Fermented Broth in Tyrosinase- and Melanogenesis Inhibition
Abstract
:1. Introduction
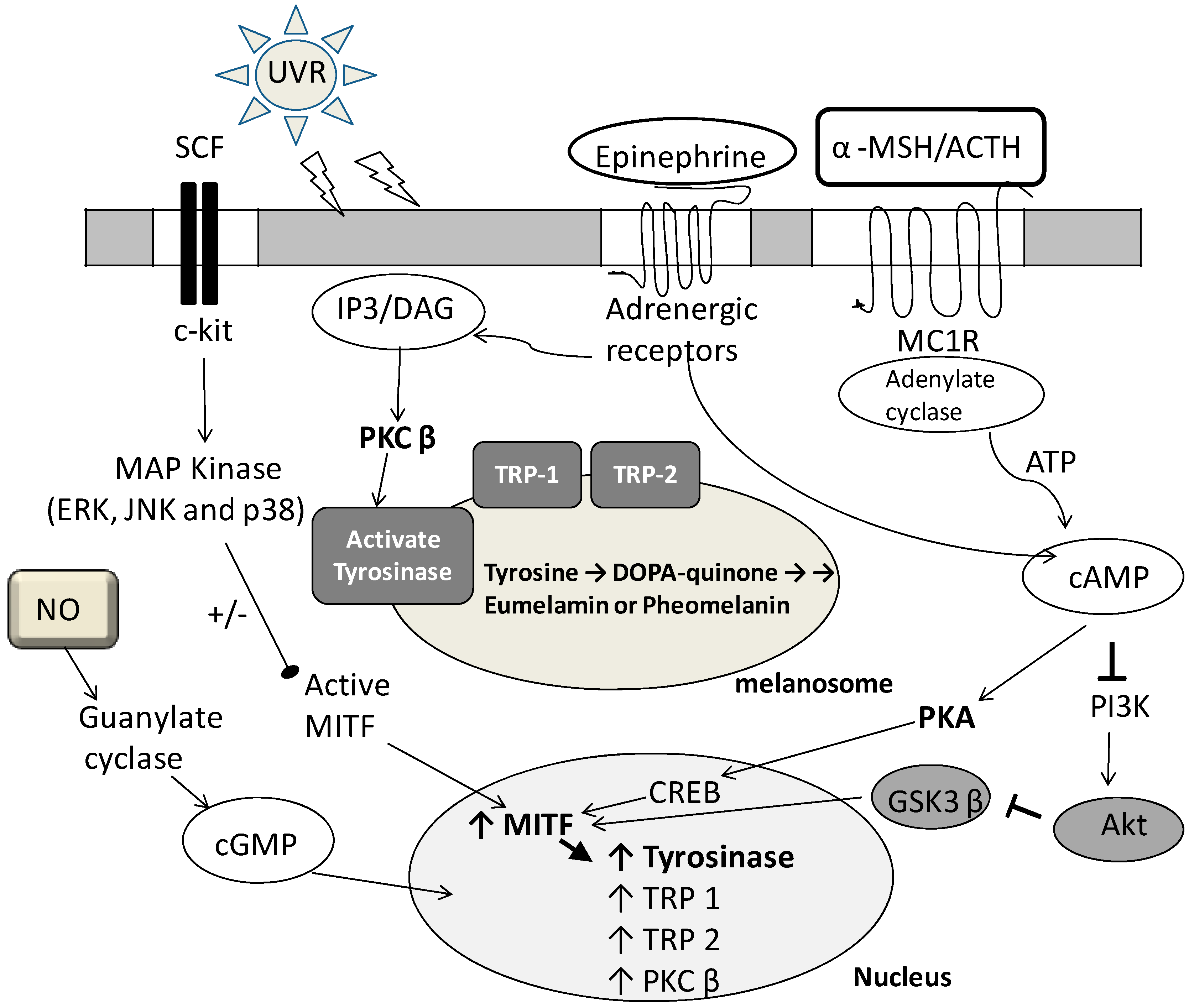
2. Fermented Broth in Inhibiting Melanogenesis
2.1. Fermentation Process
| Broth | Microorganisms | Temperature | Time | Tyrosinase Activity (Mushroom) | Melanogenesis (B16 Cells) | Ref. |
|---|---|---|---|---|---|---|
| MRS * | Bifidobacterium bifidum | 37 °C | 48 h | ↓ | ↓ | [9] |
| MRS | Bifidobacterium adolescentis | 37 °C | 48 h | ↓ | ↓ | [11] |
| MRS | Lactobacillus rhamnosus | 37 °C | 20 h | ↓ | N.D. # | [71] |
| MRS | Bifidobacterium infantis | 37 °C | 48 h | ↓ | ↓ | [79] |
| MRS | Lactobacillus brevis | 37 °C | 24 h | ↓ | N.D. | [80] |
| MRS | Leuconostoc mesenteroides | 30 °C | 24 h | X § | ↓ | [46] |
| Soy milk | Lactobacillus plantarum | 37 °C | 48 h | ↓ | ↓ | [74] |
| Soybean | Aspergillus oryzae | - | 4 months | 50% ↓ 50% X | ↓ | [47] |
| Soy germ | Aspergillus oryzae | 25 °C | 1 week | ↓ | ↓ | [75] |
| Soybean | Bacillus subtilis | 40 °C | 36 h | ↓ | N.D. | [81] |
| Seed medium | Streptomyces | 27 °C | 96 h | X | ↓ | [48] |
| Seed medium | Enterobacter sp. B20 | 28 °C | 5 days | ↓ | ↓ | [76] |
| Rice bran | Lactobacillus rhamnosus and Sac charomyce cerevisiae | 15 °C | 15 days | X | ↓ | [49] |
| Rice, black rice, sweet potato and barley | Saccharomyces cerevisiae and Aspergillus niger | - | - | N.D. | ↓ | [89] |
| Rice, soybean, or soygerm | Aspergillus oryzae | 25 °C | 1 week | ↓ | N.D. | [82] |
| Rice Bran papaya, and seaweed | Lactobacillaceae, saccharomycetes, funguses, actinomyces and photosynthetic bacteria | 35 °C | 7 days | ↓ | N.D. | [83] |
| P. linteus complex culture | Phellinus linteus | 28 °C | 9 days | ↓ | ↓ | [77] |
| Purple plain rice | Look Pang (yeasts and molds) | - | 8 days | ↓ | N.D. | [84] |
| Squid pen | Burkholderia cepacia | 30 °C | 3 days | ↓ | N.D. | [85] |
| Rhodiola rosea | Alcaligenes piechaudii | 30 °C | 5 days | ↓ | N.D. | [86] |
| Lonicera japonica | Alcaligenes piechaudii | 30 °C | 5 days | ↓ | N.D. | [86] |
| Codonopsis lanceolata | Bifidobacterium longum | 37 °C | 7 days | ↓ | N.D. | [87] |
| Codonopsis lanceolata | Lactobacillus rhamnosus | 37 °C | 7 days | ↓ | N.D. | [87] |
| Potato dextrose agar | Aspergillus oryzae | 30 °C | 2 days | ↓ | N.D. | [88] |
| Viola mandshurica | Microorganisms | - | 6 months | ↓ | ↓ | [90] |
2.2. Possible Functional Components of Fermented Broth
2.2.1. Lactic Acid
| Broth/Microorganism | Possible Active Ingredients (PAI) | Levels of PAI | Hypopigmentation Mechanism | Refs. |
|---|---|---|---|---|
| P. linteus complex culture/Phellinus linteus | Phenolics and flavonoids | ↑ | Inhibit tyrosinase activity, down regulate MITF, TRP1 and TRP2; activation of the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3beta | [77] |
