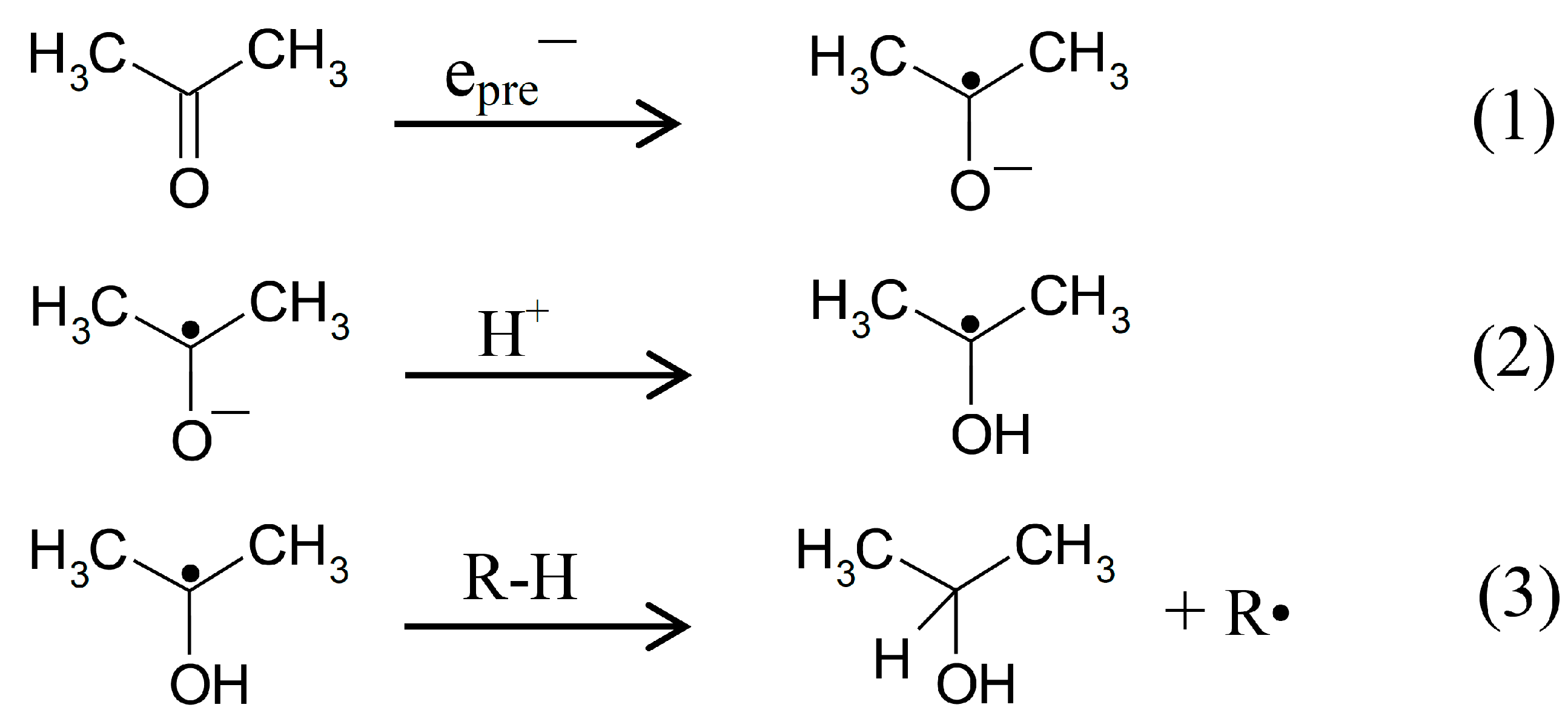
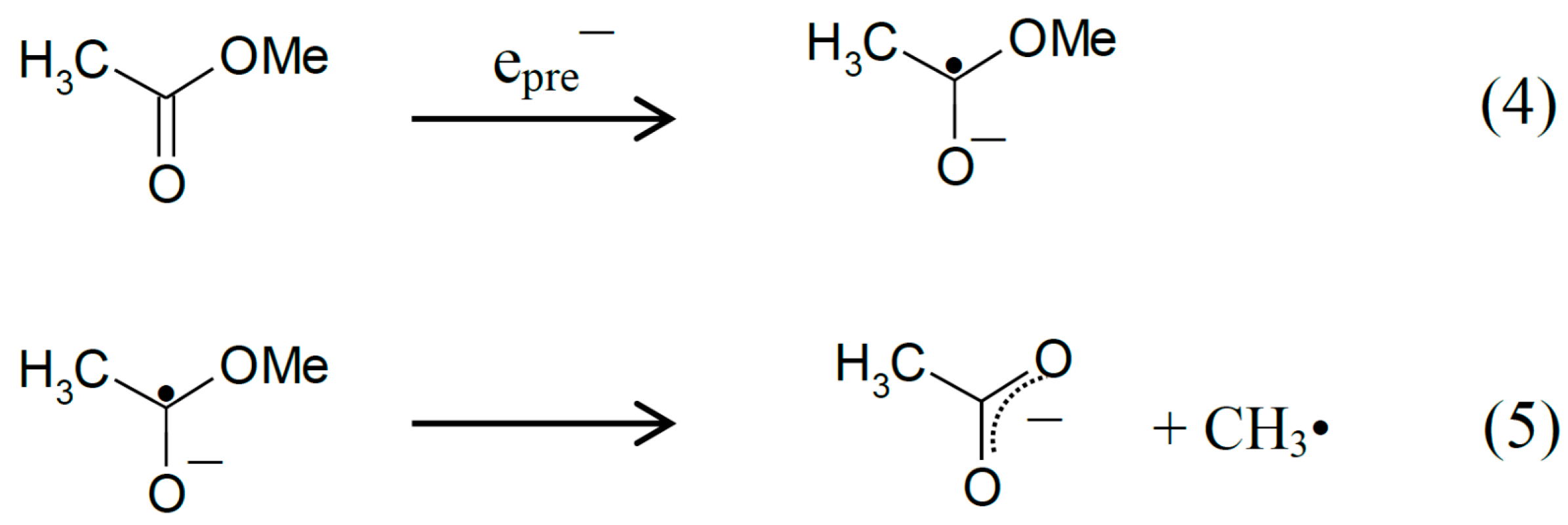
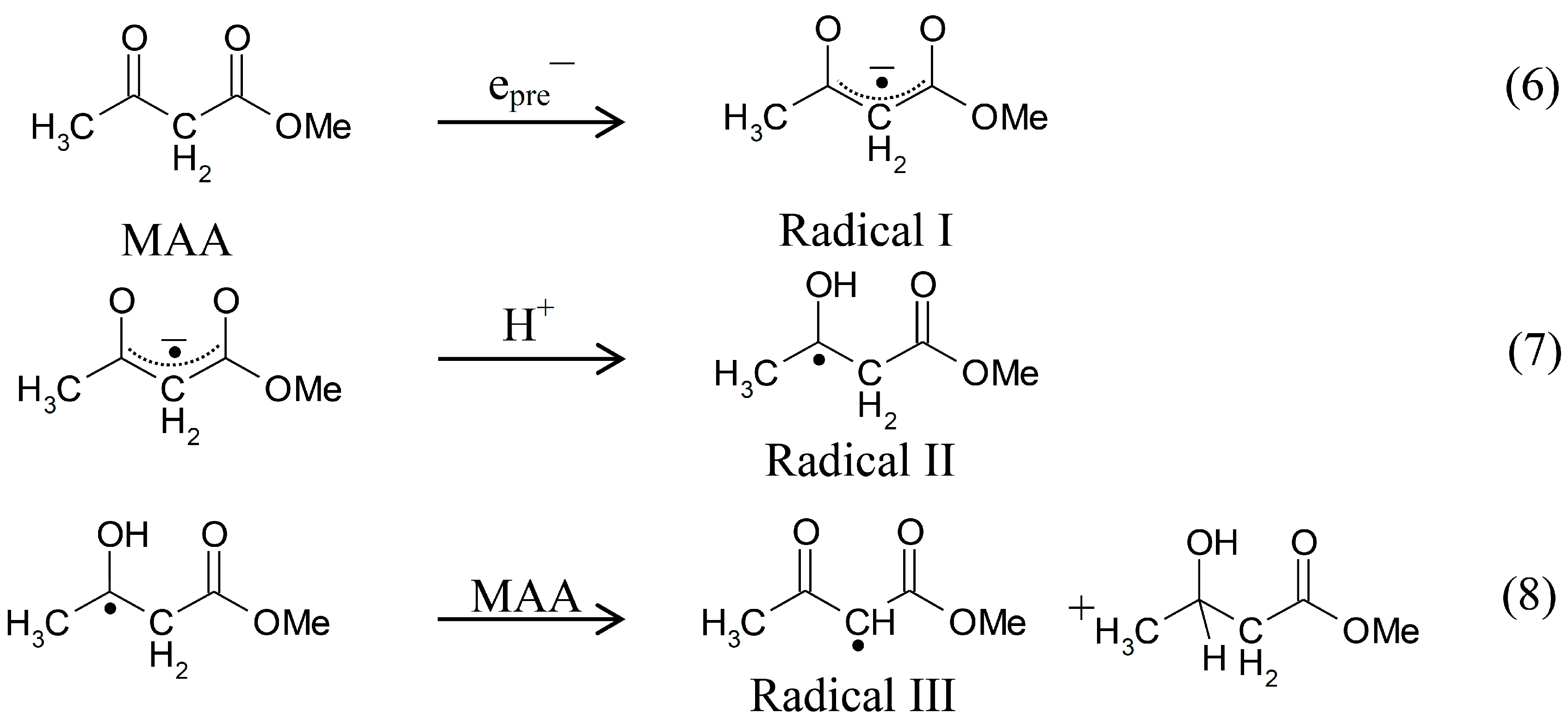
2.1. ESR Studies in H2O
In
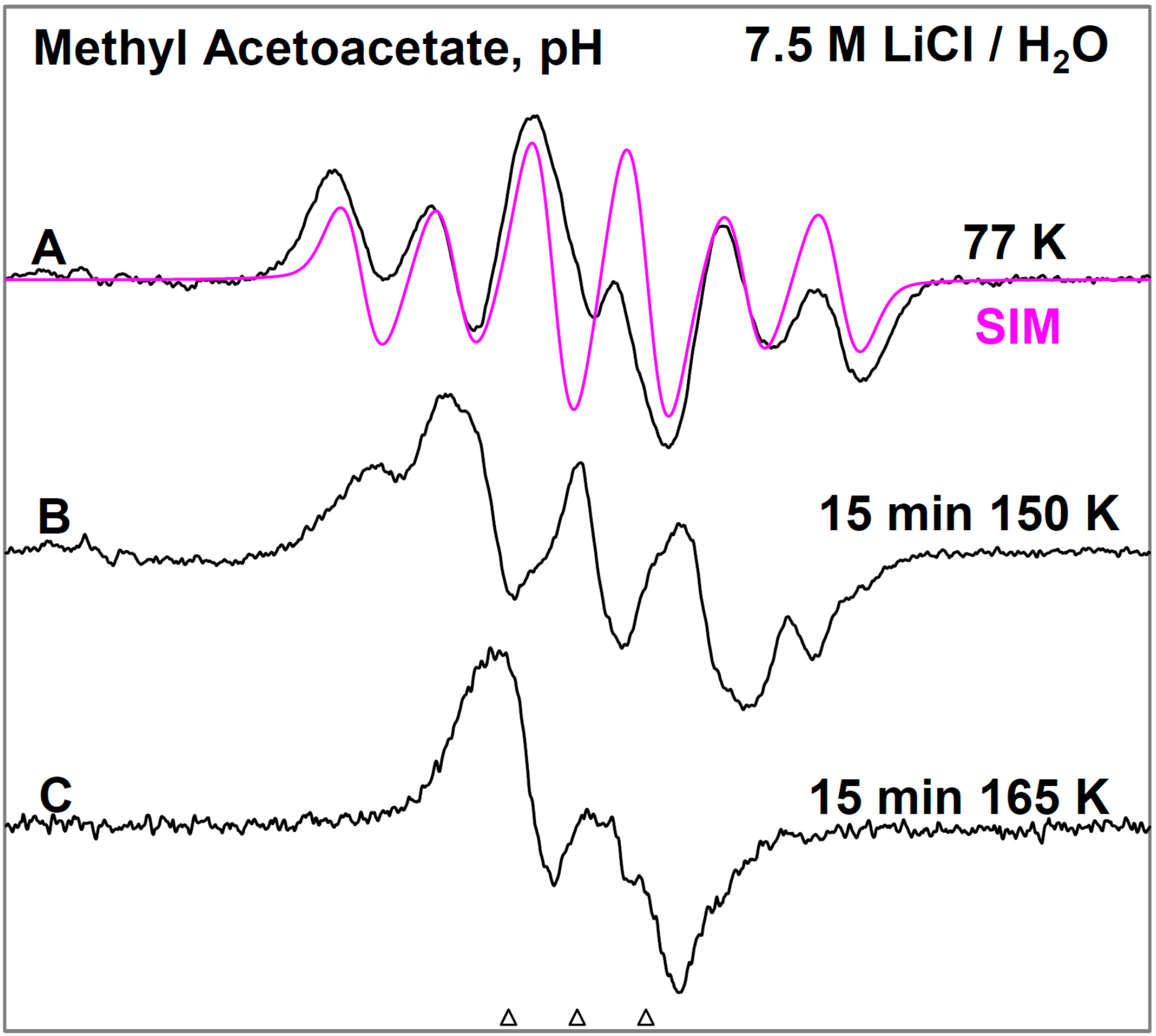
Figure 1, the ESR spectra found after γ-irradiation (600 Gy) at 77 K of a sample of MAA in 7.5 M LiCl in H
2O are shown. The black spectrum in
Figure 1A resulting from electron attachment to MAA clearly shows a six line multiplet which is assigned to three
ca. 18 G proton hyperfine couplings from a methyl group and a single proton coupling of
ca. 37 G from one of the methylene group protons. The experimental spectrum (black) has been simulated using four lines of equal intensity separated by 18 G to account for the methyl proton hyperfine couplings, a single proton hyperfine coupling of 37 G, g-value = 2.0029, and a mixed ((Lorentzian/Gaussian) = 0.2) line-width = 8 G. The simulated spectrum (pink) has been superimposed on the experimental spectrum for comparison. This spectrum is assigned to radical II formed after protonation of the anion