Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties
Abstract
:1. Introduction
The Concept of Wettability
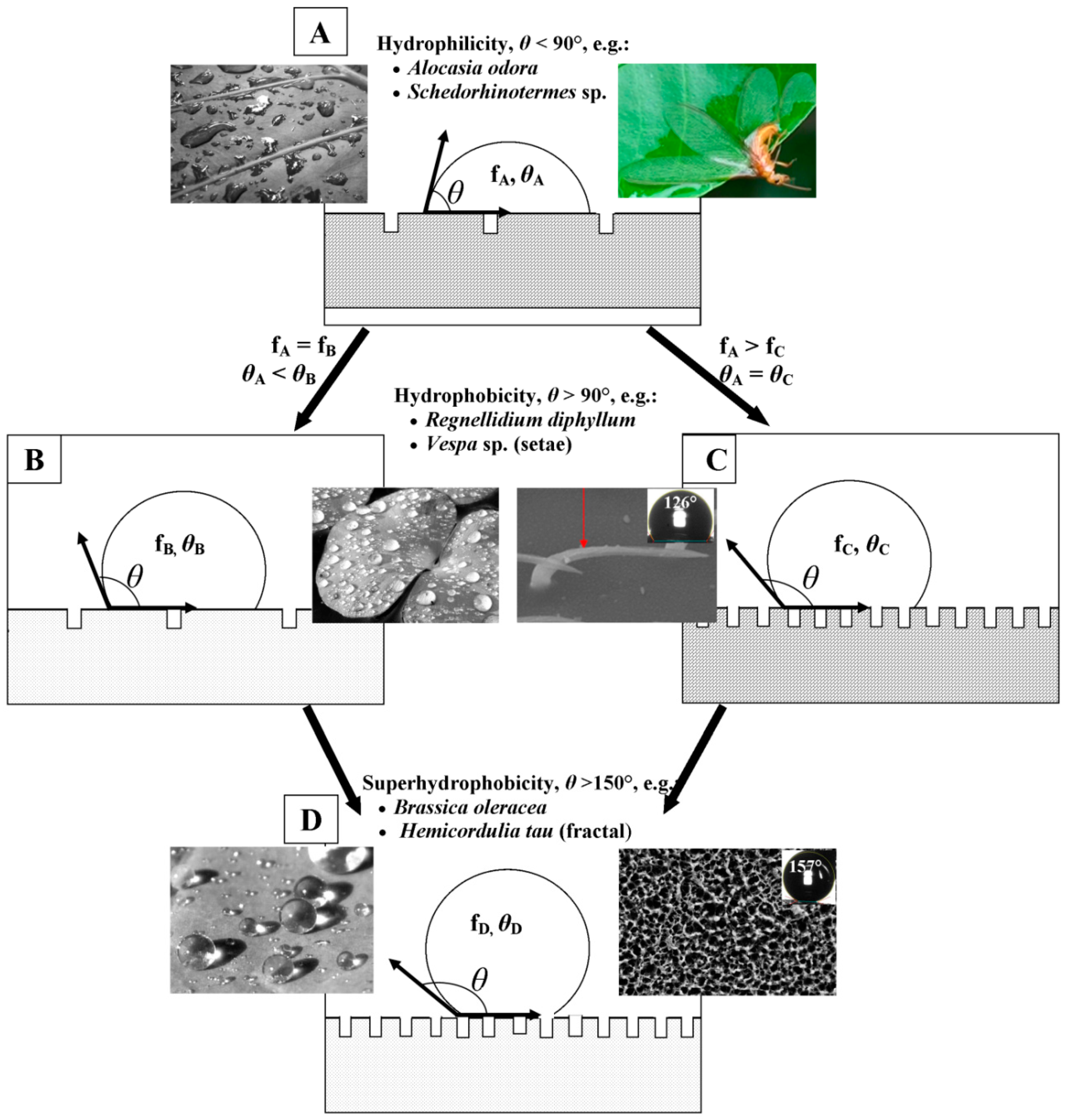
2. Cuticular Layer of Insects and Plants
2.1. Surface Morphology of Insect Wings
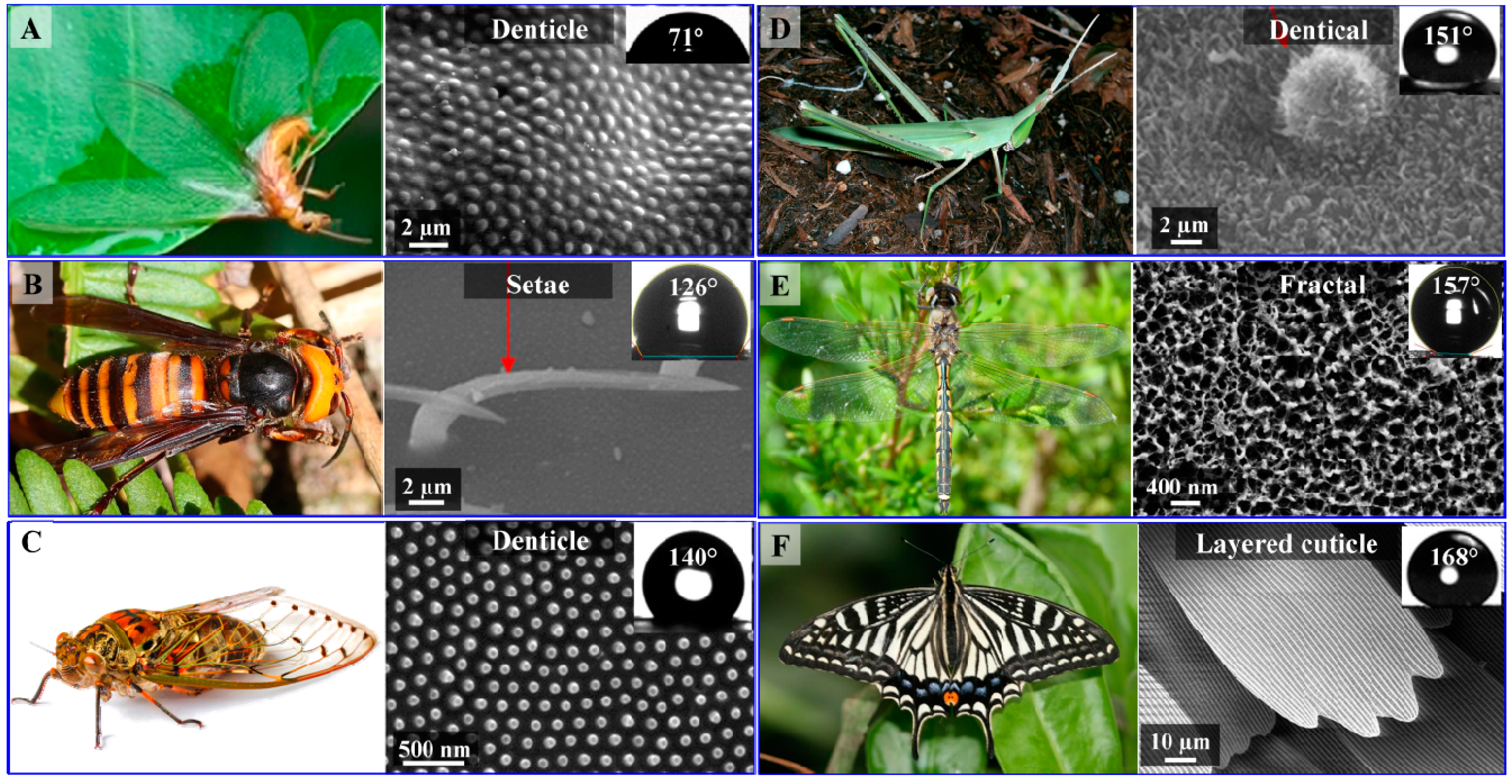
| Order | Species | Structural Morphology | WCA (°) |
|---|---|---|---|
| Coleoptera | Allomyrina dichotoma | Setae | 54 |
| Coleoptera | Chrysolina virgata | Setae | 71 |
| Isoptera | Schedorhinotermes sp. | Setae | 71 |
| Coleoptera | Amphizoa sinica | Setae | 109 |
| Hymenoptera | Vespa simillima xanthoptera | Setae | 121 |
| Hymenoptera | Vespa dybowskii | Setae | 126 |
| Hemiptera | Meimuna microdon | Denticle | 140 |
| Orthoptera | Atractomorpha lata | Denticle | 148 |
| Orthoptera | Acrida cinerea cinerea | Denticle | 151 |
| Odonata | Hemicordulia tau | Fractal | 157 |
| Odonata | Hemianax papuensis | Fractal | 161 |
| Lepidoptera | Artogeia canidia | Layered cuticle | 162 |
| Lepidoptera | Papilio xuthus | Layered cuticle | 168 |
2.2. Plant Surface Structures
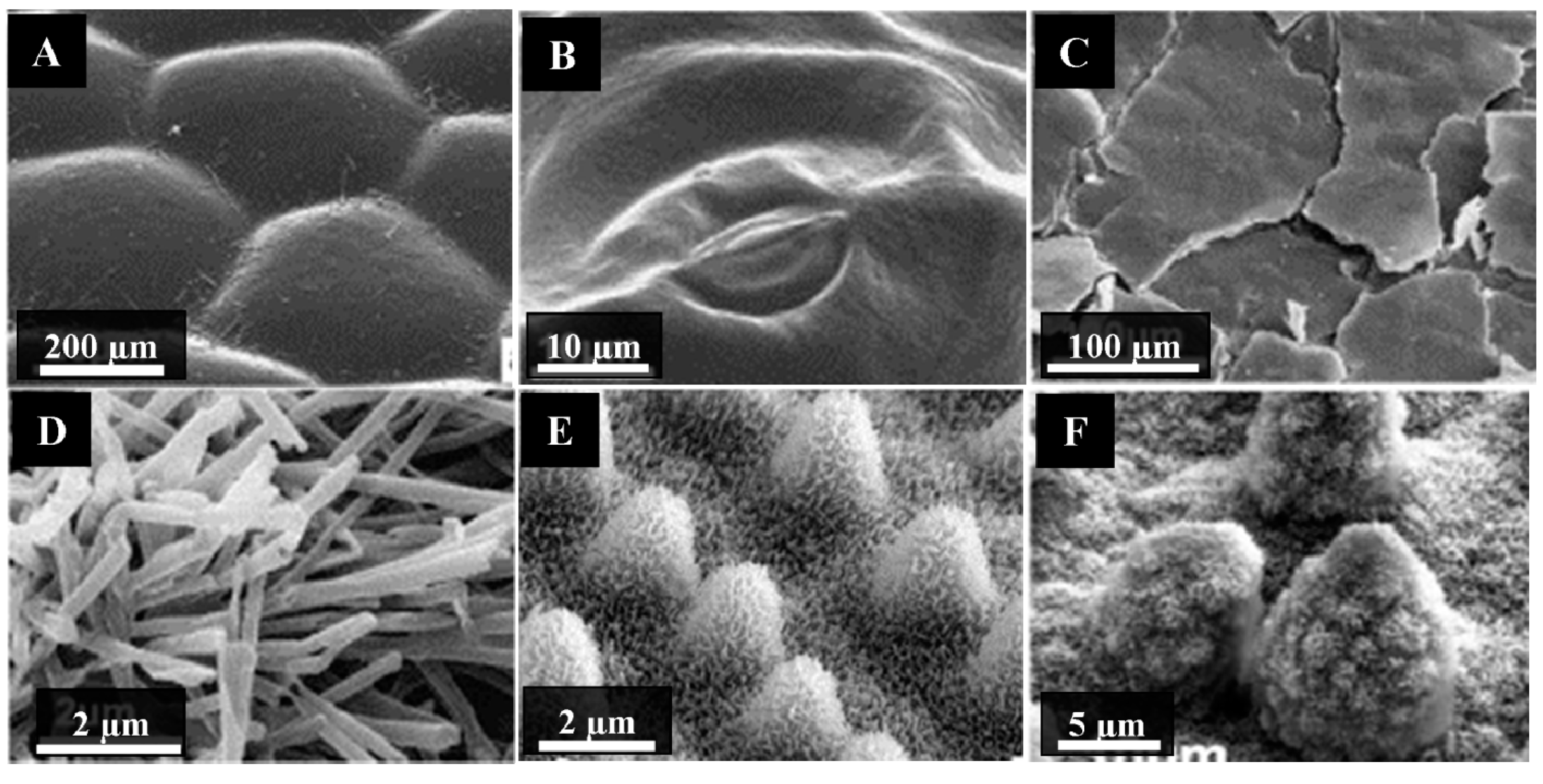
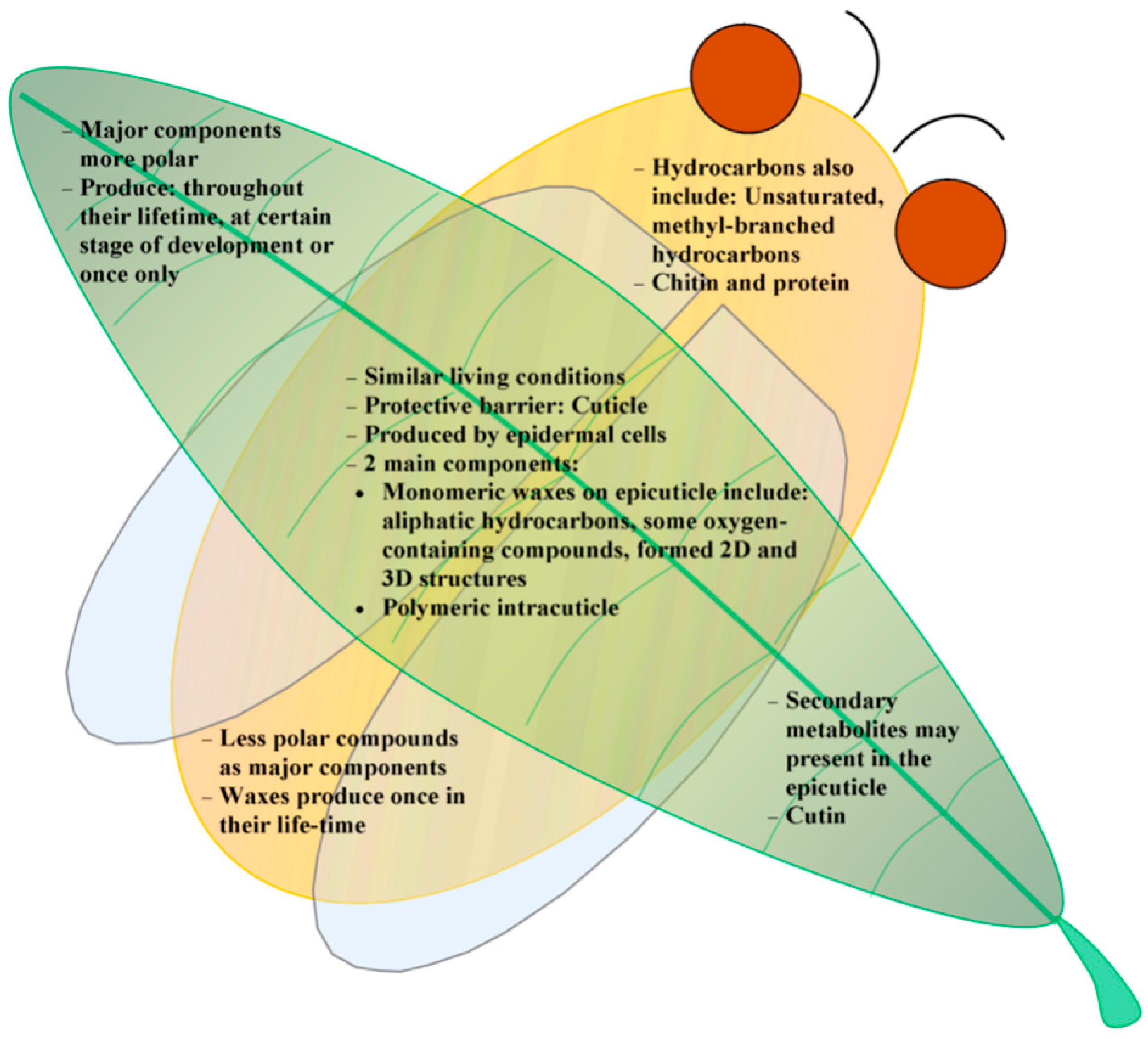
3. Correlation between the Chemistry and Morphology of Cuticular Waxes
| Species | Structural Morphology | Major Compounds | CA (°) |
|---|---|---|---|
| Crassula ovate | Crust | Aldehydes C30, C32; alkane C31 | NA (b) |
| Eucalyptus sp. | Di-ketone tubules | β-diketone C33 | 162 |
| Magnolia grandiflora | Films | Fatty acids C24-C30, prim. Alcohols C24-C28 | NA |
| N. nucifera | Nonacosanol tubules | Sec. alkane-diol C29 | 162 |
| Thalictrum sp. | Nonacosanol tubules | 154 | |
| Oryza sativa | Platelets | Aldehydes | >150 |
| Aristolochia sp. | Transversely ridged rodlets | Ketones; alkanes C31 | 160 |
| Order | Species | Major Compounds | WCA (°) | References |
|---|---|---|---|---|
| Coleoptera | Leptinotarsa decemlineata | C25-C53 straight & branched alkane (dimethyl branch) | NA (a) | [78] |
| Coleoptera | Zygogramma exclamationis | C23-C56 alkanes and alkenes | [79] | |
| Isoptera | Zootermopsis nevadensis | C19-C41 straight and branched alkanes | NA | [80,81] |
| Isoptera | Schedorhinotermes sp. | NA | 71 | [64] |
| Orthoptera | Habrobracon hebetor | Homologous series of n-alkanes, 11-, 13-, and 15-methyl alkanes, 13,17-dimethyl alkanes, and Z-5, Z-7, and Z-9-alkenes | NA | [82] |
