Self-Assembled Nanoparticles of Glycyrrhetic Acid-Modified Pullulan as a Novel Carrier of Curcumin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of GAP NPs
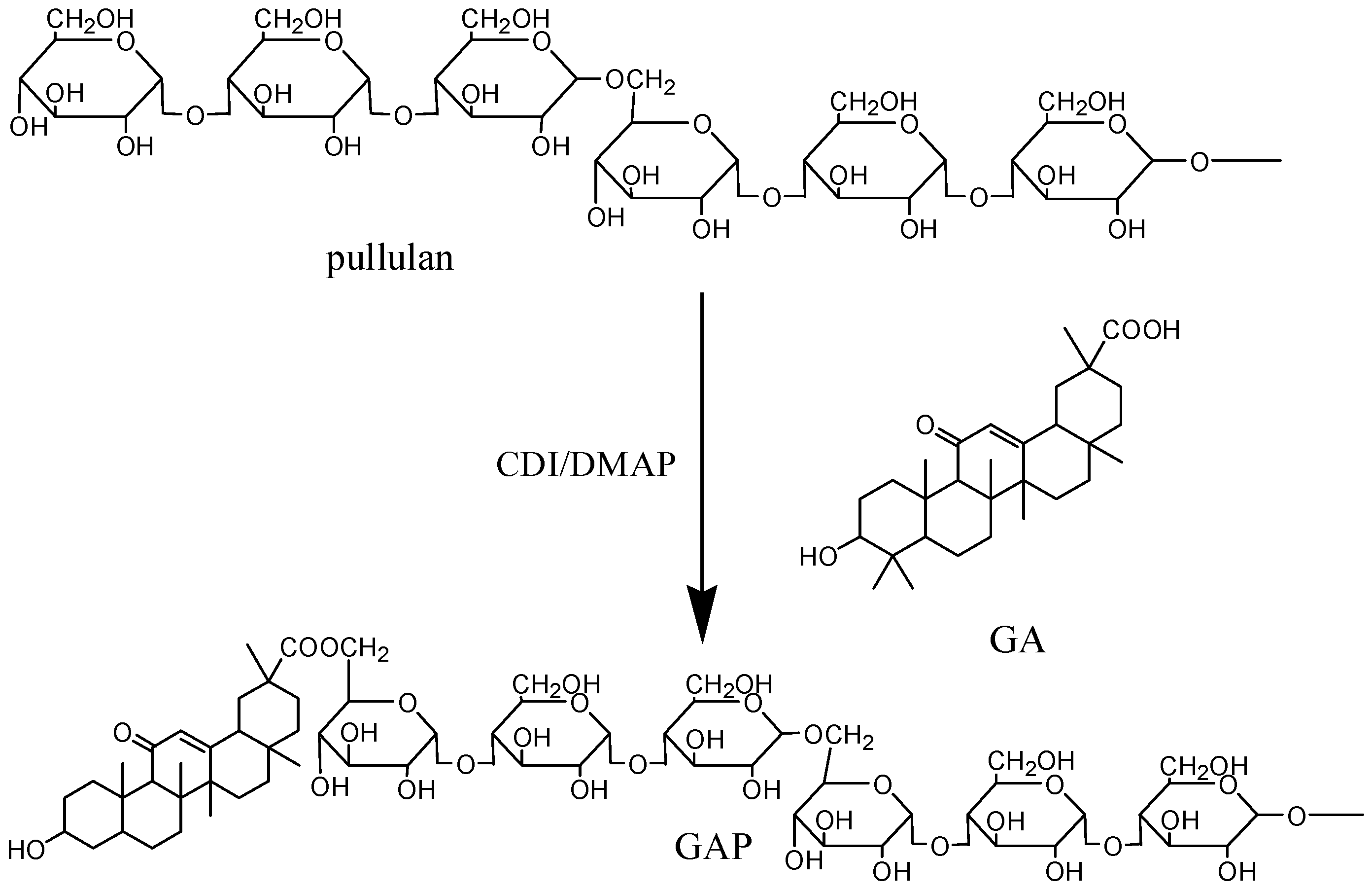
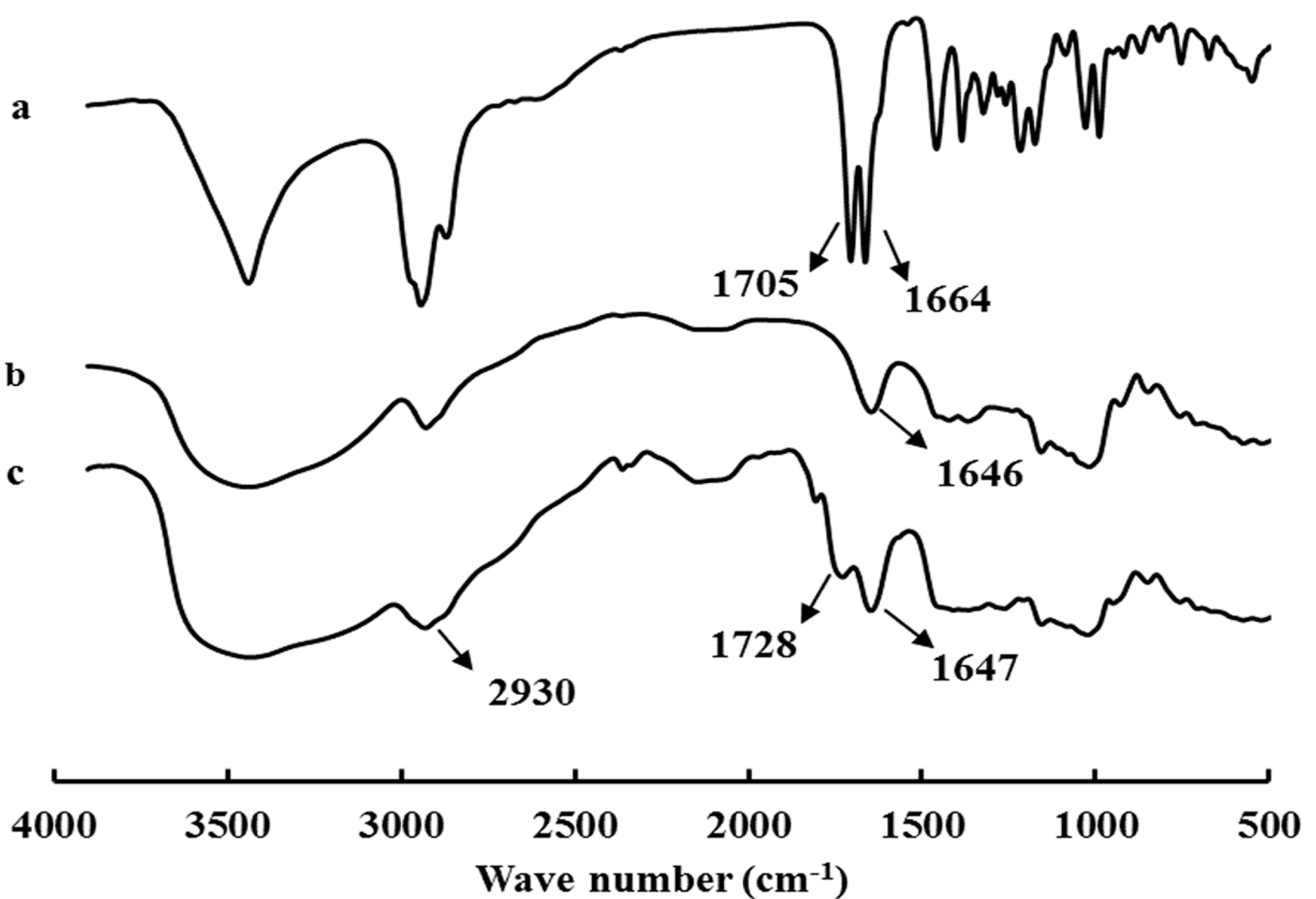

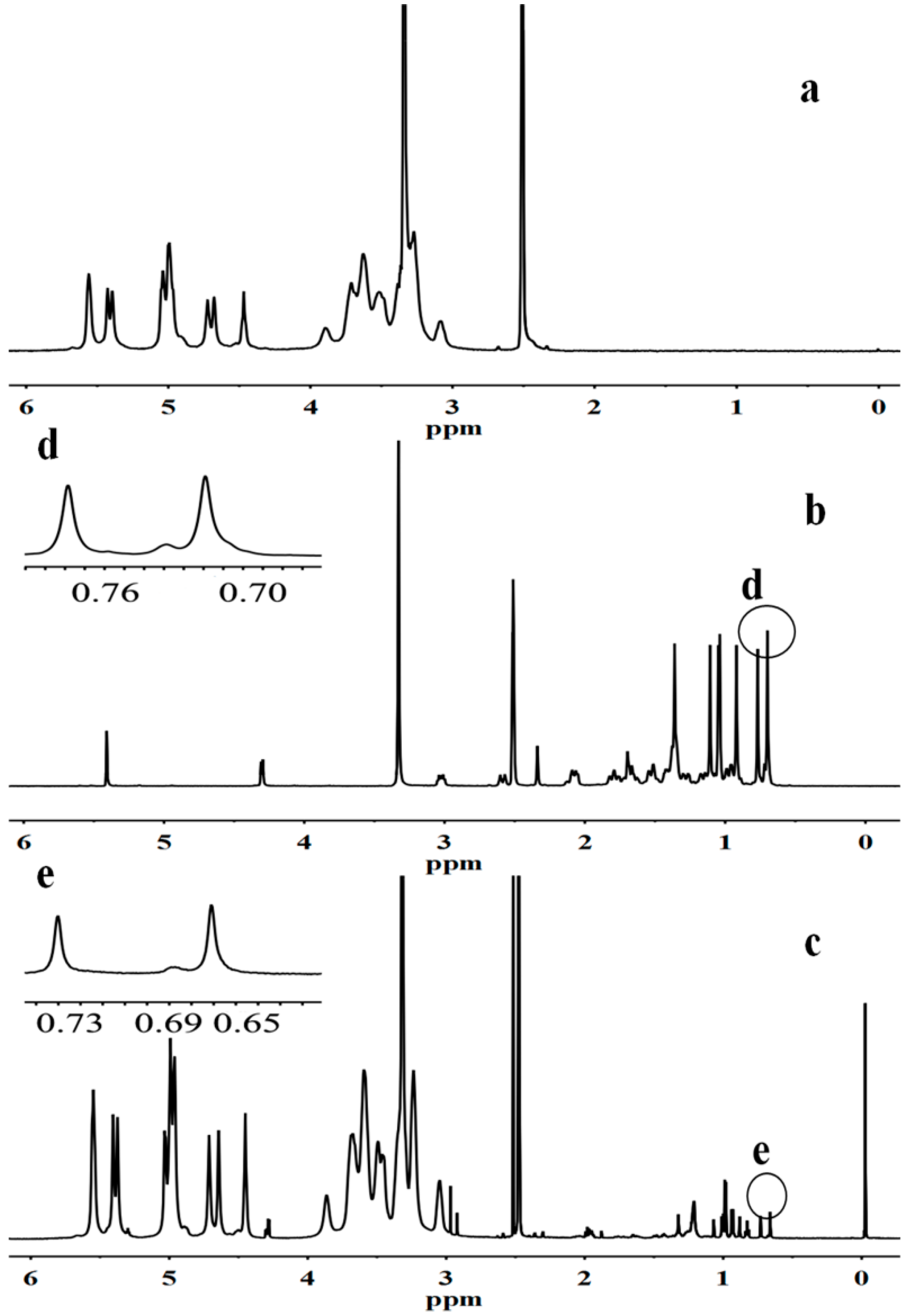
| Samples | DS | Diameter of GAP NPs (nm) | Drug/Carrier (w/w %) | LC% | EE% | Diameter of CUR-GAP NPs (nm) |
|---|---|---|---|---|---|---|
| 5 | 4.97 ± 0.13 | 78.3 ± 3.05 | 97.1 ± 3.7 | |||
| GAP1 | 6.2 | 63.7 ± 4.8 | 10 | 9.98 ± 0.28 | 72.7 ± 1.45 | 109.3 ± 8.6 |
| 20 | 10.29 ± 0.21 | 68.9 ± 2.52 | 123.6 ± 9.5 | |||
| GAP2 | 4.5 | 68.5 ± 5.3 | 10 | 6.75 ± 0.32 | 62.4 ± 3.10 | 132.7 ± 3.8 |
| GAP3 | 1.2 | 82.1 ± 6.2 | 10 | 4.16 ± 0.17 | 48.3 ± 1.73 | 153.8 ± 6.2 |
2.2. Characterization and Drug Encapsulation
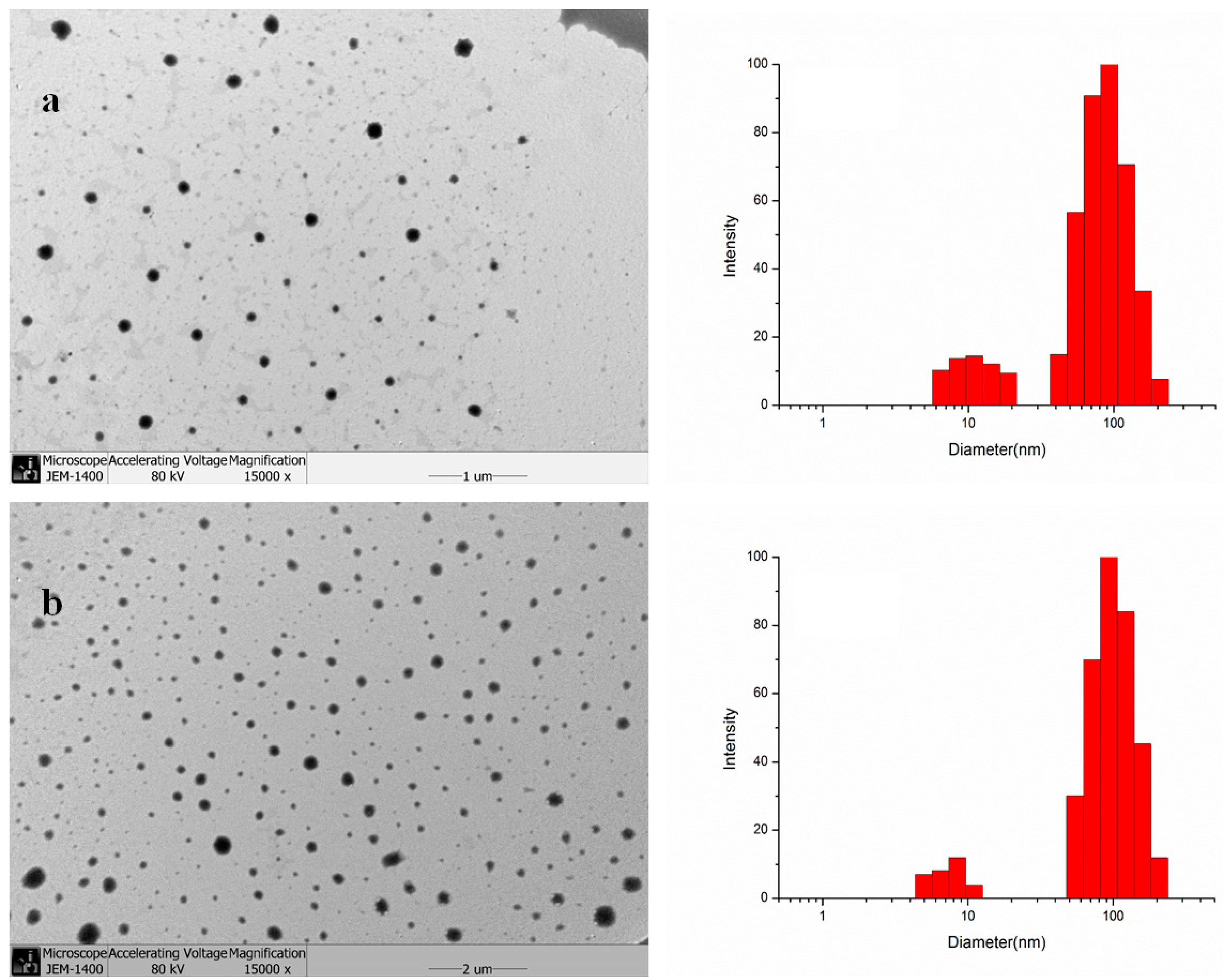
2.3. In Vitro Drug Release

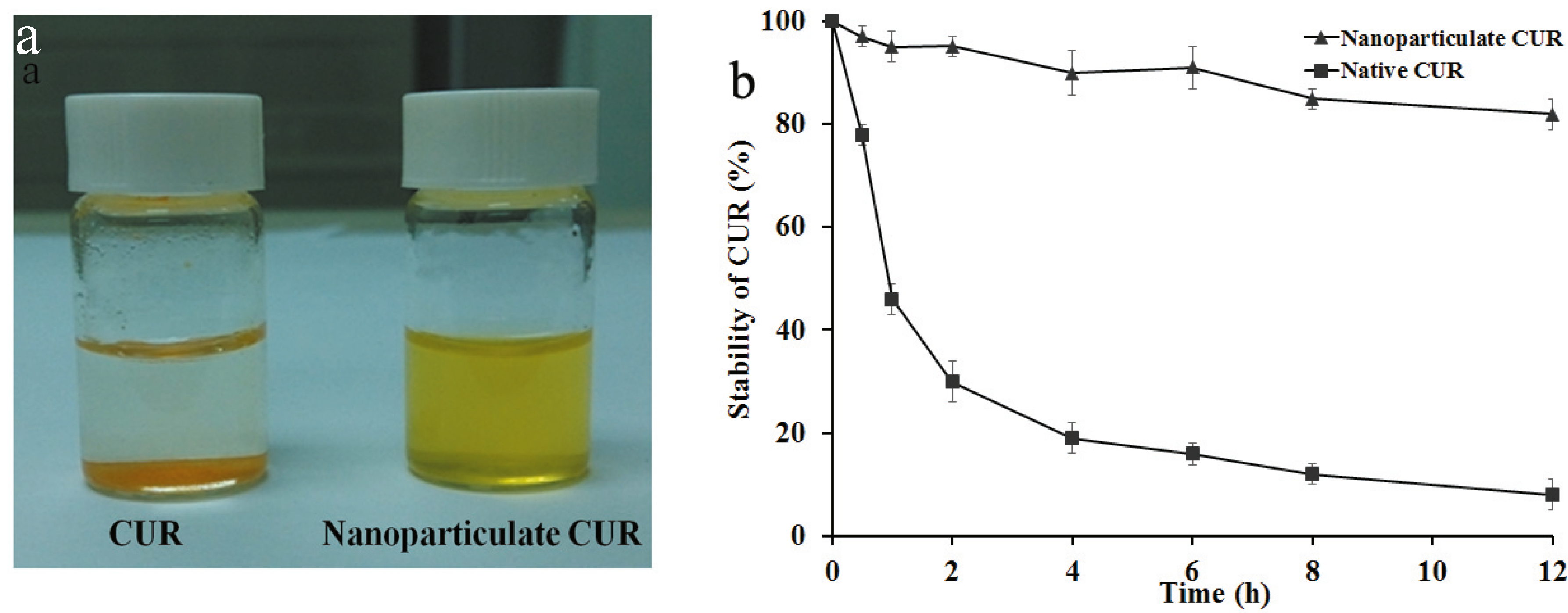
2.4. Solubility and Stability of CUR
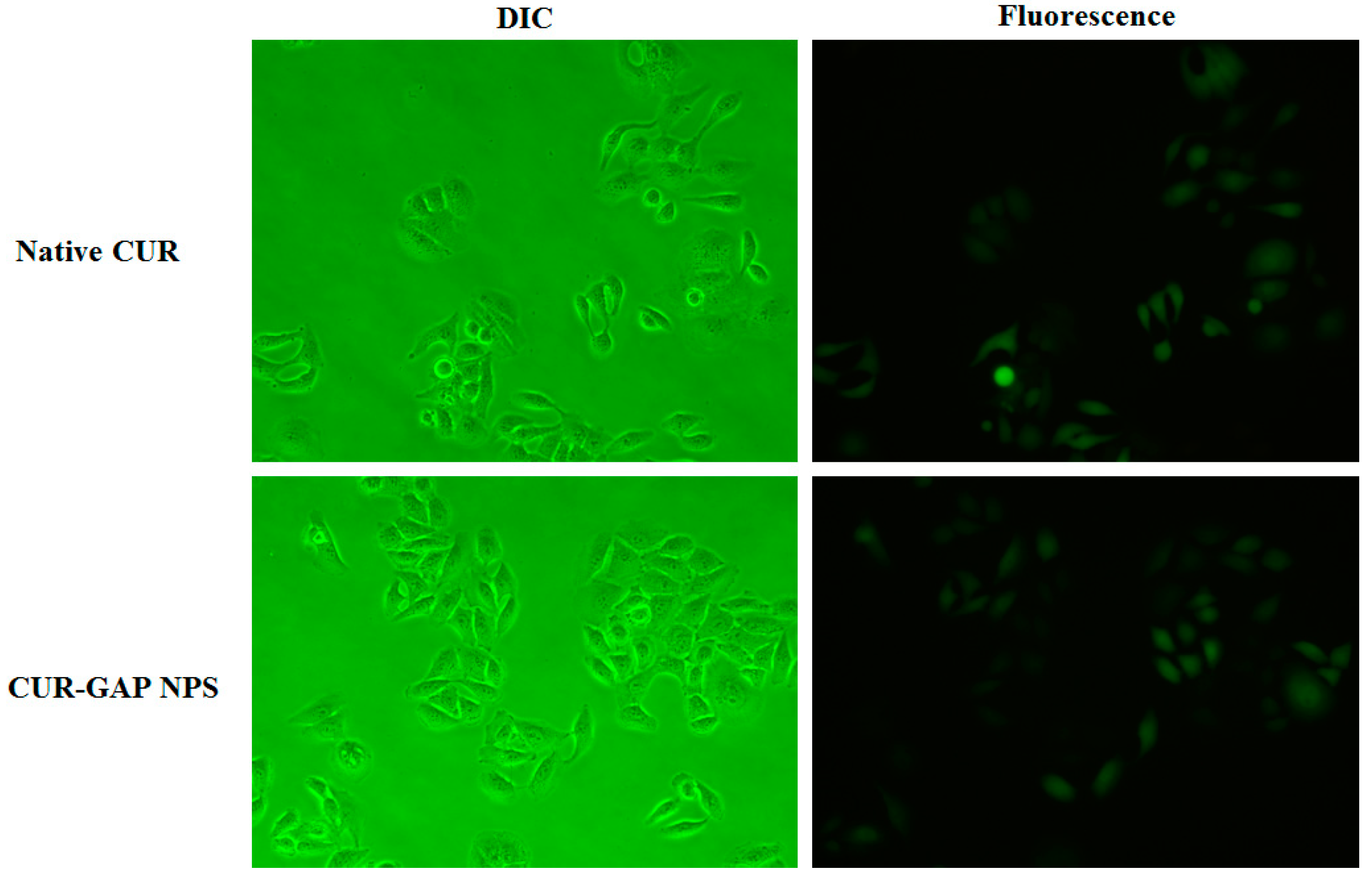
2.5. Cell Uptake of CUR-GAP NPs
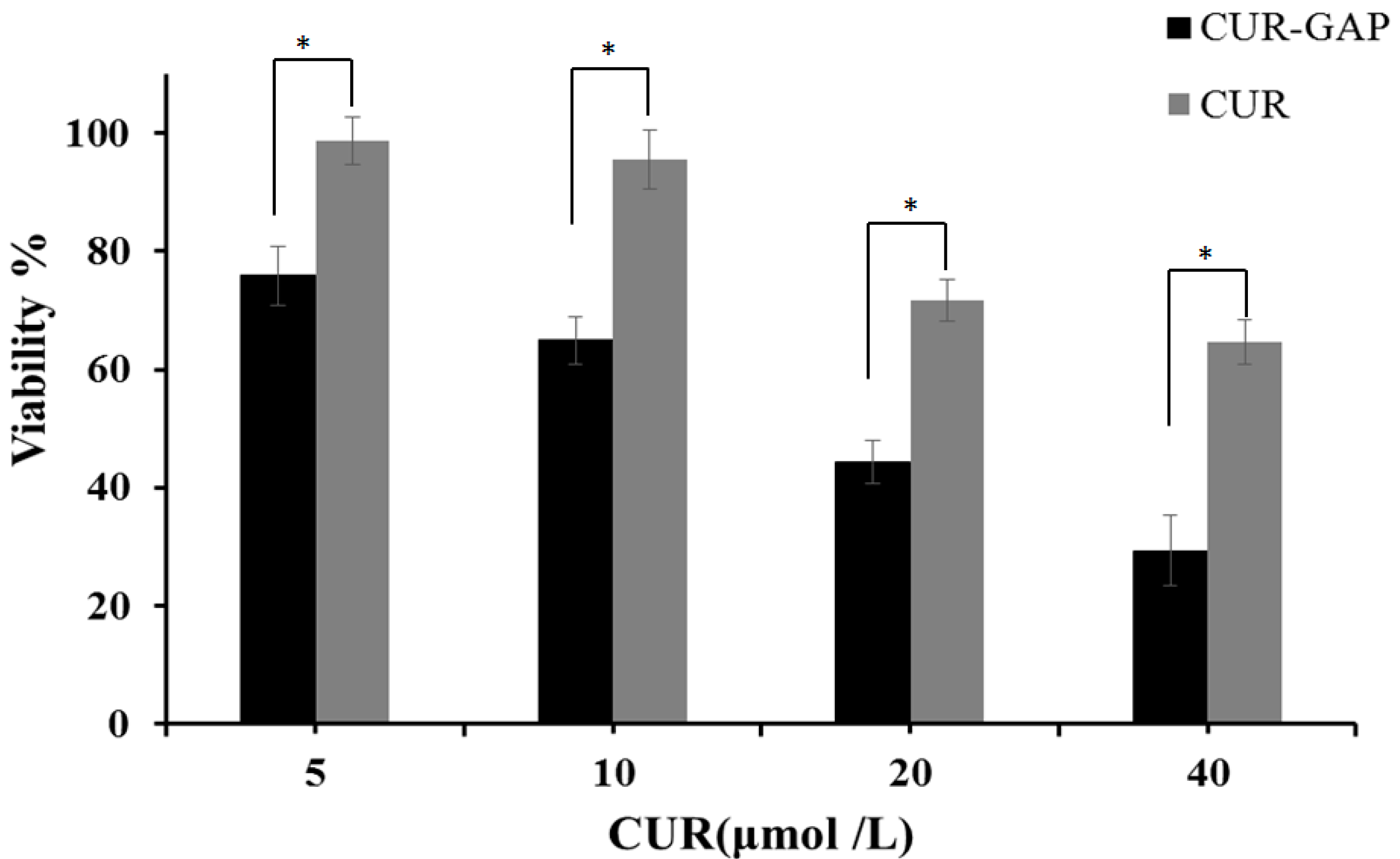
2.6. In Vitro Cytotoxicity Assay
3. Experimental
3.1. Materials
3.2. Synthesis and Characterization of GA-Modified Pullullan (GAP)