radical, reactions 6 and 7. The line intensities of the methyl protons are close to 1:1:1:1 rather than the expected 1:3:3:1 line intensities owing to the well-known tunneling rotation of the methyl groups at 77 K [
15]. Such restricted rotation of methyl group is expected at low temperatures and has been observed after electron addition to CH
3CO- groups such as in acetic acid, acetamide and N-acetylmethionine at 77 K [
11,
15,
16]. These anion radicals exhibit the normal and expected 1:3:3:1 line intensity ratios of the methyl protons at higher temperatures (provided the anion radicals are stable at that elevated temperature [
15]. Hyperfine couplings from both the methyl group and the methylene in spectrum 1A show that the electron addition is to the carbonyl group at 77 K. No evidence is found for electron addition to the acetate linkage of MAA even though esters are known to readily form anion radicals at low temperatures [
9]. The trapping of the electron at the carbonyl group in MAA at 77 K might suggest that the carbonyl group in MAA has a higher electron affinity than its acetate moiety. However, DFT calculations presented here (
Section 2.3) show that it is the protonation of the carbonyl oxygen in MAA that helps to localize the electron to the carbonyl group.
Figure 1.
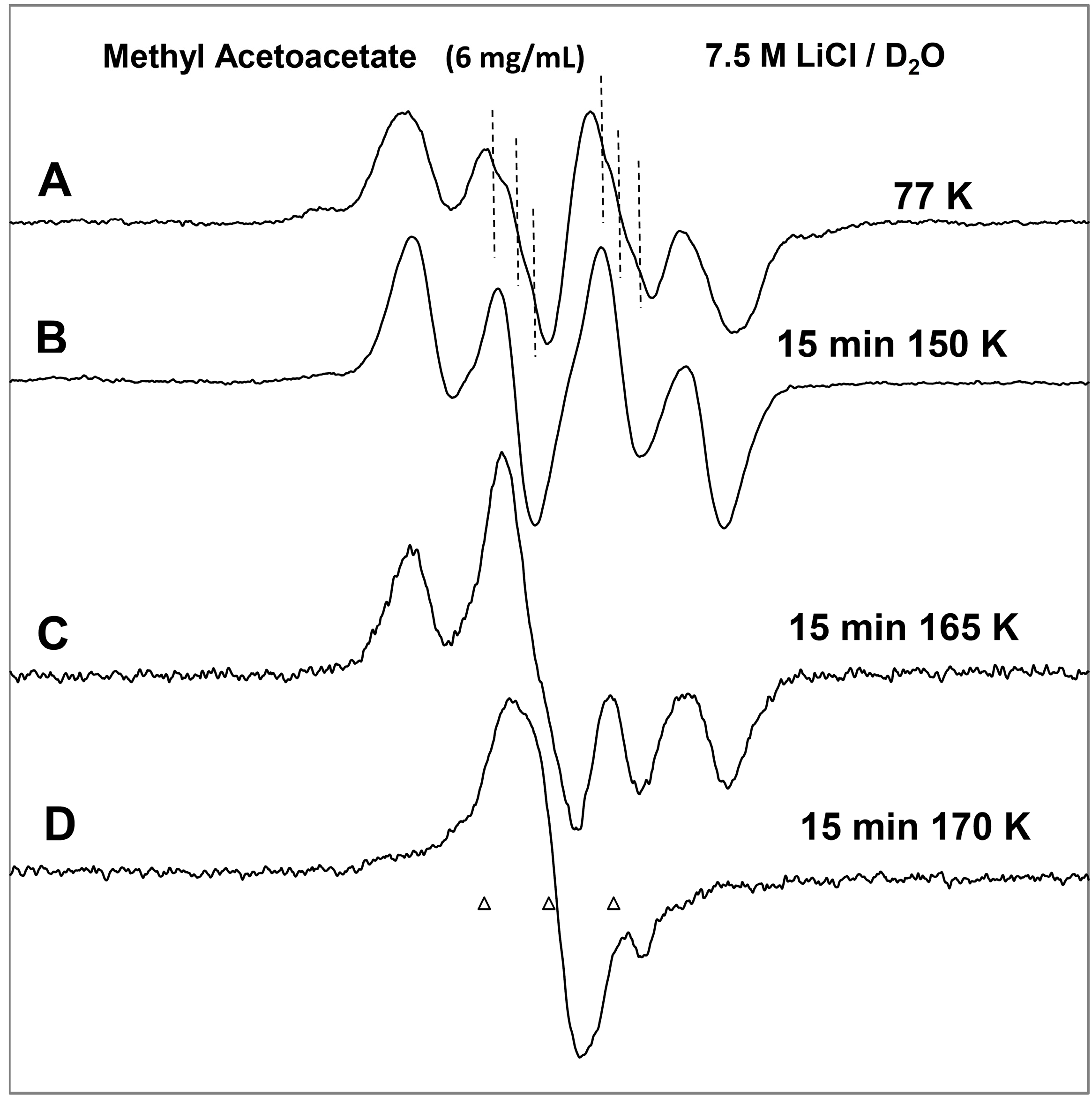
ESR spectra of the prehydrated electron addition to MAA at 77 K and subsequent reactions of the MAA anion radical on annealing to 165 K in a 7.5 M LiCl H2O glass. (A) The black spectrum of the protonated MAA anion radical (radical II). The pink spectrum is the simulated spectrum of radical II. For simulation parameters, see text; (B) Radical II, after conformational rearrangement; (C) Radical III, CH3-CO-C•H-CO-OCH3, formed by H-atom abstraction from the parent MAA molecule by radical II at 165 K. The three reference markers (open triangles) in this figure and in the subsequent figures show the position of Fremy’s salt resonance with the central marker at g = 2.0056. Each of these markers is separated from each other by 13.09 G.
Figure 1.
