Hypericum japonicum Thunb. ex Murray: Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics of an Important Herbal Medicine
Abstract
:1. Introduction
2. Phytochemistry
| No. | Compounds | Classes | References | |
|---|---|---|---|---|
| 1 | Quercetin | Flavonoid | [6] | |
| 2 | Quercitrin | Flavonoid | [6] | |
| 3 | Isoquercitrin | Flavonoid | [7] | |
| 4 | Quercetin-7-O-α-l-rhamnoside | Flavonoid | [7] | |
| 5 | Quercetin-3-O-α-l-rhamnosyl(1→2)-O-α-l-rhamnoside | Flavonoid | [7] | |
| 6 | Rutin | Flavonoid | [8] | |
| 7 | Kaempferol | Flavonoid | [7] | |
| 8 | Kaempferol-7-O-α-l-rhamnoside | Flavonoid | [7] | |
| 9 | 5,7,3',4'-Tetrahydroxy-3-methoxyflavone | Flavonoid | [9] | |
| 10 | Taxifolin-3,7-O-α-l-dirhamnoside | Flavonoid | [10] | |
| 11 | Sarothranol | Flavonoid | [11] | |
| 12 | 7,8-(2'',2''-Dimethylpyrano)-5,3',4'-trihydroxy-3-methoxyflavone | Flavonoid | [7] | |
| 13 | 3,5,7,3',5'-Pentahydroxyflavonol | Flavonoid | [9] | |
| 14 | Dihydrokaempferol | Flavonoid | [12] | |
| 15 | (2R,3R)-Dihydroquercetin-3,7-O-α-l-dirhamnoside | Flavonoid | [7] | |
| 16 | (2R,3R)-Dihydroquercetin-7-O-α-l-rhamnoside | Flavonoid | [7] | |
| 17 | (2R,3R)-Dihydroquercetin | Flavonoid | [7] | |
| 18 | 2,3- Trans-dihydro-3,5,4'-trihydroxyflavonol-7-O-α-l-rhamnoside | Flavonoid | [7] | |
| 19 | 3,8''-Biapigenin | Flavonoid | [6] | |
| 20 | Japonicin A | Phloroglucinol | [13] | |
| 21 | Japonicin B | Phloroglucinol | [13] | |
| 22 | Japonicin C | Phloroglucinol | [13] | |
| 23 | Japonicin D | Phloroglucinol | [13] | |
| 24 | Sarothralen A | Phloroglucinol | [14] | |
| 25 | Sarothralen B | Phloroglucinol | [14] | |
| 26 | Sarothralen C | Phloroglucinol | [15] | |
| 27 | Sarothralen D | Phloroglucinol | [15] | |
| 28 | Saroaspidin A | Phloroglucinol | [16] | |
| 29 | Sarothralin G | Phloroglucinol | [17] | |
| 30 | Sarothralin | Phloroglucinol | [18] | |
| 31 | 4,6-Dimethyl-1-O-[α-l-rhamnosyl(1→6)-β-d-glucosyl] multifidol | Phloroglucinol | [19] | |
| 32 | 1,5,6-Trihydroxyxanthone | Xanthone | [7] | |
| 33 | 1,3,5,6-Tetrahydroxy-4-prenylxanthone | Xanthone | [7] | |
| 34 | 1,5-Dihydroxyxanthone-6-O-β-d-glucoside | Xanthone | [7] | |
| 35 | 1,3,5,6-Tetrahydroxyxanthonin | Xanthone | [20] | |
| 36 | 1,3,6,7-Tetrahydroxyxanthonin | Xanthone | [20] | |