3.3. Preparation of GAP Self-aggregated NPs
3.4. Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS)
3.5. Preparation and Characterization of CUR-Loaded GAP NPs


3.6. Solubility and Stability of CUR
3.7. Release Kinetics of CUR from CUR-GAP NPs in Vitro
3.8. In Vitro Cellular Uptake
3.9. In Vitro Cytotoxicity

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Cui, S.-X.; Qu, X.-J.; Xie, Y.-Y.; Zhou, L.; Nakata, M.; Makuuchi, M.; Tang, W. Curcumin inhibits telomerase activity in human cancer cell lines. Int. J. Mol. Med. 2006, 18, 227–231. [Google Scholar]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin. Hemorheol. Microcirc. 2005, 33, 127–135. [Google Scholar]
- Darvesh, A.S.; Aggarwal, B.B.; Bishayee, A. Curcumin and liver cancer: A review. Curr. Pharm. Biotechnol. 2012, 13, 218–228. [Google Scholar] [CrossRef]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar]
- Anand, P.; Nair, H.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharm. 2010, 79, 330–338. [Google Scholar]
- Sahu, A.; Bora, U.; Kasoju, N.; Goswami, P. Synthesis of novel biodegradable and self-assembling methoxy poly (ethylene glycol)—Palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008, 4, 1752–1761. [Google