Effects of Single Amino Acid Substitution on the Biophysical Properties and Biological Activities of an Amphipathic α-Helical Antibacterial Peptide Against Gram-Negative Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Design and the Biophysical Properties
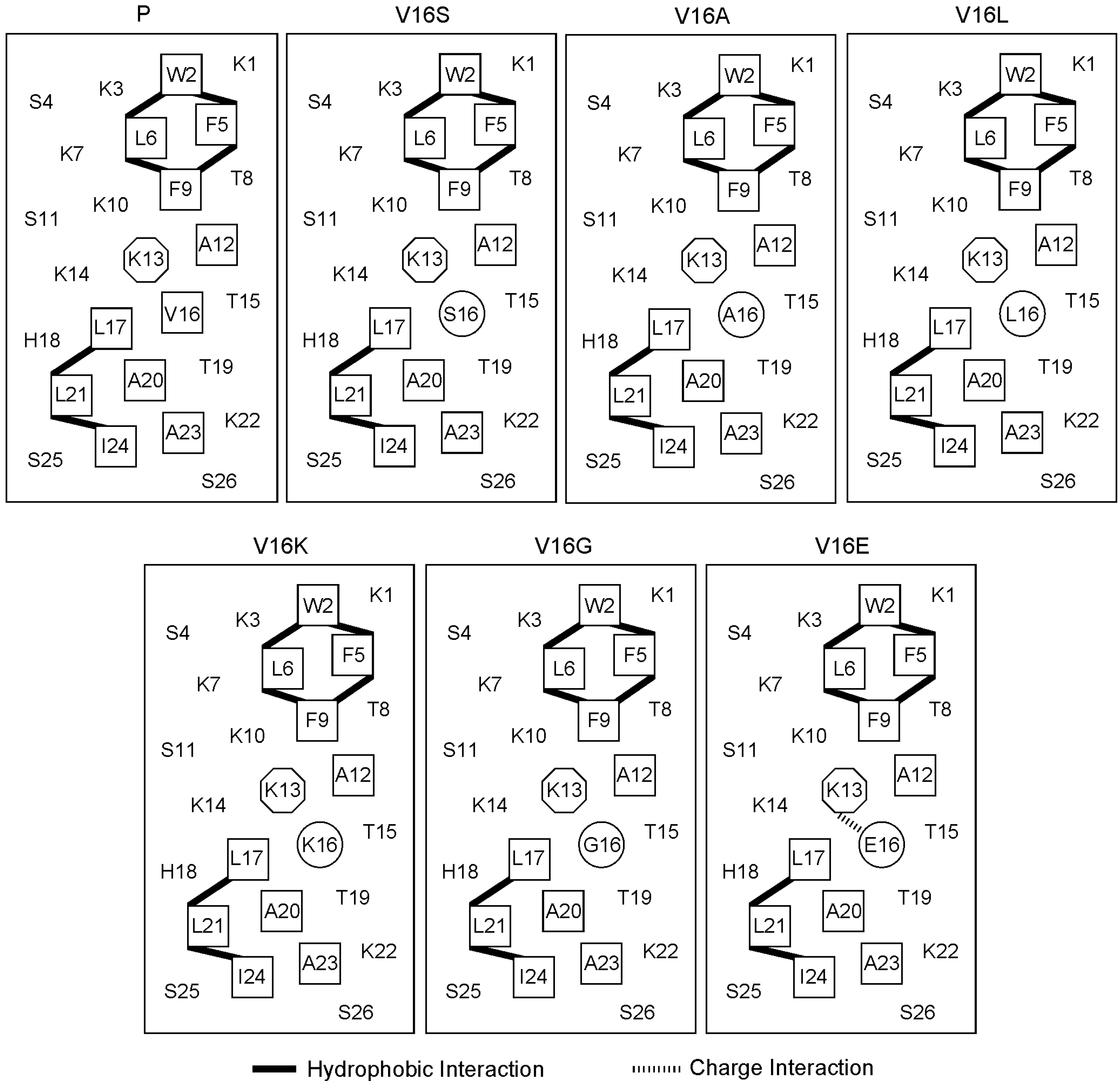
| Peptide | Amino Acid Sequence a |
|---|---|
| P | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-V-L-H-T-A-L-K-A-I-S-S-amide |
| V16K | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-K-L-H-T-A-L-K-A-I-S-S-amide |
| V16G | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-G-L-H-T-A-L-K-A-I-S-S-amide |
| V16S | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-S-L-H-T-A-L-K-A-I-S-S-amide |
| V16E | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-E-L-H-T-A-L-K-A-I-S-S-amide |
| V16A | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-A-L-H-T-A-L-K-A-I-S-S-amide |
| V16L | Ac-K-W-K-S-F-L-K-T-F-K-S-A-K-K-T-L-L-H-T-A-L-K-A-I-S-S-amide |
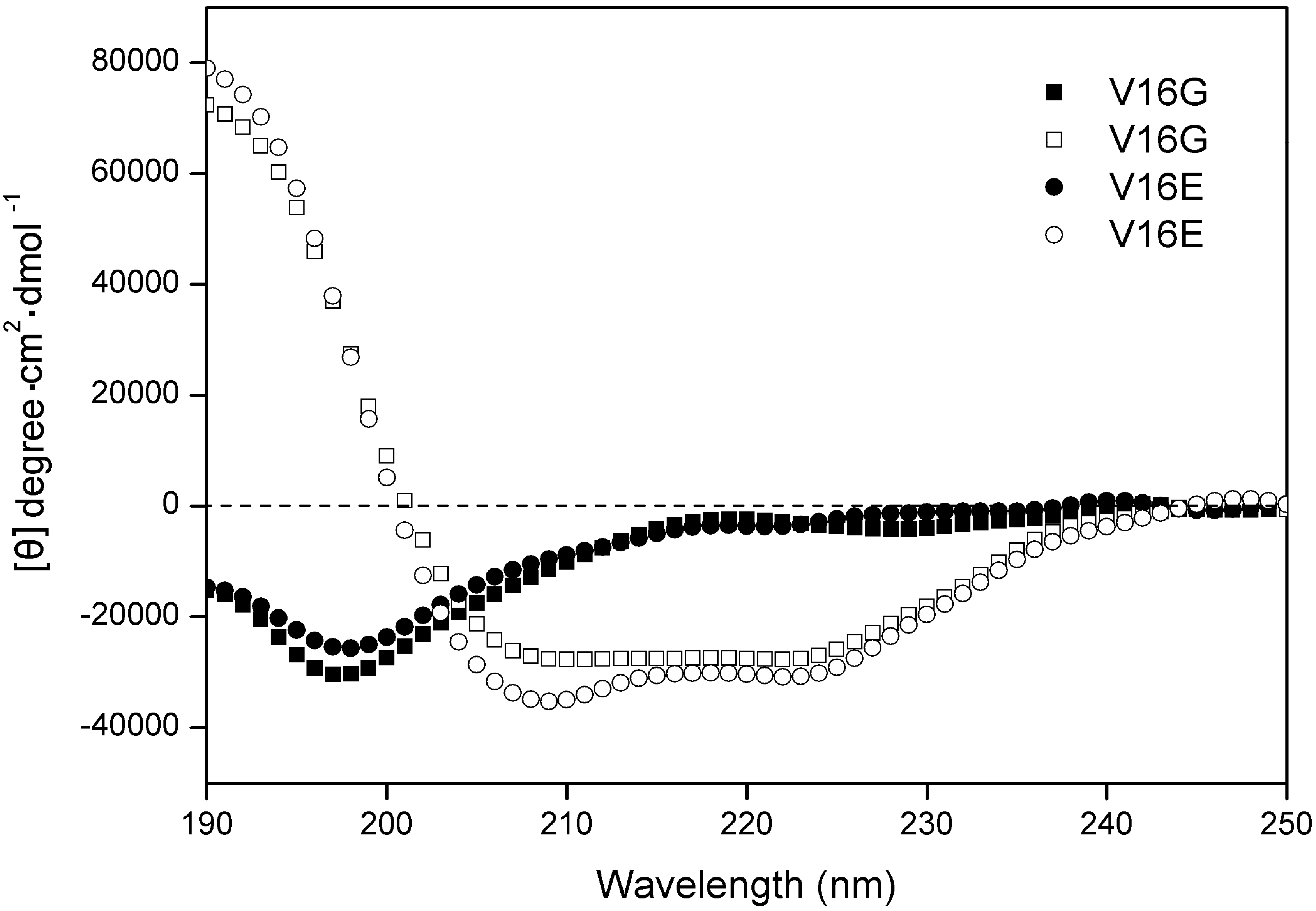
| Peptide a | tR(min) b | Benign c | 50%TFE d | ||
|---|---|---|---|---|---|
| [θ]222 | % helix e | [θ]222 | % helix e | ||
| V16K | 32.2 | −1,990 | 6.6 | −29,313 | 97.5 |
| V16G | 33.6 | −2,839 | 9.4 | −27,857 | 92.7 |
| V16S | 34.3 | −1,760 | 5.9 | −28,949 | 96.3 |
| V16E | 34.6 | −3,465 | 11.5 | −30,063 | 100.0 |
| V16A | 36.6 | −3,057 | 10.2 | −28,769 | 95.7 |
| P | 38.2 | −2,592 | 8.6 | −29,726 | 98.9 |
| V16L | 40.4 | −3,595 | 12.0 | −29,803 | 99.1 |
2.2. Biological Activities of Peptides Analogs