| 37 | 1,3,5-Trihydroxyxanthone | Xanthone | [20] | |
| 38 | Isojacareubin | Xanthone | [7] | |
| 39 | Deoxyisojacareubin | Xanthone | [7] | |
| 40 | 4',5'-Dihydro-1,5,6-trihydroxy-4',4',5'-trimethylfurano(2'3':4,5) xanthone | Xanthone | [7] | |
| 41 | Bijaponicaxanthone | Xanthone | [7] | |
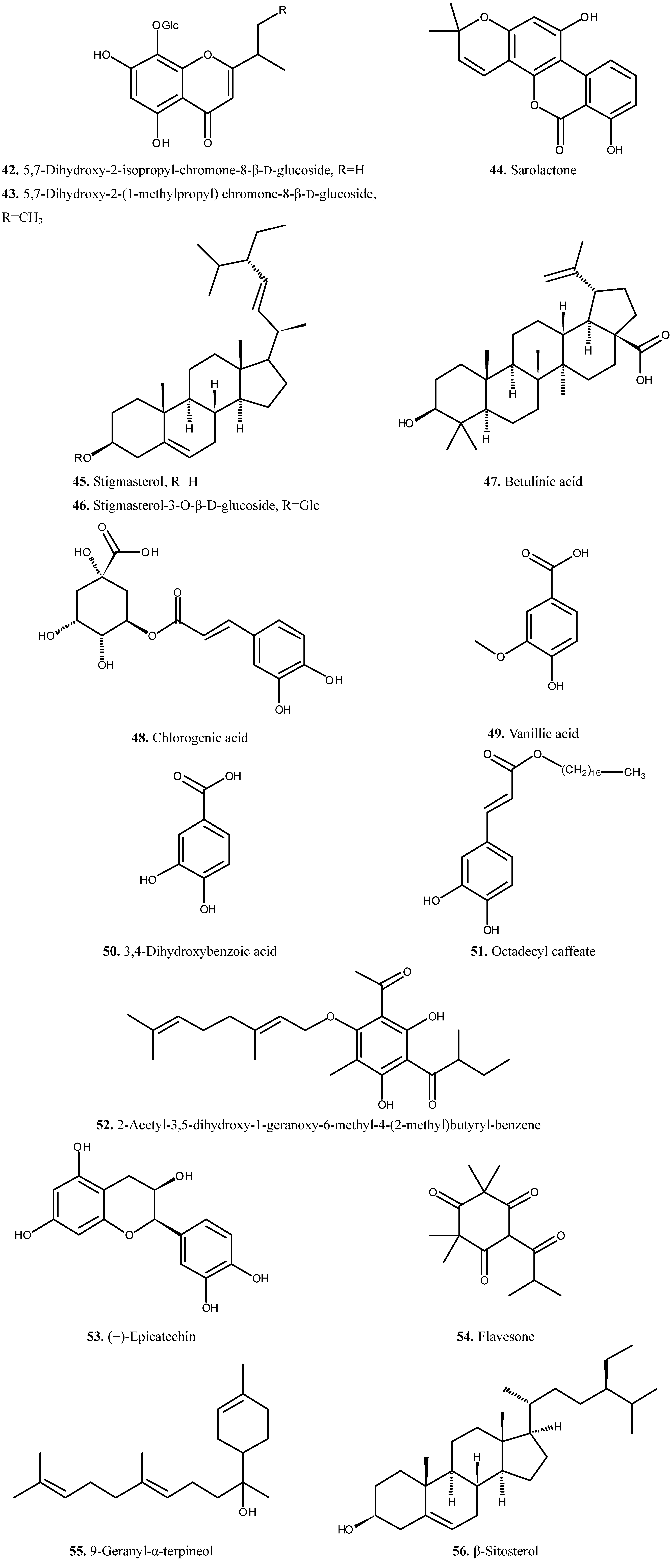
| 42 | 5,7-Dihydroxy-2-isopropyl-chromone-8-β-d-glucoside | Chromone | [7] | |
| 43 | 5,7-Dihydroxy-2-(1-methylpropyl) chromone-8-β-d-glucoside | Chromone | [7] | |
| 44 | Sarolactone | Chromone | [21] | |
| 45 | Stigmasterol | Triterpene | [6] | |
| 46 | Stigmasterol-3-O-β-d-glucoside | Triterpene | [6] | |
| 47 | Betulinic acid | Triterpene | [6] | |
| 48 | Chlorogenic acid | Phenolic acid | [12] | |
| 49 | Vanillic acid | Phenolic acid | [22] | |
| 50 | 3,4-Dihydroxybenzoic acid | Phenolic acid | [23] | |
| 51 | Octadecyl caffeate | Phenol | [23] | |
| 52 | 2-Acetyl-3,5-dihydroxy-1-geranoxy-6-methyl-4-(2-methyl)butyryl-benzene | Phenol | [24] | |
| 53 | (−)-Epicatechin | Phenol | [23] | |
| 54 | Flavesone | Ketone | [24] | |
| 55 | 9-Geranyl-α-terpineol | Alcohol | [24] | |
| 56 | β-Sitosterol | Sterol | [23] | |
2.1. Flavonoids

2.2. Phloroglucinols



2.3. Xanthones
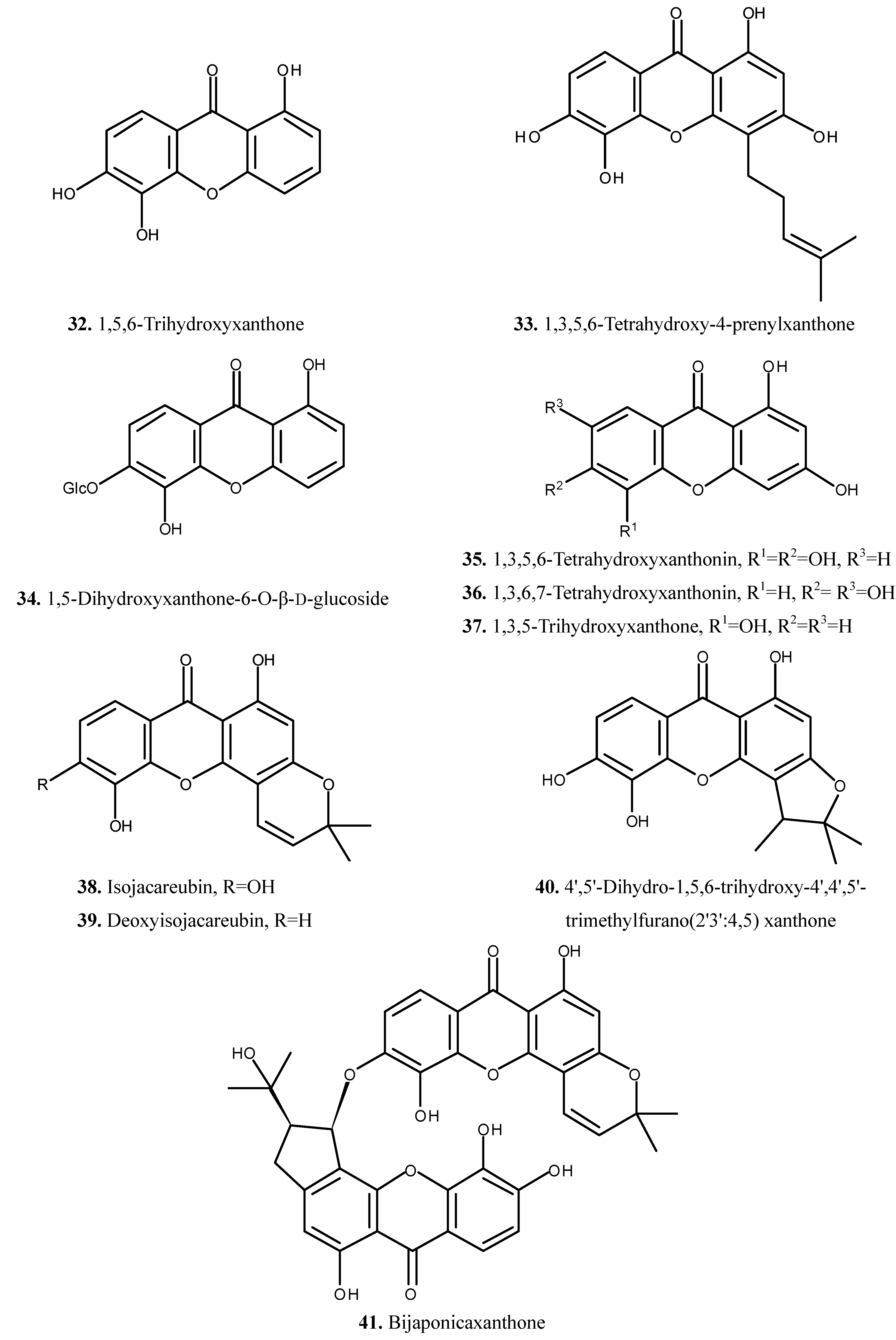
2.4. Other Compounds
| Roots | Aerial Parts | ||
|---|---|---|---|
| Compound | Relative Percentage | Compound | Relative Percentage |
| Dodecyl acetate | 20.59% | Undecane | 19.25% |
| Decyl dichlorocetate | 13.09% | Dodecyl acetate | 16.86% |
| 3-Methyl oxirane-2-methanol | 9.37% | (E)-β-Farnesene | 10.84% |
| Capraldehyde | 8.41% | β-Curcumin | 10.32% |
| β-Caryophyllene | 8.13% | Tetradecanol | 6.54% |
| (E)-β-Farnesene | 5.74% | 2,6-Bimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene | 6.15% |
| Nonane | 5.18% | ||
2.5. Volatile Oil
2.6. Metallic Elements
3. Pharmacology
3.1. Antioxidant Activity
3.2. Hepatoprotective Activity
3.3. Anti-Cancerous Activity
3.4. Antibacterial Activity
3.6. Effects on Cardiovascular System
3.7. Effects on Immunity
4. Quality Control
| Analytes | Extraction Methods | Columns | Mobile Phase | Analytical Time | Detections | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isojacareubin | Refluxing extraction with 75% (v/v) methanol aqueous solution | C18 (Diamonsil, 4.6 mm × 200 mm, 5 μm) | Acetonitrile-methanol-water-phosphoric acid (45:15:50:0.05); flow rate: 1.0 mL/min | 40 min | UV 254 nm | [49] | ||||