Scholar]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. A 2008, 86, 300–310. [Google Scholar]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 1–18. [Google Scholar] [CrossRef]
- Sou, K.; Inenaga, S.; Takeoka, S.; Tsuchida, E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int. J. Pharm. 2008, 352, 287–293. [Google Scholar] [CrossRef]
- Vemula, P.K.; Li, J.; John, G. Enzyme catalysis: Tool to make and break amygdalin hydrogelators from renewable resources: A delivery model for hydrophobic drugs. J. Am. Chem. Soc. 2006, 128, 8932–8938. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Yuen, S. Pullulan and its applications. Proc. Biochem. 1974, 9, 7–9. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar]
- Kaneo, Y.; Tanaka, T.; Nakano, T.; Yamaguchi, Y. Evidence for receptor-mediated hepatic uptake of pullulan in rats. J. Control. Release 2001, 70, 365–373. [Google Scholar] [CrossRef]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Body distribution profile of polysaccharides after intravenous administration. Drug Deliv. 1993, 1, 75–82. [Google Scholar] [CrossRef]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Comparison of body distribution of poly (vinyl alcohol) with other water-soluble polymers after intravenous administration. J. Pharm. Pharmacol. 1995, 47, 479–486. [Google Scholar] [CrossRef]
- Lee, I.; Akiyoshi, K. Single molecular mechanics of a cholesterol-bearing pullulan nanogel at the hydrophobic interfaces. Biomaterials 2004, 25, 2911–2918. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control. Release 1998, 54, 313–320. [Google Scholar]
- Nishikawa, T.; Akiyoshi, K.; Sunamoto, J. Supramolecular assembly between nanoparticles of hydrophobized polysaccharide and soluble protein complexation between the self-aggregate of cholesterol-bearing pullulan and alpha-chymotrypsin. Macromolecules 1994, 27, 7654–7659. [Google Scholar]
- Akiyoshi, K.; Nishikawa, T.; Mitsui, Y.; Miyata, T.; Kodama, M.; Sunamoto, J. Self-assembly of polymer amphiphiles: Thermodynamics of complexation between bovine serum albumin and self-aggregate of cholesterol-bearing pullulan. Colloids Surf. A 1996, 112, 91–95. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Na, H.-S.; Oh, J.-S.; Choi, K.-C.; Song, C.-E.; Lee, H.-C. Adriamycin release from self-assembling nanospheres of poly(dl-lactide-co-glycolide)-grafted pullulan. Int. J. Pharm. 2006, 322, 154–160. [Google Scholar]