| Hymenoptera | Muscidifurax sp. | Methyl alkane C29-C39 | NA | [83] |
| Odonata | Hemicordulia tau | 86% C10-C34 straight and branched alkanes, 14% hexadecanoic acid | 157 | [76] |
| Odonata | Hemianax papuensis | 38% C14-C30 straight and branched alkanes, 38% carboxylic acid | 161 | [84] |
4. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Bartell, F.E.; Bartell, L.S. Quantitative correlation of interfacial free surface energies. J. Am. Chem. Soc. 1934, 56, 2205–2210. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Fogg, G.E. Diurnal fluctuation in a physical property of leaf cuticle. Nature 1944, 154, 515. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics inspired surfaces for drag reduction and oleophobicity/philicity. Beilstein J. Nanotechnol. 2011, 2, 66–84. [Google Scholar] [CrossRef]
- Bhushan, B. Bioinspired structured surfaces. Langmuir 2012, 28, 1698–1714. [Google Scholar] [CrossRef]
- Liu, K.; Du, J.; Wu, J.; Jiang, L. Superhydrophobic gecko feet with high adhesive forces towards water and their bio-inspired materials. Nanoscale 2012, 4, 768–772. [Google Scholar] [CrossRef]
- Bhushan, B.; Sayer, R.A. Gecko feet: Natural attachment systems for smart adhesion-mechanism, modeling, and development of bio-inspired materials. In Applied Scanning Probe Methods x: Biomimetics and Industrial Applications; Bhushan, B., Fuchs, H., Tomitori, M., Eds.; Springer: Berlin, Germany, 2008; Volume 10. [Google Scholar]
- Stark, A.Y.; Badge, I.; Wucinich, N.A.; Sullivan, T.W.; Niewiarowski, P.H.; Dhinojwala, A. Surface wettability plays a significant role in gecko adhesion underwater. Proc. Natl. Acad. Sci. USA 2013, 110, 6340–6345. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Xin, J.H. Hydrophobic duck feathers and their simulation on textile substrates for water repellent treatment. Bioinspiration Biomim. 2008, 3, 046007. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y.; Stein, T.; Whyman, G.; Bormashenko, E. Why do pigeon feathers repel water? Hydrophobicity of pennae, cassie-baxter wetting hypothesis and cassie-wenzel capillarity-induced wetting transition. J. Colloid Interface Sci. 2007, 311, 212–216. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 172, 1103–1112. [Google Scholar] [CrossRef]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef]