| Soy milk/Lactobacillus plantarum | Aglycone isoflavones (such as dadzein and genistein) | ↑ | Inhibit tyrosinase activity, down regulate MITF, inactive MAPK and p38 | [74] |
| Soybean/Aspergillus oryzae | 8-Hydroxydaidzein and 3-hydroxydaidzein | ↑ | Repress MITF, decrease expression of tyrosinase, TRP1 and TRP2 | [47] |
| Soy germ/Aspergillus oryzae | 8-Hydroxydaidzein | ↑ | Inhibit tyrosinase activity | [75] |
| MRS/Bifidobacterium bifidum; MRS/Bifidobacterium adolescentis | Unknown | N.D. | Antioxidative activity | [9,11] |
| Rice, black rice, sweet potato and barley/Saccharomyces cerevisiae and Aspergillus niger | Polyphenolic compounds | barley > black rice > sweet potato > rice | Antioxidative activity | [89] |
| Seed edium/Enterobacter sp. B20 | Byelyankacin | ↑ | Inhibit tyrosinase activity | [76] |
| Seed medium/Stveptomyces sp. | Albocycline K3 | ↑ | Unknown | [48] |
| MRS/Leuconostoc mesenteroides | Crude self-digestion (autolysis) extract | N.D. | Inhibits tyrosinase activity, tyrosinase translation, or accelerating its degradation | [46] |
2.2.2. Flavonoids
2.2.3. Antioxidants
2.2.4. Novel Melanogenesis Inhibitors
3. Future Outlook of Fermented Broth in Cosmetic Industries
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, Q.; Zhang, M. White or tan? A cross-cultural analysis of skin beauty advertisements between China and the United States. Asian J. Commun. 2013, 23, 538–554. [Google Scholar]
- Li, E.P.; Min, H.J.; Belk, R.W.; Kimura, J.; Bahl, S. Skin lightening and beauty in four asian cultures. Adv. Consum. Res. 2008, 35, 444–449. [Google Scholar]
- Ashikari, M. Urban middle-class Japanese women and their white faces: Gender, ideology, and representation. Ethos 2003, 31, 3–37. [Google Scholar]
- Cao, S. Intertextuality and glocalization a corpus-based analysis of advertisement texts of an international female fashion magazine. J. Arts Hum. 2014, 3, 39–49. [Google Scholar]
- Li, H. TCM in skin whitening and lightening: The eternal pursuit in east asia. Cosmet. Toiletries 2013, 128, 104–109. [Google Scholar]
- Dixson, B.J.; Dixson, A.F.; Li, B.; Anderson, M. Studies of human physique and sexual attractiveness: Sexual preferences of men and women in China. Am. J. Hum. Biol. 2007, 19, 88–95. [Google Scholar]
- Ashikari, M. Cultivating Japanese whiteness: The ‘whitening’ cosmetics boom and the Japanese identity. J. Mater. Cult. 2005, 10, 73–91. [Google Scholar]
- Lagouvardos, P.E.; Tsamali, I.; Papadopoulou, C.; Polyzois, G. Tooth, skin, hair and eye colour interrelationships in Greek young adults. Odontology 2013, 101, 75–83. [Google Scholar] [CrossRef]
- Huang, H.C.; Huang, W.Y.; Chiu, S.H.; Ke, H.J.; Chiu, S.W.; Wu, S.Y.; Kuo, F.S.; Chang, T.M. Antimelanogenic and antioxidative properties of Bifidobacterium bifidum. Arch. Dermatol. Res. 2011, 303, 527–531. [Google Scholar]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar]