- Zhang, H.-Z.; Gao, F.-P.; Liu, L.-R.; Li, X.-M.; Zhou, Z.-M.; Yang, X.-D.; Zhang, Q.-Q. Pullulan acetate nanoparticles prepared by solvent diffusion method for epirubicin chemotherapy. Colloids Surf. B 2009, 71, 19–26. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Davidson, J.S.; Baumgarten, I.M. Glycyrrhetinic acid derivatives: A novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J. Pharmacol. Exp. Ther. 1988, 246, 1104–1107. [Google Scholar]
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008, 22, 141–148. [Google Scholar] [CrossRef]
- Negishi, M.; Irie, A.; Nagata, N.; Ichikawa, A. Specific binding of glycyrrhetinic acid to the rat liver membrane. BBA-Biomembranes 1991, 1066, 77–82. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.-H.; Wang, W.; Zhang, C.-N.; Wang, P.; Yuan, Z. Self-assembly and liver targeting of sulfated chitosan nanoparticles functionalized with glycyrrhetinic acid. Nanomed. Nanotechnol. 2012, 8, 870–879. [Google Scholar] [CrossRef]
- Shi, L.; Tang, C.; Yin, C. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials 2012, 33, 7594–7604. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Liu, T.; Wu, Y.; Guo, H.; Wang, P.; Tian, Q.; Wang, Y.; Yuan, Z. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials 2012, 33, 2187–2196. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, J.; Zhou, J.; Wang, T.; Zhang, Q. Glycyrrhetinic acid-graft-hyaluronic acid conjugate as a carrier for synergistic targeted delivery of antitumor drugs. Int. J. Pharm. 2013, 441, 654–664. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry; Cengage Learning: Saunders, Orlando, FL, USA, 1979. [Google Scholar]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Maatooq, G.T.; Marzouk, A.M.; Gray, A.I.; Rosazza, J.P. Bioactive microbial metabolites from glycyrrhetinic acid. Phytochemistry 2010, 71, 262–270. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jo, W.H.; Kwon, I.C.; Kim, Y.-H.; Jeong, S.Y. Physicochemical characteristics of self-aggregates of hydrophobically modified chitosans. Langmuir 1998, 14, 2329–2332. [Google Scholar]
- Kwon, S.; Park, J.H.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Kim, I.-S. Physicochemical characteristics of self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid. Langmuir 2003, 19, 10188–10193. [Google Scholar] [CrossRef]
- Nishikawa, T.; Akiyoshi, K.; Sunamoto, J. Macromolecular complexation between bovine serum albumin and the self-assembled hydrogel nanoparticle of hydrophobized polysaccharides. J. Am. Chem. Soc. 1996, 118, 6110–6115. [Google Scholar] [CrossRef]
- Choure, N.; Bhoite, S. Synthesis and kinetic study of hydrolysis of di-2-chloroaniline phosphate ester in acidic medium. Int. J. Chem. Kinet. 2010, 42, 126–131. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar]
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Microencapsulation of curcumin in cells of Saccharomyces cerevisiae. Food Chem. 2011, 125, 892–902. [Google Scholar] [CrossRef]
- Guo, H.; Lai, Q.; Wang, W.; Wu, Y.; Zhang, C.; Liu, Y.; Yuan, Z. Functional alginate nanoparticles for efficient intracellular release of doxorubicin and hepatoma carcinoma cell targeting therapy. Int. J. Pharm. 2013, 451, 1–11. [Google Scholar]
- Jeong, Y.I.; Kim, S.H.; Jung, T.Y.; Kim, I.Y.; Kang, S.S.; Jin, Y.H.; Ryu, H.H.; Sun, H.S.; Jin, S.; Kim, K.K.; et al. Polyion complex micelles composed of all-trans retinoic acid and poly(ethylene glycol)-grafted-chitosan. J. Pharm. Sci. 2006, 95, 2348–2360. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar]