- Su, Y.; Ji, B.; Zhang, K.; Gao, H.; Huang, Y.; Hwang, K. Nano to micro structural hierarchy is crucial for stable superhydrophobic and water-repellent surfaces. Langmuir 2010, 26, 4984–4989. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Ferrari, M.; Ravera, F. Surfactants and wetting at superhydrophobic surfaces: Water solutions and non aqueous liquids. Adv. Colloid Interface Sci. 2010, 161, 22–28. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Biologically inspired surfaces: Broadening the scope of roughness. Adv. Funct. Mater. 2008, 18, 843–855. [Google Scholar] [CrossRef]
- Mei, H.; Luo, D.; Guo, P.; Song, C.; Liu, C.; Zheng, Y.; Jiang, L. Multi-level micro-/nanostructures of butterfly wings adapt at low temperature to water repellency. Soft Matter 2011, 7, 10569–10573. [Google Scholar] [CrossRef]
- Webb, H.K.; Truong, V.K.; Hasan, J.; Fluke, C.; Crawford, R.J.; Ivanova, E.P. Roughness parameters for standard description of surface nanoarchitecture. Scanning 2012, 34, 257–263. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Huang, Y.F.; Jen, Y.J.; Ganguly, A.; Chen, K.H.; Chen, L.C. Anti-reflecting and photonic nanostructures. Mater. Sci. Eng. R Rep. 2010, 69, 1–35. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Self-cleaning efficiency of artificial superhydrophobic surfaces. Langmuir 2009, 25, 3240–3248. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro- and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970–4980. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness makes superhydrophobic states stable. Microelectron. Eng. 2007, 84, 382–386. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Isects take to the skies. In Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; pp. 155–178. [Google Scholar]
- Kenrick, P.; Crane, P.R. The origin and early evolution of plants on land. Nature 1997, 389, 33–39. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef]
- Lockey, K.H. Insect cuticular hydrocarbons. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1980, 65, 457–462. [Google Scholar] [CrossRef]
- Lockey, K.H. Insect cuticular lipids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1985, 81, 263–273. [Google Scholar] [CrossRef]
- Nelson, D.R.; Blomquist, L.G. Insect waxes . In Waxes: Chemistry,Molecular Biology and Functions; Hamilton, R.J., Christie, W.W., Eds.; The Oily Press Ltd Hamilton RJ: Dundee, UK, 1995; pp. 1–90. [Google Scholar]