- Huang, H.C.; Chang, T.M. Antioxidative properties and inhibitory effect of Bifidobacterium adolescentis on melanogenesis. World J. Microbiol. Biotechnol. 2012, 28, 2903–2912. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar]
- Badreshia-Bansal, S.; Draelos, Z.D. Insight into skin lightening cosmeceuticals for women of color. J. Drugs Dermatol. 2007, 6, 32–39. [Google Scholar]
- Sato, K.; Morita, M.; Ichikawa, C.; Takahashi, H.; Toriyama, M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Biosci. Biotechnol. Biochem. 2008, 72, 2589–2597. [Google Scholar]
- Ando, H.; Itoh, A.; Mishima, Y.; Ichihashi, M. Correlation between the number of melanosomes, tyrosinase mRNA levels, and tyrosinase activity in cultured murine melanoma cells in response to various melanogenesis regulatory agents. J. Cell. Physiol. 1995, 163, 608–614. [Google Scholar] [CrossRef]
- Wiechers, J.; Rawlings, A.; Garcia, C.; Chesne, C.; Balaguer, P.; Nicolas, J.; Corre, S.; Galibert, M.D. A new mechanism of action for skin whitening agents: Binding to the peroxisome proliferator-activated receptor1. Int. J. Cosmet. Sci. 2005, 27, 123–132. [Google Scholar]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar]
- Picardo, M.; Carrera, M. New and experimental treatments of cloasma and other hypermelanoses. Dermatol. Clin. 2007, 25, 353–362. [Google Scholar]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigm. Cell Res. 2006, 19, 550–571. [Google Scholar]
- Wood, J.M.; Schallreuter, K.U. Studies on the reactions between human tyrosinase, superoxide anion, hydrogen peroxide and thiols. Biochim. Biophys. Acta 1991, 1074, 378–385. [Google Scholar]
- Shivhare, S.; Malviya, K.; Malviya, K.; Jain, V. A review: Natural skin lighting and nourishing agents. Res. J. Top. Cosmet. Sci. 2013, 4, 21–25. [Google Scholar]
- Huang, C.H.; Sung, H.C.; Hsiao, C.Y.; Hu, S.; Ko, Y.S. Transdermal delivery of three vitamin C derivatives by Er: YAG and carbon dioxide laser pretreatment. Lasers Med. Sci. 2013, 28, 807–814. [Google Scholar]
- Yao, C.L.; Lin, Y.M.; Mohamed, M.S.; Chen, J.H. Inhibitory effect of ectoine on melanogenesis in B16-F0 and A2058 melanoma cell lines. Biochem. Eng. J. 2013, 78, 163–169. [Google Scholar]
- Kumar, K.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar] [CrossRef]
- Gonçalez, M.; Corrêa, M.; Chorilli, M. Skin delivery of kojic acid-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. BioMed Res. Int. 2013, 2013, 271276. [Google Scholar]
- Ki, D.H.; Jung, H.C.; Noh, Y.W.; Thanigaimalai, P.; Kim, B.H.; Shin, S.C.; Jung, S.H.; Cho, C.W. Preformulation and formulation of newly synthesized QNT3-18 for development of a skin whitening agent. Drug Dev. Ind. Pharm. 2013, 39, 526–533. [Google Scholar]
- Won, Y.-K.; Loy, C.-J.; Randhawa, M.; Southall, M.D. Clinical efficacy and safety of 4-hexyl-1, 3-phenylenediol for improving skin hyperpigmentation. Arch. Dermatol. Res. 2014, 306, 455–465. [Google Scholar]