| Peptide a | MIC (μg/mL) | Hemolysis Percentage b | GM c | ||||
|---|---|---|---|---|---|---|---|
| E. coli ATCC25922 | E. coli DH5α | E. coli Clinical Isolate | P. aeruginosa ATCC27853 | P. aeruginosa H188 | |||
| V16K | 32 | 16 | 8 | 32 | 16 | 6.2 | 18.4 |
| V16G | 8 | 8 | 16 | 32 | 8 | 8.1 | 12.1 |
| V16S | 8 | 8 | 16 | 32 | 4 | 11.3 | 10.6 |
| V16E | 16 | 64 | 125 | 125 | 64 | 3.9 | 63.4 |
| V16A | 2 | 4 | 1 | 4 | 4 | 14.3 | 2.6 |
| P | 1 | 2 | 1 | 4 | 4 | 28.3 | 2.0 |
| V16L | 1 | 4 | 1 | 2 | 2 | 53.9 | 1.7 |
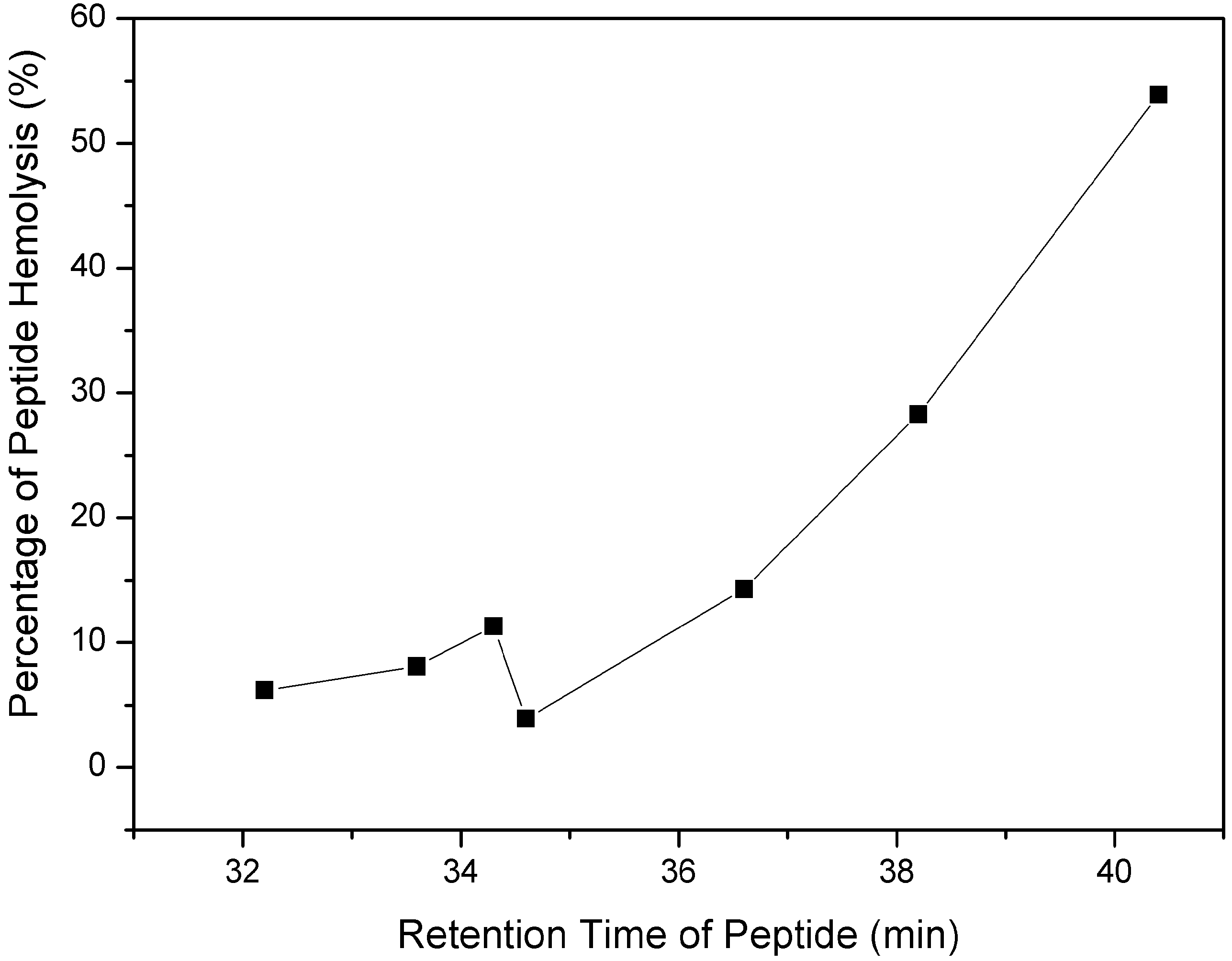
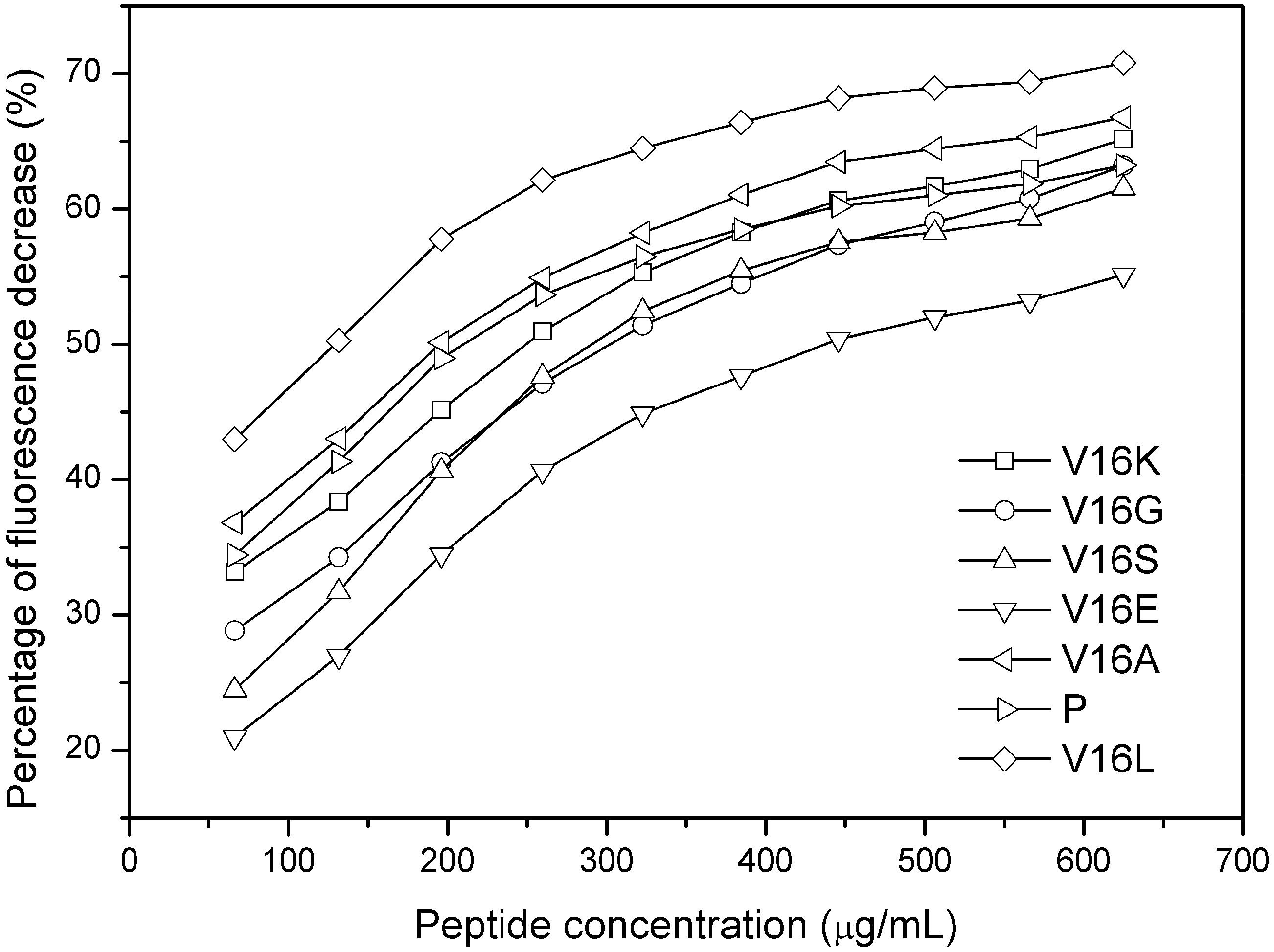
2.3. Outer Membrane Permeabilization and LPS Binding Affinity

2.4. Membrane Binding
| Peptide | HEPES buffer | PC/PG (7:3, w/w) a | PC/cholesterol (8:1,w/w) |
|---|---|---|---|
| nm | |||
| V16K | 350 | 329 (21 b) | 349 (1) |
| V16G | 350 | 329 (21) | 349 (1) |
| V16S | 350 | 328 (22) | 349 (1) |
| V16E | 350 | 333 (17) | 349 (1) |
| V16A | 350 | 328 (22) | 349 (1) |
| P | 350 | 328 (22) | 349 (1) |
| V16L | 350 | 326 (23) | 348 (2) |
3. Experimental Section
3.1. Materials
3.2. Peptide Synthesis and Purification
3.3. Analytical RP-HPLC of Peptides
3.4. CD Spectroscopy
3.5. Measurement of Antibacterial Activity
3.6. Measurement of Hemolytic Activity
3.7. Permeabilization of Bacterial Outer Membranes
3.8. Dansyl-Polymyxin B Displacement Assay
3.9. Preparation of Lipid Vesicles
3.10. Fluorescence Spectroscopy