ESR spectra of the prehydrated electron addition to MAA at 77 K and subsequent reactions of the MAA anion radical on annealing to 165 K in a 7.5 M LiCl H2O glass. (A) The black spectrum of the protonated MAA anion radical (radical II). The pink spectrum is the simulated spectrum of radical II. For simulation parameters, see text; (B) Radical II, after conformational rearrangement; (C) Radical III, CH3-CO-C•H-CO-OCH3, formed by H-atom abstraction from the parent MAA molecule by radical II at 165 K. The three reference markers (open triangles) in this figure and in the subsequent figures show the position of Fremy’s salt resonance with the central marker at g = 2.0056. Each of these markers is separated from each other by 13.09 G.
From the p
Ka of the acetone anion radical, p
Ka =
ca. 12 [
12], it is expected that the anion of carbonyl group in MAA anion radical has a p
Ka less than 12 owing to delocalization of spin and charge (see
Section 2.3). Thus, the carbonyl group of the MAA anion radical is expected to be protonated on warming; however, DFT calculations suggest that protonation has occurred even at 77 K and hence, we have assigned spectrum 1A to radical II.
On annealing from 77 K (spectrum 1A) to 150 K (spectrum 1B), we find that the spectrum changes substantially. Note that all spectra are recorded at 77 K after annealing at each temperature. The broad lines in
Figure 1B make the spectral analyses difficult. However, it appears that in spectrum 1B, the methylene proton coupling drops from
ca. 37 G to
ca. 25 G (
Table 1). Upon increasing the temperature from 77 to 150 K, the methylene protons likely take on a range of values that somewhat broaden the spectrum in
Figure 1B. However, the methyl protons hyperfine couplings remain at 18 G (see spectrum 2B). These results are suggestive of a further change in the conformation of radical II on annealing from 77 K to 150 K. On this basis, spectrum 1B is assigned to the conformationally rearranged radical II (
Scheme 3, reaction (7)).
Table 1.
DFT Calculated Proton hyperfine couplings (B3LYP/6-31G*).