- Son, K.; Heo, M. The evaluation of depigmenting efficacy in the skin for the development of new whitening agents in Korea. Int. J. Cosmet. Sci. 2013, 35, 9–18. [Google Scholar]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y.; Wu, M.C.; Shi, W.L. Kinetic study on the tyrosinase and melanin formation inhibitory activities of carthamus yellow isolated from Carthamus tinctorius L. J. Biosci. Bioeng. 2013, 115, 242–245. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Chen, W.Y.; Aljuffali, A.; Chen, C.C.; Fang, J.Y. Co-drug strategy for promoting skin targeting and minimizing the transdermal diffusion of hydroquinone and tranexamic acid. Curr. Med. Chem. 2013, 20, 4080–4092. [Google Scholar]
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66. [Google Scholar]
- Eimpunth, S.; Wanitphadeedecha, R.; Manuskiatti, W. A focused review on acne-induced and aesthetic procedure-related postinflammatory hyperpigmentation in Asians. J. Eur. Acad. Dermatol. 2013, 27, 7–18. [Google Scholar]
- Amer, M.; Metwalli, M. Topical hydroquinone in the treatment of some hyperpigmentary disorders. Int. J. Dermatol. 1998, 37, 449–450. [Google Scholar] [CrossRef]
- Haddad, A.L.; Matos, L.F.; Brunstein, F.; Ferreira, L.M.; Silva, A.; Costa, D. A clinical, prospective, randomized, double-blind trial comparing skin whitening complex with hydroquinone vs. placebo in the treatment of melasma. Int. J. Dermatol. 2003, 42, 153–156. [Google Scholar] [CrossRef]
- Josefina, N.S.; Pablo, C.C.J.; Bertha, T.Á.; Cuauhtemoc, O.O.; Cornelia, F.A.; González, F.J.; David, M.R.J.; Benjamin, M. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol. Res. Pract. 2011, 2011, 379173. [Google Scholar]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs. iodometric titration as analytical methods. LWT-Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- Ookubo, N.; Michiue, H.; Kitamatsu, M.; Kamamura, M.; Nishiki, T.; Ohmori, I.; Matsui, H. The transdermal inhibition of melanogenesis by a cell-membrane-permeable peptide delivery system based on poly-arginine. Biomaterials 2014, 35, 4508–4516. [Google Scholar]
- Fujimoto, N.; Watanabe, H.; Nakatani, T.; Roy, G.; Ito, A. Induction of thyroid tumours in (C57BL/6N × C3H/N) F1 mice by oral administration of kojic acid. Food Chem. Toxicol. 1998, 36, 697–703. [Google Scholar]
- Takizawa, T.; Mitsumori, K.; Tamura, T.; Nasu, M.; Ueda, M.; Imai, T.; Hirose, M. Hepatocellular tumor induction in heterozygous p53-deficient CBA mice by a 26-week dietary administration of kojic acid. Toxicol. Sci. 2003, 73, 287–293. [Google Scholar]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B 2013, 110, 313–320. [Google Scholar]
- Findlay, G.H.; Morrison, J.; Simson, I. Exogenous ochronosis and pigmented colloid milium from hydroquinone bleaching creams. Brit. J. Dermatol. 1975, 93, 613–622. [Google Scholar]