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Gera, L.; Vasil, M.L.; Hodges, R.S. Rational design of α-helical antimicrobial peptides to target gram-negative pathogens, acinetobacter baumannii and pseudomonas aeruginosa: Utilization of charge, “specificity determinants,” total hydrophobicity, hydrophobe type and location as design parameters to improve the therapeutic ratio. Chem. Biol. Drug Des. 2011, 77, 225–240. [Google Scholar] [CrossRef]
- Oyston, P.C.; Fox, M.A.; Richards, S.J.; Clark, G.C. Novel peptide therapeutics for treatment of infections. J. Med. Microbiol. 2009, 58, 977–987. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Huang, H.W. Action of antimicrobial peptides: Two-state model. Biochemistry 2000, 39, 8347–8352. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. α-Helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Ehrenstein, G.; Lecar, H. Electrically gated ionic channels in lipid bilayers. Quar. Rev. Biophys. 1977, 10, 1–34. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim.Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Nicoletto, S.F. Antimicrobial peptides: An overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Tytler, E.M.; Anantharamaiah, G.M.; Walker, D.E.; Mishra, V.K.; Palgunachari, M.N.; Segrest, J.P. Molecular basis for prokaryotic specificity of magainin-induced lysis. Biochemistry 1995, 34, 4393–4401. [Google Scholar]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim.Biophys. Acta 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, M.H.; Hossain, M.A.; Shin, S.Y.; Kim, Y.; Stella, L.; Wade, J.D.; Park, Y.; Hahm, K.S. Amphipathic α-helical peptide, HP (2-20), and its analogues derived from helicobacter pylori: Pore formation mechanism in various lipid compositions. Biochim.Biophys. Acta 2008, 1778, 229–241. [Google Scholar] [CrossRef]