| Isojacareubin | Ultrasonic extraction with 80% (v/v) ethanol aqueous solution | C18 (Hypersil, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.04% phosphoric acid (47:53); flow rate: 1.0 mL/min | 28 min | UV 254 nm | [50] | ||||
| Quercetin-7-O-α- l-rhamnoside | Refluxing extraction with 60% (v/v) ethanol aqueous solution | C18 (Hypersil, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.04% phosphoric acid (77:23); flow rate: 1.0 mL/min | 20 min | UV 371 nm | [51] | ||||
| Quercetin | Refluxing extraction with methanol and 25% hydrochloric acid aqueous solution (3:1) | C18 (Diamonsil, 4.6 mm × 250 mm, 5 μm) | Methanol-0.06% phosphoric acid (52:48); flow rate: 1.0 mL/min | 20 min | UV 360 nm | [52] | ||||
| Quercitrin | Ultrasonic extraction with ethanol | C18 (Agilent, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.05 mol/L potassium dihydrogenphosphate (19:81); flow rate: 1.0 mL/min | 40 min | UV 256 nm | [53] | ||||
| Quercetin, quercitrin and isoquercitrin | Ultrasonic extraction with 80% (v/v) methanol aqueous solution | C18 (Hypersil, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.02 mol/L potassium dihydrogenphosphate (14:86) with gradient elution; flow rate: 1.0 mL/min | 45 min | UV 360 nm | [54] | ||||
| Quercetin, rutin and isorhamnetin | Soxhlet extraction with methanol | BDS-C18 (Agilent, 4.6 mm × 250 mm, 5 μm) | Methanol-0.2% phosphoric acid (52:48); flow rate: 1.0 mL/min | 16 min | UV 260 nm | [8] | ||||
| Quercetin, rutin and isorhamnetin | Ultrasonic extraction with 60% (v/v) ethanol aqueous solution | C18 (Agilent, 4.6 mm × 250 mm, 5 μm) | Methanol-0.2% phosphoric acid (54:46); flow rate: 1.0 mL/min | 15 min | UV 261 nm | [55] | ||||
| Quercetin, quercitrin, isoquercitrin and quercetin-7-O-α-l-rhamnoside | Refluxing extraction with 60% (v/v) ethanol aqueous solution | SB-C18 (Agilent ZORBAX, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.5% acetic acid (12:88) with gradient elution; flow rate: 1.0 mL/min | 45 min | UV 360 nm | [56] | ||||