- Charlín, R.; Barcaui, C.B.; Kac, B.K.; Soares, D.B.; Rabello-Fonseca, R.; Azulay-Abulafia, L. Hydroquinone-induced exogenous ochronosis: A report of four cases and usefulness of dermoscopy. Int. J. Dermatol. 2008, 47, 19–23. [Google Scholar]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigm. Cell Res. 2003, 16, 101–110. [Google Scholar]
- Kiken, D.A.; Cohen, D.E. Contact dermatitis to botanical extracts. Dermatitis 2002, 13, 148–152. [Google Scholar]
- Kondo, S.; Takahashi, T.; Yoshida, K.; Mizoguchi, H. Inhibitory effects of autolysate of Leuconostoc mesenteroides isolated from kimoto on melanogenesis. J. Biosci. Bioeng. 2012, 114, 424–428. [Google Scholar] [CrossRef]
- Goh, M.J.; Park, J.S.; Bae, J.H.; Kim, D.H.; Kim, H.K.; Na, Y.J. Effects of ortho-dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesis in B16 melanoma cells and human skin equivalents. Phytother. Res. 2012, 26, 1107–1112. [Google Scholar]
- Takamatsu, S.; Kim, Y.; Hayashi, M.; Komiyama, K.; Imokawa, G.; Omura, S. A new inhibitor of melanogenesis, albocycline K3, produced by Stveptomyces sp. OH-3984. Tetrahedron Lett. 1995, 35, 2635–2636. [Google Scholar]
- Chung, S.Y.; Seo, Y.K.; Park, J.M.; Seo, M.J.; Park, J.K.; Kim, J.W.; Park, C.S. Fermented rice bran bownregulates MITF expression and leads to inhibition of α-MSH-induced melanogenesis in B16F1 melanoma. Biosci. Biotechnol. Biochem. 2009, 73, 1704–1710. [Google Scholar]
- Bae-Harboe, Y.S.; Park, H.Y. Tyrosinase: A central regulatory protein for cutaneous pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar]
- Tepper, A.W.J.W. Tyrosinase: Biology, structure and mechanism. In Structure and Mechanism of the Type-3 Copper Protein Tyrosinase; Optima Grafische Communicatie: Rotterdam, The Netherlands, 2005; pp. 19–45. [Google Scholar]
- Burchill, S.A.; Bennett, D.C.; Holmes, A.; Thody, A.J. Tyrosinase expression and melanogenesis in melanotic and amelanotic B16 mouse melanoma cells. Pathobiology 1991, 59, 335–339. [Google Scholar]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar]
- Lee, J.Y.; Choi, H.J.; Chung, T.W.; Kim, C.H.; Jeong, H.S.; Ha, K.T. Caffeic acid phenethyl ester inhibits alpha-melanocyte stimulating hormone-induced melanin synthesis through suppressing transactivation activity of microphthalmia-associated transcription factor. J. Nat. Prod. 2013, 76, 1399–1405. [Google Scholar]
- Wu, L.C.; Lin, Y.Y.; Yang, S.Y.; Weng, Y.T.; Tsai, Y.T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigm. Cell Res. 2006, 19, 595–605. [Google Scholar]
- Lin, C.; Babiarz, L.; Liebel, F.; Price, E.R.; Kizoulis, M.; Gendimenico, G.; Fisher, D.; Seiberg, M. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar]