- Ahmad, A.; Yadav, S.P.; Asthana, N.; Mitra, K.; Srivastava, S.P.; Ghosh, J.K. Utilization of an amphipathic leucine zipper sequence to design antibacterial peptides with simultaneous modulation of toxic activity against human red blood cells. J. Biol. Chem. 2006, 281, 22029–22038. [Google Scholar] [CrossRef]
- Huang, J.-F.; Xu, Y.-M.; Hao, D.-M.; Huang, Y.-B.; Liu, Y.; Chen, Y. Structure-guided de novo design of α-helical antimicrobial peptide with enhanced specificity. Pure Appl. Chem. 2010, 82, 243–257. [Google Scholar]
- Kovacs, J.M.; Mant, C.T.; Hodges, R.S. Determination of intrinsic hydrophilicity/hydrophobicity of amino acid side chains in peptides in the absence of nearest-neighbor or conformational effects. Biopolymers 2006, 84, 283–297. [Google Scholar] [CrossRef]
- Zhou, N.E.; Monera, D.M.; Kay, C.M.; Hodges, R.S. α-Helical propensity of amino acids in the hydrophobic face of an amphipathic α-helix. Prot. Pept. Lett. 1994, 1, 114–119. [Google Scholar]
- Scholtz, J.M.; Qian, H.; Robbins, V.H.; Baldwin, R.L. The energetics of ion-pair and hydrogen-bonding interactions in a helical peptide. Biochemistry 1993, 32, 9668–9676. [Google Scholar] [CrossRef]
- Monera, O.D.; Sereda, T.J.; Zhou, N.E.; Kay, C.M.; Hodges, R.S. Relationship of sidechain hydrophobicity andα-helical propensity on the stability of the single-stranded amphipathicα-helix. J. Pept. Sci. 1995, 1, 319–329. [Google Scholar] [CrossRef]
