenyl)-4-oxo-thiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7i). Yield: 77%, yellow solid; m.p. 124 °C; IR (ATR diamond, cm−1): 3246 (NH), 2928 (CH), 1668 (CONH), 1650 (COthiazolidine-4-one), 1591 (COpyrazolin-5-one), 1527 (sym. NO2), 1349 (asym. NO2), 641 (C-S); 1H-NMR: 9.44 (s, 1H, NH), 8.16 (d, J = 8.3 Hz, 2H, Ar-H), 7.59 (d, J = 7.9 Hz, 1H, Ar-H), 7.53–7.44 (m, 3H, Ar-H), 7.40 (d, J = 7.9 Hz, 2H, Ar-H), 7.30 (dd, J = 12.6, 3.9 Hz, 1H, Ar-H), 5.99 (s, 1H, CH), 3.92–3.84 (m, 1H, CH2N), 3.80 (d, J = 15.6 Hz, 1H, CH2S), 3.70 (d, J = 15.6 Hz, 1H, CH2S), 3.12 (s, 3H, CH3N), 2.91–2.83 (m, 1H, CH2N), 2.59 (dt, J = 12.9, 6.4 Hz, 1H, CH2CO), 2.43 (dt, J = 15.6, 5.9 Hz, 1H, CH2CO), 2.19 (s, 3H, CH3); 13C-NMR: 171.29, 170.44, 162.05 (3C, CO), 148.65, 142.61, 134.30 (3C, CAr), 133.18, 130.13, 124.90, 123.6, 122.27 (5C, CHAr), 129.42 (2C, CHAr), 127.46 (2C, CHAr), 150.46, 107.91 (2C, Cpyrazoline), 62.34 (CH), 39.46 (CH2N), 35.75, 12.06 (2C, CH3), 33.11 (CH2CO), 32.64 (CH2S); HRMS (EI-MS): m/z Calcd for C23H24N5O5S: 482.1492 [M+H]+, Found: 482.1493 [M+H]+.
3-{2-[(3-Hydroxi-4-methoxy)phenyl]-4-oxothiazolidin-3-yl}-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7j). Yield 62%; light yellow solid, m.p. 120–122 °C; IR (ATR diamond, cm−1): 3355 (OH), 3240 (NH), 2935 (CH), 1652 (CONH, COthiazolidine-4-one), 1591 (COpyrazolin-5-one), 667 (C-S); 1H-NMR: 9.03 (s, 1H, NH), 7.43 (dd, J = 8.4, 7.2 Hz, 2H, Ar-H, 7.35–7.32 (m, 2H, Ar-H), 7.26 (s, 1H, Ar-H), 6.88 (t, J = 4.3 Hz, 2H, Ar-H), 6.75 (d, J = 1.9 Hz, 2H, Ar-H), 5.68 (d, J = 1.9Hz, 1H, CH), 3.85–3.73 (m; 3H, OCH3; 1H, CH2S; 1H, CH2N; 1H, OH)), 3.63 (d, J = 15.5 Hz, 1H, CH2S), 3.09 (m; 3H, CH3N; 1H, CH2N), 2.54–2.38 (m, 2H, CH2CO), 2.16 (s, 3H, CH3); 13C-NMR: 171.46, 170.55, 162.04 (3C, CO), 147.81, 146.52, 134.32, 132.43 (4C, CAr), 124.88, 118.98, 113.76, 111.16 (4C, CHAr), 129.39 (2C, CHAr), 127.40 (2C, CHAr), 150.65, 107.83 (2C, Cpyrazoline), 63.63 (CH), 56.02 (CH3O), 39.66 (CH2N), 35.76, 12.02 (2C, CH3), 33.36 (CH2CO), 32.95 (CH2S); HRMS (EI-MS): m/z Calcd for C24H27N4O5S: 483.1697 [M+H]+, Found: 483.1698 [M+H]+.