- Jiang, Z.; Li, S.; Liu, Y.; Deng, P.; Huang, J.; He, G. Sesamin induces melanogenesis by microphthalmia-associated transcription factor and tyrosinase up-regulation via cAMP signaling pathway. Acta Biochim. Biophys. Sin. 2011, 43, 763–770. [Google Scholar]
- Lee, C.S.; Jang, W.H.; Park, M.; Jung, K.; Baek, H.S.; Joo, Y.H.; Park, Y.H.; Lim, K.M. A novel adamantyl benzylbenzamide derivative, AP736, suppresses melanogenesis through the inhibition of cAMP-PKA-CREB-activated microphthalmia-associated transcription factor and tyrosinase expression. Exp. Dermatol. 2013, 22, 762–764. [Google Scholar] [CrossRef]
- Shibahara, S.; Takeda, K.; Yasumoto, K.; Udono, T.; Watanabe, K.; Saito, H.; Takahashi, K. Microphthalmia-associated transcription factor (MITF): Multiplicity in structure, function, and regulation. J. Investig. Dermatol. Symp. Proc. 2001, 6, 99–104. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits alpha-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/beta-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar]
- Dong, Y.; Wang, H.; Cao, J.; Ren, J.; Fan, R.; He, X.; Smith, G.W.; Dong, C. Nitric oxide enhances melanogenesis of alpaca skin melanocytes in vitro by activating the MITF phosphorylation. Mol. Cell. Biochem. 2011, 352, 255–260. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.H.; Jeong, S.Y.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Partially purified Curcuma longa inhibits alpha-melanocyte-stimulating hormone-stimulated melanogenesis through extracellular signal-regulated kinase or Akt activation-mediated signalling in B16F10 cells. Exp. Dermatol. 2009, 18, 689–694. [Google Scholar] [CrossRef]
- Ko, H.H.; Chiang, Y.C.; Tsai, M.H.; Liang, C.J.; Hsu, L.F.; Li, S.Y.; Wang, M.C.; Yen, F.L.; Lee, C.W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar]
- Ouwehand, A.; Båtsman, A.; Salminen, S. Probiotics for the skin: A new area of potential application? Lett. Appl. Microbiol. 2003, 36, 327–331. [Google Scholar] [CrossRef]
- Mak, A.K.Y. Advertising whiteness: An assessment of skin color preferences among urban Chinese. Vis. Commun. Q. 2007, 14, 144–157. [Google Scholar]
- Tsai, C.C.; Chan, C.F.; Huang, W.Y.; Lin, J.S.; Chan, P.; Liu, H.Y.; Lin, Y.S. Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Molecules 2013, 18, 14161–14171. [Google Scholar]
- Gaden, E.L. Fermentation process kinetics. Biotechnol. Bioeng. 2000, 67, 629–635. [Google Scholar]
- Hanko, V.P.; Rohrer, J.S. Determination of carbohydrates, sugar alcohols, and glycols in cell cultures and fermentation broths using high-performance anion-exchange chromatography with pulsed amperometric detection. Anal. Biochem. 2000, 283, 192–199. [Google Scholar] [CrossRef]
- Chen, Y.M.; Shih, T.W.; Chiu, C.P.; Pan, T.M.; Tsai, T.Y. Effects of lactic acid bacteria-fermented soy milk on melanogenesis in B16F0 melanocytes. J. Funct. Foods 2013, 5, 395–405. [Google Scholar]