- Buckner, J.S. Oxygenated derivatives of hydrocarbons. In Insect Hydrocarbons: Biology,Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 187–203. [Google Scholar]
- Buschhaus, C.; Herz, H.; Jetter, R. Chemical composition of the epicuticular and intracuticular wax layers on adaxial sides of rosa canina leaves. Ann. Bot. 2007, 100, 1557–1564. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L. Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J. 2008, 54, 670–683. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Blomquist, G.J. Structure and analysis of insect hydrocarbons. In Insect Hydrocarbons: Biology,Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 19–30. [Google Scholar]
- Lockey, K.H. Lipids of the insect cuticle: Origin, composition and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1988, 89, 595–645. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef]
- Hu, D.L.; Chan, B.; Bush, J.W.M. The hydrodynamics of water strider locomotion. Nature 2003, 424, 663–666. [Google Scholar] [CrossRef]
- Haverty, M.I.; Page, M.; Nelson, L.J.; Blomquist, G.J. Cuticular hydrocarbons of dampwood termites, Zootermopsis: Intra- and intercolony variation and potential as taxonomic characters. J. Chem. Ecol. 1988, 14, 1035–1058. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Riedel, M.; Eichner, A.; Jetter, R. Slippery surfaces of carnivorous plants: Composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta 2003, 218, 87–97. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Agati, G.; Zang Cerovic, G. Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Pub.: Oxford, UK, 2006; Volume 3, pp. 216–239. [Google Scholar]
- Holmes, M.G.; Keiller, D.R. Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: A comparison of a range of species. Plant Cell Environ. 2002, 25, 85–93. [Google Scholar] [CrossRef]
- Bargel, H.; Koch, K.; Cerman, Z.; Neinhuis, C. Structure-function relationships of the plant cuticle and cuticular waxes—A smart material? Funct. Plant Biol. 2006, 33, 893–910. [Google Scholar]
- Ren, L.; Wang, S.; Tian, X.; Han, Z.; Yan, L.; Qiu, Z. Non-smooth morphologies of typical plant leaf surfaces and their anti-adhesion effects. J. Bionic Eng. 2007, 4, 33–40. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Wootton, R.J. Functional morphology of insect wings. Annu. Rev. Entomol. 1992, 37, 113–140. [Google Scholar] [CrossRef]