| Quercetin, quercitrin, isoquercitrin and quercetin-7-O-α-l-rhamnoside | Refluxing extraction with water | HC C18 (Agilent, 4.0 mm × 250 mm, 5 μm) | Methanol-2.5% acetic acid (36:64); flow rate: 1.0 mL/min | 50 min | UV 255 nm | [57] | ||||
| Quercetin, quercitrin, isoquercitrin, taxifolin-7-O-α-l-rhamnoside and kaempferol | Ultrasonic extraction with 50% (v/v) methanol aqueous solution | C18 (Luna, 4.6 mm × 250 mm, 5 μm) | Methanol-0.5% acetic acid (54:46); flow rate: 1.0 mL/min | 50 min | UV 350 nm | [58] | ||||
| Quercetin, quercitrin, isoquercitrin, quercetin-7-O-α-l-rhamnoside and taxfolin-7-O-α-l-rhamnoside | Ultrasonic extraction with 70% (v/v) methanol aqueous solution | SB-C18 (Agilent ZORBAX, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.5% formic acid (12:88) with gradient elution; flow rate: 1.0 mL/min | 70 min | UV 256 nm and MS | [59] | ||||
| Quercetin, quercitrin, isoquercitrin, rutin, kaempferol and quercetin-3-O-galactoside | Refluxing extraction with 80% (v/v) methanol aqueous solution | C18 (Alltima, 4.6 mm × 250 mm, 5 μm) | Acetonitrile-0.8% acetic acid (11:89) with gradient elution; flow rate: 0.8 mL/min | 70 min | UV 254 nm and MS | [60] | ||||
| Quercetin, quercitrin, isoquercitrin, quercetin-7-O-α-rhamnoside, 3,4-dihydroxybenzoic acid, taxifolin-7-O-α-l-rhamnoside, 5,7-dihydroxy-2-isopropyl and chormone-8-β-D-glucoside | Ultrasonic extraction with 70% (v/v) methanol aqueous solution | XB-C18 (Ultimate, 4.6 mm × 250 mm, 5 μm) | Methanol-water (5:95) with gradient elution; flow rate: 1.0 mL/min | 100 min | UV 254 nm and MS | [61] | ||||
5. Pharmacokinetics
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robson, N.K.B. Hypericum botany. In Hypericum: The Genus Hypericum; Emst, E., Ed.; Taylor and Francis: New York, NY, USA, 2003; pp. 1–22. [Google Scholar]
- Editorial board of Flora of China; The Chinese Academy of Sciences. Flora of China, 2nd ed.; Science Press: Beijing, China, 1990; Volume 50, p. 47. [Google Scholar]
- Guangdong Food and Drug Administration. Guangdong Food Standardization of Chinese Medicinal Materials; Guangdong Chemical Industry Press: Guangdong, China, 2004; pp. 73–75. [Google Scholar]
- Nanjing University of Chinese Medicine. Dictionary of Chinese Traditional Medicine (Zhong. Yao Da. Ci. Dian), 2nd ed.; Shanghai Scientific & Technical Publishers: Shanghai, China, 2006; pp. 909–911. [Google Scholar]