3-{2-[(3-Methoxy-4-hydroxi)phenyl]-4-oxothiazolidin-3-yl}-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7k). Yield 67%, white solid; m.p. 123–125 °C; IR (ATR diamond, cm−1): 3242 (OH), 3182 (NH), 2925 (CH), 1652 (CONH, COthiazolidine-4-one), 1591 (COpyrazolin-5-one), 715 (C-S); 1H-NMR: 8.96 (s, 1H, NH), 7.44 (t, J = 7.8 Hz, 2H, Ar-H), 7.36 (d, J = 7.8 Hz, 2H, Ar-H), 7.31–7.27 (m, 2H, Ar-H), 6.81 (dd, J = 4.8, 3.2 Hz, 2H, Ar-H), 6.76 (dd, J = 8.1, 1.7 Hz, 1H, Ar-H), 6.31 (s, 1H, OH), 5.76 (s, 1H, CH), 3.84–3.72 (m; 3H, OCH3; 1H, CH2S; 1H, CH2N), 3.68 (d, J = 15.4 Hz, 1H, CH2S), 3.08 (s, 3H, CH3N), 2.96 (dd, J = 13.8, 6.8 Hz, 1H, CH2N), 2.54 (dd, J = 14.4, 7.5 Hz, 1H, CH2CO), 2.40 (dd, J = 14.4, 7.5 Hz, 1H, CH2CO), 2.18 (s, 3H, CH3); 13C-NMR: 171.40, 170.30, 161.96 (3C, CO), 147.31, 146.58, 134.34, 130.72 (4C, CAr), 124.65, 120.79, 114.61, 109.46 (4C, CHAr), 129.29 (2C, CHAr), 127.25 (2C, CHAr), 150.46, 107.99 (2C, Cpyrazoline), 63.78 (CH), 56.04 (CH3O), 39.37 (CH2N), 35.77, 12.02 (2C, CH3), 33.03 (CH2CO), 32.99 (CH2S); HRMS (EI-MS): m/z Calcd for C24H27N4O5S: 483.1697 [M+H]+, Found: 483.1698 [M+H]+.
3-[2-(4-Methylphenyl)-4-oxo-thiazolidin-3-yl]-N-(2,3-dimethyl-1-phenyl-5-oxo-pyrazolin-4-yl) propionamide (7l). Yield 66%, light yellow solid; m.p.70–72 °C; IR (ATR diamond, cm−1): 3247 (NH), 3024.16 (C-H), 1668 (CONH), 1652 (COthiazolidine-4-one), 1591 (COpyrazolin-5-one) 632 (S-C); 1H-NMR: 9.46 (s, 1H, NH), 7.45 (t, J = 7.9 Hz, 2H, Ar-H), 7.39 (d, J = 7.9 Hz, 2H, 2Ar-H), 7.29 (s, 1H, Ar-H), 7.12 (s, 4H, Ar-H), 5.81 (s, 1H, CH), 3.90 (dt, J = 13.6, 6.9 Hz, 1H, CH2N), 3.76 (d, J = 15.5 Hz, 1H, CH2S), 3.66 (d, J = 15.5 Hz, 1H, CH2S), 3.09 (s, 3H, CH3N), 2.94–2.85 (m, 1H, CH2N), 2.51 (m, 1H, CH2CO), 2.45–2.38 (m, 1H, CH2CO), 2.33 (s, 3H, CH3), 2.19 (s, 3H, CH3); 13C-NMR: 171.30, 170.31, 162.13 (3C, CO), 138.82, 136.72, 134.38, (3C, CAr), 124.65 (CHAr), 129.66 (2C, CHAr), 129.29 (2C, CHAr), 127.19 (2C, CHAr), 126.96 (2C, CHAr), 150.65, 108.18 (2C, Cpyrazoline), 63.10 (CH), 39.33 (CH2N), 35.78, 21.20, 12.01 (3C, CH3), 32.91 (CH2CO), 32.81 (CH2S); HRMS (EI-MS): m/z Calcd fot C24H27N4O3S: 451.1798 [M+H]+, Found: 451.1801 [M+H]+.