- Kreuz, P.; Arnold, W.; Kesel, A.B. Acoustic microscopic analysis of the biological structure of insect wing membranes with emphasis on their waxy surface. Ann. Biomed. Eng. 2001, 29, 1054–1058. [Google Scholar] [CrossRef]
- Gorb, S.N. Serial elastic elements in the damselfly wing: Mobile vein joints contain resilin. Naturwissenschaften 1999, 86, 552–555. [Google Scholar] [CrossRef]
- Hu, H.M.S.; Watson, G.S.; Cribb, B.W.; Watson, J.A. Non-wetting wings and legs of the cranefly aided by fine structures of the cuticle. J. Exp. Biol. 2011, 214, 915–920. [Google Scholar] [CrossRef]
- Ditsche-Kuru, P.; Barthlott, W.; Koop, J.H.E. At which surface roughness do claws cling? Investigations with larvae of the running water mayfly Epeorus assimilis (Heptageniidae, Ephemeroptera). Zoology 2012, 115, 379–388. [Google Scholar] [CrossRef]
- Andersen, S.O.; Weis-Fogh, T. Resilin A rubberlike protein in arthropod cuticle. Adv. Insect Physiol. 1964, 2, 1–65. [Google Scholar] [CrossRef]
- Neville, A.C.; Parry, D.A.; Woodhead-Galloway, J. The chitin crystallite in arthropod cuticle. J. Cell Sci. 1976, 21, 73–82. [Google Scholar]
- Dirks, J.H.; Taylor, D. Fracture toughness of locust cuticle. J. Exp. Biol. 2012, 215, 1502–1508. [Google Scholar] [CrossRef]
- Kramer, K.J.; Muthukrishnan, S. Insect chitinases: Molecular biology and potential use as biopesticides. Insect Biochem. Mol. Biol. 1997, 27, 887–900. [Google Scholar] [CrossRef]
- Watson, G.S.; Cribb, B.W.; Watson, J.A. How micro/nanoarchitecture facilitates anti-wetting: An elegant hierarchical design on the termite wing. ACS Nano 2010, 4, 129–136. [Google Scholar] [CrossRef]
- Byun, D.; Hong, J.; Saputra; Ko, J.H.; Lee, Y.J.; Park, H.C.; Byun, B.K.; Lukes, J.R. Wetting characteristics of insect wing surfaces. J. Bionic Eng. 2009, 6, 63–70. [Google Scholar] [CrossRef]
- Watson, G.S.; Cribb, B.W.; Watson, J.A. Contrasting micro/nano architecture on termite wings: Two divergent strategies for optimising success of colonisation flights. PLoS One 2011, 6, e24368. [Google Scholar] [CrossRef]
- Sun, M.; Watson, G.S.; Zheng, Y.; Watson, J.A.; Liang, A. Wetting properties on nanostructured surfaces of cicada wings. J. Exp. Biol. 2009, 212, 3148–3155. [Google Scholar] [CrossRef]