Table 1.
DFT Calculated Proton hyperfine couplings (B3LYP/6-31G*).
| Radical | Phase | Proton Hyperfine Couplings 1,2 (Gauss) |
|---|
| | | CH3 | CH2 | O-H [xx, yy, zz] 1 |
|---|
| I (opt) | vacuum | 11.6 | 18.7, 6.6 | - |
| PCM | 13.1 | 25.4, 4.9 | - |
| II (opt) | vacuum | 18.5 | 35.6, 0.8 | [−6.5, −5.5, 5.4] |
| PCM | 18.7 | 35.9, 0.9 | [−6.7, −5.7, 5.0] |
| II (Exp) | Glass 3 | 18 | 37, <5 | unresolved |
| III (opt) | vacuum | C-H [xx, yy, zz] | - |
| [−29.4, −20.7, −8.1] | - |
| PCM | [−29.6, −20.9, −8.0] | - |
| III (Exp) | Glass 3 | ca. −22 | - |
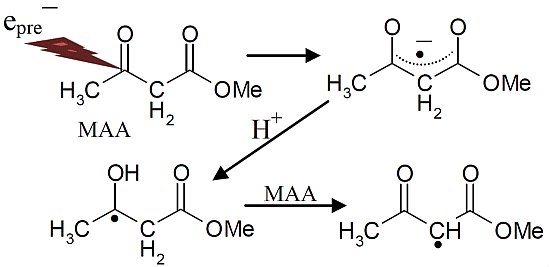
Scheme 3.
Reactions initiated by presolvated electron attachment to MAA.
Scheme 3.
Reactions initiated by presolvated electron attachment to MAA.
On annealing to 165 K (
Figure 1C), we find that a
ca. 22 G doublet (
Table 1) builds in with the loss of the signal of the protonated electron adduct radical (II). This species is assigned to the radical (III) (CH
3-CO-CH•-COOCH
3) formed by the biomolecular H-atom abstraction of a methylene proton of the parent MAA molecule by the radical II (
Scheme 3, reaction (8)).
2.2. ESR Studies in D2O
Our results in D
2O glasses, for otherwise identical (
i.
e., matched) samples clarify and add strong support for the above analyses (
Scheme 3, reactions (6) to (8)). Owing to the p
Ka of -CH
2- group of MAA as 11 [
17] and pD of the 7.5 M LiCl/D
2O solution being
ca. 5 [
18], the CH
2 group in MAA has undergone complete deuterium exchange in D
2O solutions of 7.5 M LiCl
i.
e., MAA is converted to CH
3-CO-CD
2-COOCH
3 in 7.5 M LiCl/D
2O.
In
Figure 2, the results in D
2O glasses are shown. At 77 K, only four major lines expected from the methyl group hyperfine couplings (
ca. 18 G) are observed in spectrum 2A. Owing to the smaller magnetic moment of deuterons than protons, deuterons show hyperfine couplings that are only 15% (1/6.514) that of protons in the same environment [
18,
19,
20]. Hence, the one methylene proton coupling of
ca. 37 G, is reduced to 5.7 G deuterium coupling. Also any unresolved couplings from the other remaining methylene proton and the hyperfine coupling due to –OH in radical II (
Scheme 3, reaction (7)) are lost as expected since they are now deuterons in CH
3-C•OD-CD
2-COOCH
3. Analyses of the central two lines in
Figure 2A (77 K) do show a poorly resolved triplet (marked with lines) of 5.7 G as expected. Therefore, spectrum 2A is assigned to CH
3-C•OD-CD
2-COOCH
3.
Figure 2.
ESR spectra of prehydrated electron addition to MAA and subsequent reactions of the MAA anion radical on annealing to 170 K in a 7.5 M LiCl/D2O glass. (A) The deuterated electron adduct, radical II. Note the central components show distinct deuterium couplings of the exchanged methylene deuteron (marked with hash marks); (B) Deuterated radical II after conformational rearrangement in which the deuterium couplings are lost; (C) Gradual formation of deuterated radical III as radical II is lost; (D) Deuterated radical III, CH3-CO-C•D-COOCH3, formed by hydrogen (deuteron) abstraction from the parent molecule by II at 170 K.
Figure 2.
ESR spectra of prehydrated electron addition to MAA and subsequent reactions of the MAA anion radical on annealing to 170 K in a 7.5 M LiCl/D2O glass. (A) The deuterated electron adduct, radical II. Note the central components show distinct deuterium couplings of the exchanged methylene deuteron (marked with hash marks); (B) Deuterated radical II after conformational rearrangement in which the deuterium couplings are lost; (C) Gradual formation of deuterated radical III as radical II is lost; (D) Deuterated radical III, CH3-CO-C•D-COOCH3, formed by hydrogen (deuteron) abstraction from the parent molecule by II at 170 K.