- Tai, S.S.; Lin, C.G.; Wu, M.H.; Chang, T.S. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int. J. Mol. Sci. 2009, 10, 4257–4266. [Google Scholar]
- Takahashi, S.; Iwai, H.; Kosaka, K.; Miyazaki, T.; Osanai, Y.; Arao, N.; Tanaka, K.; Nagai, K.; Nakagawa, A. Byelyankacin: A novel melanogenesis inhibitor produced by Enterobacter sp. B20. J. Antibiot. 2007, 60, 717–720. [Google Scholar] [CrossRef]
- Cha, J.Y.; Yang, H.J.; Moon, H.I.; Cho, Y.S. Inhibitory effect and mechanism on melanogenesis from fermented herbal composition for medical or food uses. Food Res. Int. 2012, 45, 225–231. [Google Scholar]
- Kwak, Y.J.; Kim, K.S.; Kim, K.M.; Yu, H.Y.; Chung, E.; Kim, S.J.; Cha, J.Y.; Lee, Y.C.; Lee, J.H. Fermented Viola mandshurica inhibits melanogenesis in B16 melanoma cells. Biosci. Biotechnol. Biochem. 2011, 75, 841–847. [Google Scholar] [CrossRef]
- Huang, H.C.; Chiu, S.H.; Ke, H.J.; Chiu, S.W.; Wu, S.Y.; Chang, T.M. Antimelanogenic and antioxidant activities of Bifidobacterium infantis. Afr. J. Microbiol. Res. 2011, 5, 3150–3156. [Google Scholar]
- Lee, H.S.; Kim, M.R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: In vitro and animal study. J. Med. Food 2012, 15, 1015–1023. [Google Scholar] [CrossRef]
- Chae, G.Y.; Ha, B.J. The comparative evaluation of fermented and non-fermented soybean extract on antioxidation and whitening. Toxicol. Res. 2011, 27, 205–209. [Google Scholar]
- Chang, T.S.; Ding, H.Y.; Tai, S.S.K.; Wu, C.Y. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem. 2007, 105, 1430–1438. [Google Scholar]
- Li, Z.; Lee, J.; Cho, M.H. Antioxidant, antibacterial, tyrosinase inhibitory, and biofilm inhibitory activities of fermented rice bran broth with effective microorganisms. Biotechnol. Bioprocess Eng. 2010, 15, 139–144. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Manosroi, J. Free radical scavenging and tyrosinase inhibition activities of fermented Thai rice for cosmeceuticals. J. Thai. Tradit. Altern. Med. 2008, 6 (Suppl. S1), 71. [Google Scholar]
- Hsu, C.H.; Nguyen, A.D.; Chen, Y.W.; Wang, S.L. Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res. Chem. Intermed. 2014, 40, 2249–2258. [Google Scholar] [CrossRef]
- Chen, Y.S.; Liou, H.C.; Chan, C.F. Tyrosinase inhibitory effect and antioxidative activities of fermented and ethanol extracts of Rhodiola rosea and Lonicera japonica. Sci. World J. 2013, 2013, 612739. [Google Scholar]
- He, X.; Zou, Y.; Yoon, W.B.; Park, S.J.; Park, D.S.; Ahn, J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar]
- Chang, T.S.; Lin, M.Y.; Lin, H.J. Identifying 8-hydroxynaringenin as a suicide substrate of mushroom tyrosinase. J. Cosmet. Sci. 2010, 61, 205–210. [Google Scholar]
- Ohgidani, M.; Komizu, Y.; Goto, K.; Ueoka, R. Antimelanogenic and antioxidative effects of residual powders from Shochu distillation remnants. Food Chem. 2012, 132, 2140–2143. [Google Scholar]