- Sun, M.; Liang, A.; Watson, G.S.; Watson, J.A.; Zheng, Y.; Jiang, L. Compound microstructures and wax layer of beetle elytral surfaces and their influence on wetting properties. PLoS One 2012, 7, e46710. [Google Scholar]
- Nosonovsky, M.; Bhushan, B. Roughness optimization for biomimetic superhydrophobic surfaces. Microsyst. Technol. 2005, 11, 535–549. [Google Scholar] [CrossRef]
- Verho, T.; Korhonen, J.T.; Sainiemi, L.; Jokinen, V.; Bower, C.; Franze, K.; Franssila, S.; Andrew, P.; Ikkala, O.; Rasa, R.H.A. Reversible switching between superhydrophobic states on a hierarchically structured surface. Proc. Natl. Acad. Sci. USA 2012, 109, 10210–10213. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Mammen, L.; Deng, X.; Vollmer, D.; Butt, H.J. How superhydrophobicity breaks down. Proc. Natl. Acad. Sci. USA 2013, 110, 3254–3258. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Multiscale friction mechanisms and hierarchical surfaces in nano- and bio-tribology. Mater. Sci. Eng. R Rep. 2007, 58, 162–193. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Riederer, M.; Müller, C. Biology of the Plant Cuticle; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Ensikat, H.J.; Boese, M.; Mader, W.; Barthlott, W.; Koch, K. Crystallinity of plant epicuticular waxes: Electron and x-ray diffraction studies. Chem. Phys. Lipids 2006, 144, 45–59. [Google Scholar] [CrossRef]
- Barthlott, W.; Theisen, I.; Borsch, T.; Neinhuis, C. Epicuticular waxes and vascular plant systematics: tegrating micromorphological and chemical data. In Deep Morphology. Toward a Renaissance of Morphology in Plant Systematics; Stuessy, T.F., Mayer, V., Horandl, E., Eds.; Gantner Verlag: Ruggell, Liechtenstein, 2003; pp. 189–206. [Google Scholar]
- Koch, K.; Bhushan, B.; Barthlott, W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 2008, 4, 1943–1963. [Google Scholar] [CrossRef]
- Jeffree, C.E. Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Pub.: Oxford, UK, 2006; pp. 11–125. [Google Scholar]
- Nguyen, S.H.; Webb, H.K.; Hasan, J.; Tobin, M.J.; Crawford, R.J.; Ivanova, E.P. Dual role of outer epicuticular lipids in determining the wettability of dragonfly wings. Colloids Surf. B Biointerfaces 2013, 106, 126–134. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Yocum, G.D.; Buckner, J.S.; Fatland, C.L. A comparison of internal and external lipids of nondiapausing and diapause initiation phase adult colorado potato beetles, Leptinotarsa decemlineata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 159, 163–170. [Google Scholar] [CrossRef]