On annealing to 150 K, this poorly resolved triplet is lost in spectrum 2B which is expected since the rearrangement found in H2O at this temperature suggests only a 3.8 G deuteron coupling will be observed from the methylene, i.e., 25/6.514 G and this is too small to observe. However, the methyl proton hyperfine coupling remains at ca. 18 G in D2O as it was in H2O glasses.
On annealing to higher temperatures, 165 K (
Figure 2C) and then to 170 K (
Figure 2D), a singlet gradually builds in with the loss of the signal of four line spectrum of the deuterated radical II. This species is assigned to the deuterated radical III (reaction 8),
i.
e., CH
3-CO-CD•-COOCH
3, formed by deuterium atom abstraction from the deuterated methylene of the parent MAA (
Scheme 3, reaction (8)). Comparison of
Figure 1C (H
2O) with
Figure 2C (D
2O) clearly shows that reaction (8) takes place more slowly in D
2O over that found in H
2O. This kinetic isotope effect is expected because a C-D bond is
ca. 1 kcal/mole stronger than a C-H bond simply from vibrational zero point energy considerations [
21].
In summary, the overall experimental results shown in
Figure 1 and
Figure 2 strongly support the following mechanism: prehydrated electron addition to the carbonyl group in MAA (
Scheme 3, reaction (6)) which is followed by protonation of the electron adduct of MAA at 77 K (reaction 7) and the subsequent H-atom abstraction from the parent MAA by the protonated electron adduct (
Scheme 3, reaction (8)).
The effect of pH has also been investigated in identically prepared samples in 7.5 M LiCl/H2O glasses but at pH ca. 8.5. The initial spectrum found at 77 K after the prehydrated electron addition shows the identical spectrum of the protonated electron adduct (II) found at pH 5. On annealing to 165 K, similar to the spectrum 1C, the ca. 22 G doublet is formed. Thus, the mechanism shown in reactions (6) to (8) is not affected by increasing the pH from ca. 5 to ca. 8.5.
2.3. DFT Calculations
DFT calculations for each of radical intermediates (I, II, and III) have been performed using the B3LYP functional and a 6-31G* basis set. The DFT/B3LYP/6-31G* method is known to provide hyperfine couplings that are in excellent agreement with the experimentally obtained ones [
1,
13,
14,
18,
19,
22]. In our calculations, the solvent has been treated by use of the polarized continuum model (PCM) as implemented in Gaussian 09 [
23]. The geometry of each of the radicals has been optimized employing the DFT/B3LYP/6-31G* method and the hyperfine coupling constant values for each optimized radical intermediate were calculated. These theoretically calculated hyperfine coupling constants along with the corresponding experimentally obtained ones are shown in
Table 1.
In radical I, the carbonyl group is not protonated and the isotropic hyperfine couplings of the β-protons of the methyl group and the methylene group are found to be significantly smaller than those obtained by experiment (
Table 1). For the optimized protonated radical II, the isotropic hyperfine couplings are quite close to those found experimentally (
Table 1: 18.7 G (PCM)
vs. 18 G (experimental) and 35.9 G (PCM)
vs. 37 G (experimental)).
The poor fit of the theoretically predicted hyperfine coupling constant values of the anion radical (I) with the experimentally obtained ones and the excellent fit of the protonated radical (II) with experiment indicates that the carbonyl functional group protonates even at low temperatures in the glassy system. Calculations employing PCM do alter hyperfine couplings of the anion radical (I) marginally but have a negligible effect on hyperfine couplings of the protonated radical (II) (
Table 1). The protonated carbonyl is predicted to show anisotropic proton hyperfine couplings but of a magnitude that would simply broaden the line components in the ESR spectra somewhat and this is in agreement with experiment (
Section 2.1 and
Figure 1).