- Lew, L.C.; Gan, C.Y.; Liong, M.T. Dermal bioactives from lactobacilli and bifidobacteria. Ann. Microbiol. 2012, 63, 1047–1055. [Google Scholar]
- Hasegawa, S.; Azuma, M.; Takahashi, K. Stabilization of enzyme activity during the esterification of lactic acid in hydrophobic ethers and ketones as reaction media that are miscible with lactic acid despite their high hydrophobicity. Enzym. Microb. Technol. 2008, 43, 309–316. [Google Scholar]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12, 43–50. [Google Scholar]
- Bowe, W.P.; Shalita, A.R. Effective over-the-counter acne treatments. Semin. Cutan. Med. Surg. 2008, 27, 170–176. [Google Scholar]
- Lim, Y.H.; Kim, I.H.; Seo, J.J.; Kim, J.K. Tyrosinase inhibitor from the flowers of Impatiens balsamina. J. Microbiol. Biotechnol. 2006, 16, 1977–1983. [Google Scholar]
- Ken, J.; Jennifer, H.; Mei, H.; Qi, J.; Steve, O. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigm. Cell Res. 2002, 15, 335–340. [Google Scholar]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.; Cui, S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar]
- Del Marmol, V.; Solano, F.; Sels, A.; Huez, G.; Libert, A.; Lejeune, F.; Ghanem, G. Glutathione depletion increases tyrosinase activity in human melanoma cells. J. Investig. Dermatol. 1993, 101, 871–874. [Google Scholar]
- Galván, I.; Alonso-Alvarez, C. An intracellular antioxidant determines the expression of a melanin-based signal in a bird. PLoS One 2008, 3, e3335. [Google Scholar]
- Foligne, B.; Daniel, C.; Pot, B. Probiotics from research to market: the possibilities, risks and challenges. Curr. Opin. Microbiol. 2013, 16, 284–292. [Google Scholar]
- Tsai, H.H.; Chen, Y.C.; Lee, W.R.; Hu, C.H.; Hakozaki, T.; Yoshii, T.; Shen, S.C. Inhibition of inflammatory nitric oxide production and epidermis damages by Saccharomycopsis ferment filtrate. J. Dermatol. Sci. 2006, 42, 249–257. [Google Scholar] [CrossRef]
- Pang, J.H.; Wong, W.R.; Hakozaki, T.; Yoshii, T.; Chen, T.Y. Up-regulation of tight junction-related proteins and increase of human epidermal keratinocytes barrier function by Saccharomycosis ferment filtrate. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 15–24. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chan, C.-F.; Huang, C.-C.; Lee, M.-Y.; Lin, Y.-S. Fermented Broth in Tyrosinase- and Melanogenesis Inhibition. Molecules 2014, 19, 13122-13135. https://doi.org/10.3390/molecules190913122
Chan C-F, Huang C-C, Lee M-Y, Lin Y-S. Fermented Broth in Tyrosinase- and Melanogenesis Inhibition. Molecules. 2014; 19(9):13122-13135. https://doi.org/10.3390/molecules190913122
Chicago/Turabian StyleChan, Chin-Feng, Ching-Cheng Huang, Ming-Yuan Lee, and Yung-Sheng Lin. 2014. "Fermented Broth in Tyrosinase- and Melanogenesis Inhibition" Molecules 19, no. 9: 13122-13135. https://doi.org/10.3390/molecules190913122