- Nelson, D.R.; Charlet, L.D. Cuticular hydrocarbons of the sunflower beetle, Zygogramma exclamationis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 273–284. [Google Scholar] [CrossRef]
- Sevala, V.L.; Bagnères, A.G.; Kuenzli, M.; Blomquist, G.J.; Schal, C. Cuticular hydrocarbons of the dampwood termite, zootermopsis nevadensis: Caste differences and role of lipophorin in transport of hydrocarbons and hydrocarbon metabolites. J. Chem. Ecol. 2000, 26, 765–789. [Google Scholar] [CrossRef]
- Saïd, I.; Costagliola, G.; Leoncini, I.; Rivault, C. Cuticular hydrocarbon profiles and aggregation in four Periplaneta species (insecta: Dictyoptera). J. Insect Physiol. 2005, 51, 995–1003. [Google Scholar] [CrossRef]
- Howard, R.W.; Baker, J.E. Cuticular hydrocarbons and wax esters of the ectoparasitoid Habrobracon hebetor: Ontogenetic, reproductive, and nutritional effects. Arch. Insect Biochem. Physiol. 2003, 53, 1–18. [Google Scholar] [CrossRef]
- Bernier, U.R.; Carlson, D.A.; Geden, C.J. Gas chromatography/mass spectrometry analysis of the cuticular hydrocarbons from parasitic wasps of the genus Muscidifurax. J. Am. Soc. Mass Spectrom. 1998, 9, 320–332. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Webb, H.K.; Hasan, J.; Truong, V.K.; Lamb, R.N.; Duan, X.; Tobin, M.J.; Mahon, P.J.; Crawford, R.J. Molecular organization of the nanoscale surface structures of the dragonfly hemianax papuensis wing epicuticle. PLoS One 2013, 8, e67893. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Superhydrophobic surfaces and emerging applications: Non-adhesion, energy, green engineering. Curr. Opin. Colloid Interface Sci. 2009, 14, 270–280. [Google Scholar] [CrossRef]
- Berne, B.J.; Weeks, J.D.; Zhou, R. Dewetting and hydrophobic interaction in physical and biological systems. Annu. Rev. Phys. Chem. 2009, 60, 85–103. [Google Scholar] [CrossRef]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Wagner, T.; Neinhuis, C.; Barthlott, W. Wettability and contaminability of insect wings as a function of their surface sculptures. Acta Zool. 1996, 77, 213–223. [Google Scholar] [CrossRef]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Ferveur, J.F. Cuticular hydrocarbons: Their evolution and roles in drosophila pheromonal communication. Behav. Genet. 2005, 35, 279–295. [Google Scholar] [CrossRef]