- Hancock, R.E.; Farmer, S.W.; Li, Z.S.; Poole, K. Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and ompf porin of escherichia coli. Antimicrob. Agents Chemother. 1991, 35, 1309–1314. [Google Scholar] [CrossRef]
- Dewan, P.C.; Anantharaman, A.; Chauhan, V.S.; Sahal, D. Antimicrobial action of prototypic amphipathic cationic decapeptides and their branched dimers. Biochemistry 2009, 48, 5642–5657. [Google Scholar] [CrossRef]
- Nishida, M.; Imura, Y.; Yamamoto, M.; Kobayashi, S.; Yano, Y.; Matsuzaki, K. Interaction of a magainin-pgla hybrid peptide with membranes: Insight into the mechanism of synergism. Biochemistry 2007, 46, 14284–14290. [Google Scholar] [CrossRef]
- Moore, R.A.; Bates, N.C.; Hancock, R.E. Interaction of polycationic antibiotics with pseudomonas aeruginosa lipopolysaccharide and lipid a studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 1986, 29, 496–500. [Google Scholar] [CrossRef]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef]
- Lee, D.L.; Mant, C.T.; Hodges, R.S. A novel method to measure self-association of small amphipathic molecules: Temperature profiling in reversed-phase chromatography. J. Biol. Chem. 2003, 278, 22918–22927. [Google Scholar]
- Stark, M.; Liu, L.P.; Deber, C.M. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob. Agents Chemother. 2002, 46, 3585–3590. [Google Scholar] [CrossRef]