- Sample Availability: Samples of the GAP and CUR-GAP NPs are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yuan, R.; Zheng, F.; Zhong, S.; Tao, X.; Zhang, Y.; Gao, F.; Yao, F.; Chen, J.; Chen, Y.; Shi, G. Self-Assembled Nanoparticles of Glycyrrhetic Acid-Modified Pullulan as a Novel Carrier of Curcumin. Molecules 2014, 19, 13305-13318. https://doi.org/10.3390/molecules190913305
Yuan R, Zheng F, Zhong S, Tao X, Zhang Y, Gao F, Yao F, Chen J, Chen Y, Shi G. Self-Assembled Nanoparticles of Glycyrrhetic Acid-Modified Pullulan as a Novel Carrier of Curcumin. Molecules. 2014; 19(9):13305-13318. https://doi.org/10.3390/molecules190913305
Chicago/Turabian StyleYuan, Roufen, Fuchun Zheng, Shuping Zhong, Xiaojun Tao, Yanmei Zhang, Fenfei Gao, Fen Yao, Jiaxiong Chen, Yicun Chen, and Ganggang Shi. 2014. "Self-Assembled Nanoparticles of Glycyrrhetic Acid-Modified Pullulan as a Novel Carrier of Curcumin" Molecules 19, no. 9: 13305-13318. https://doi.org/10.3390/molecules190913305
APA StyleYuan, R., Zheng, F., Zhong, S., Tao, X., Zhang, Y., Gao, F., Yao, F., Chen, J., Chen, Y., & Shi, G. (2014). Self-Assembled Nanoparticles of Glycyrrhetic Acid-Modified Pullulan as a Novel Carrier of Curcumin. Molecules, 19(9), 13305-13318. https://doi.org/10.3390/molecules190913305