For the final radical III, the only
ca. 22 G doublet from the methylene α-proton is observed. The calculation provides a typical anisotropic proton coupling tensor with a
ca. 21 G middle component that is in excellent agreement with the experimentally obtained overall value (
Table 1,
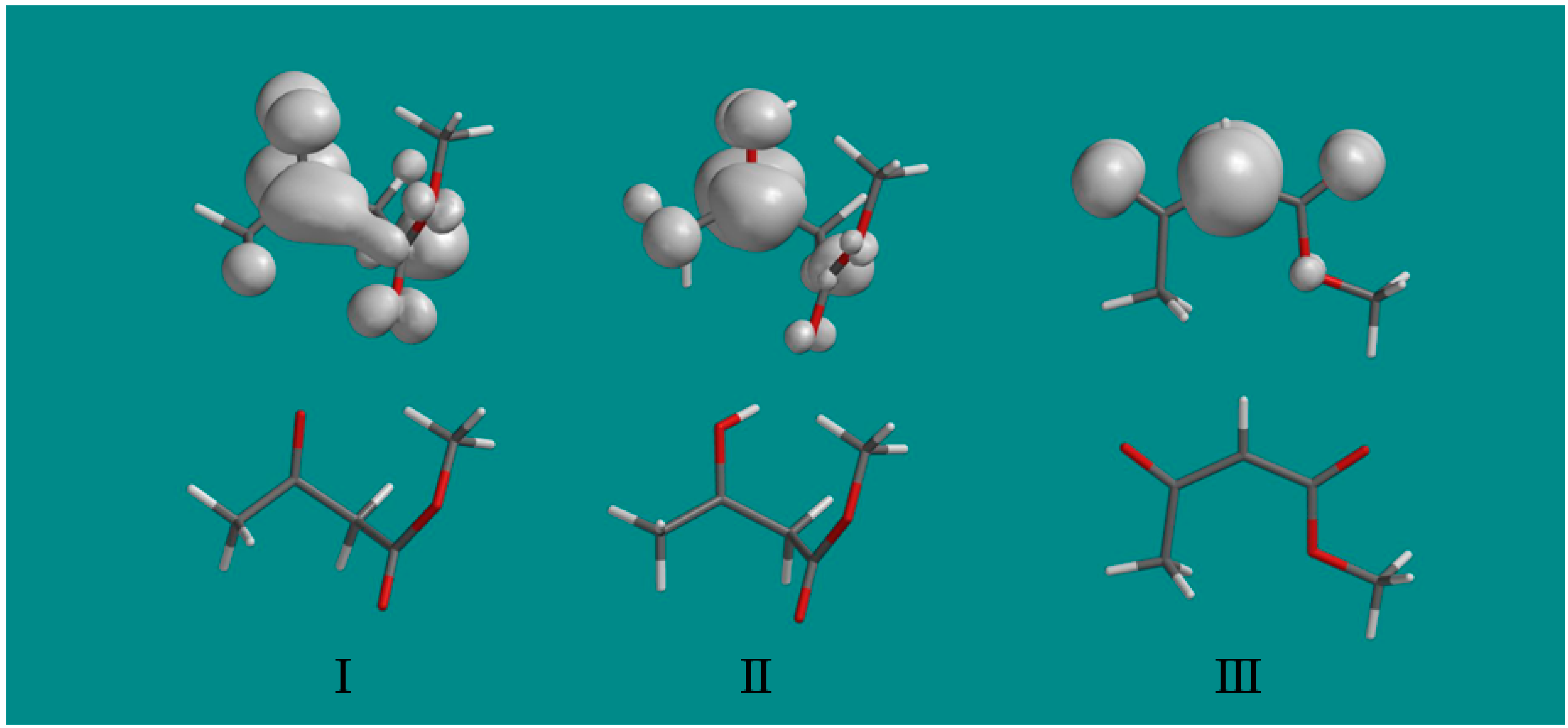
Figure 1). The spin density distributions in the three radicals found in this work are shown in
Figure 3. They clearly show that on protonation of radical I to radical II, the unpaired spin localizes on the carbonyl group and substantially increases the interaction with the methyl group as indicated by the hyperfine couplings in
Table 1. The final radical III shows the expected spin localization at the methylene carbon radical site.
Figure 3.
The spin density distributions of radicals I, II and III are shown above the optimized structure of each radical. Note that protonation of the oxygen at the carbonyl group of radical I substantially localizes the spin at the carbonyl group in radical II and increases the coupling to the methyl protons. Only a fixed methyl group is shown with spin density largely on one methyl proton, however, experimentally the methyl group rotates and averages the spin over the three protons.
Figure 3.
The spin density distributions of radicals I, II and III are shown above the optimized structure of each radical. Note that protonation of the oxygen at the carbonyl group of radical I substantially localizes the spin at the carbonyl group in radical II and increases the coupling to the methyl protons. Only a fixed methyl group is shown with spin density largely on one methyl proton, however, experimentally the methyl group rotates and averages the spin over the three protons.