- Sedgwick, E.G.; Bragg, P.D. Distinct phases of the fluorescence response of the lipophilic probe n-phenyl-1-naphthylamine in intact cells and membrane vesicles of escherichia coli. Biochim.Biophys. Acta 1987, 894, 499–506. [Google Scholar] [CrossRef]
- Schindler, P.R.G.; Teuber, M. Action of polymyxin b on bacterial membranes: Morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli b. Antimicrob. Agents Chemother. 1975, 8, 94–104. [Google Scholar]
- Zhang, L.; Rozek, A.; Hancock, R.E. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 2001, 276, 35714–35722. [Google Scholar] [CrossRef]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, J.; Huang, J.; Huang, Y.; Chen, Y. Effects of Single Amino Acid Substitution on the Biophysical Properties and Biological Activities of an Amphipathic α-Helical Antibacterial Peptide Against Gram-Negative Bacteria. Molecules 2014, 19, 10803-10817. https://doi.org/10.3390/molecules190810803
Tan J, Huang J, Huang Y, Chen Y. Effects of Single Amino Acid Substitution on the Biophysical Properties and Biological Activities of an Amphipathic α-Helical Antibacterial Peptide Against Gram-Negative Bacteria. Molecules. 2014; 19(8):10803-10817. https://doi.org/10.3390/molecules190810803
Chicago/Turabian StyleTan, Juanjuan, Jinfeng Huang, Yibing Huang, and Yuxin Chen. 2014. "Effects of Single Amino Acid Substitution on the Biophysical Properties and Biological Activities of an Amphipathic α-Helical Antibacterial Peptide Against Gram-Negative Bacteria" Molecules 19, no. 8: 10803-10817. https://doi.org/10.3390/molecules190810803