- Endler, A.; Liebig, J.; Schmitt, T.; Parker, J.E.; Jones, G.R.; Schreier, P.; Hölldobler, B. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl. Acad. Sci. USA 2004, 101, 2945–2950. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Animal Behaviour: Communication by Smell and Taste; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Van Dooremalen, C.; Ellers, J. A moderate change in temperature induces changes in fatty acid composition of storage and membrane lipids in a soil arthropod. J. Insect Physiol. 2010, 56, 178–184. [Google Scholar] [CrossRef]
- Arsene, C.; Schulz, S.; van Loon, J.J.A. Chemical polymorphism of the cuticular lipids of the cabbage white Pieris rapae. J. Chem. Ecol. 2002, 28, 2627–2631. [Google Scholar] [CrossRef]
- Van Maarseveen, C.; Han, H.; Jetter, R. Development of the cuticular wax during growth of kalanchoe daigremontiana (hamet et perr. De la bathie) leaves. Plant Cell Environ. 2009, 32, 73–81. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, K.W.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; Bactericidal activity of black silicon. Nat. Commun. 2013. [Google Scholar] [CrossRef]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nguyen, S.H.; Webb, H.K.; Mahon, P.J.; Crawford, R.J.; Ivanova, E.P. Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties. Molecules 2014, 19, 13614-13630. https://doi.org/10.3390/molecules190913614
Nguyen SH, Webb HK, Mahon PJ, Crawford RJ, Ivanova EP. Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties. Molecules. 2014; 19(9):13614-13630. https://doi.org/10.3390/molecules190913614
Chicago/Turabian StyleNguyen, Song Ha, Hayden K. Webb, Peter J. Mahon, Russell J. Crawford, and Elena P. Ivanova. 2014. "Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties" Molecules 19, no. 9: 13614-13630. https://doi.org/10.3390/molecules190913614
APA StyleNguyen, S. H., Webb, H. K., Mahon, P. J., Crawford, R. J., & Ivanova, E. P. (2014). Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties. Molecules, 19(9), 13614-13630. https://doi.org/10.3390/molecules190913614