- Group of Compilation of Chinese Herbal Medicine. Compilation of Chinese Herbal Medicine in China; People’s Medical Publishing House: Beijing, China, 1996; p. 4. [Google Scholar]
- Zhang, L.; Jin, Y.Y.; Tian, J.K. Studies on chemical constituents of Hypericum japonicum. Chin. Pharm. J. 2007, 42, 341–344. [Google Scholar]
- Wu, Q.L.; Wang, S.P.; Du, L.J.; Zhang, S.M.; Yang, J.S.; Xiao, P.G. Chromone glycosides and flavonoids from Hypericum japonicum. Phytochemistry 1998, 49, 1417–1420. [Google Scholar] [CrossRef]
- Xi, Z.H.; Yu, J.B.; Lv, W.Q.; Li, J.; Li, C.T. Determination of flavonoids in different parts and different harvesting seasons of Hypericum japonicum. China Pharm. 2009, 20, 1635–1637. [Google Scholar]
- Fu, P.; Li, T.Z.; Liu, R.H.; Zhang, W.; Zhang, C.; Zhang, W.D.; Chen, H.S. Studies on the flavonoids of Hypericum japonicum Thunb. ex Murray. Chin. J. Nat. Med. 2004, 2, 283–284. [Google Scholar]
- Wu, Q.L.; Wang, S.P.; Liao, Y.H.; Wang, L.W.; Feng, Y.X.; Yang, J.S.; Xiao, P.G. New constituents from Hypericum japonicum. Chin. Chem. Lett. 1996, 7, 1011–1012. [Google Scholar]
- Ishiguro, K.; Nagata, S.; Fukumoto, H.; Yamaki, M.; Isoi, K.; Oyama, Y. A flavanonol rhamnoside from Hypericum japonicum. Phytochemistry 1991, 30, 3152–3153. [Google Scholar] [CrossRef]
- Wang, X.W.; Mao, Y.; Fan, M.; Ding, A.S.; Wang, N.L.; Yao, X.S. Isolation, identification and activity determination on the anti-hypoxic components of Hypericum japonicum Thunb. J. Shenyang Pharm. Univ. 2009, 26, 701–703. [Google Scholar]
- Gu, G.M.; Feng, S.Z.; Wang, X.Y. Antimalarial constituents of Hypericum japonicum Thunb.: Isolation and structure of japonicins A, B, C and D. Acta Chim. Sin. 1988, 46, 246–251. [Google Scholar]
- Ishiguro, K.; Yamaki, M.; Kashihara, M.; Takagi, S. Sarothralen A and B, new antibiotic compounds from Hypericum japonicum. Planta Med. 1986, 4, 288–290. [Google Scholar]
- Ishiguro, K.; Nagata, S.; Fukumoto, H.; Yamaki, M.; Isoi, K. Phloroglucinol derivatives from Hypericum japonicum. Phytochemistry 1994, 35, 469–471. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yamaki, M.; Kashihara, M.; Takagi, S. Saroaspidin A, B, and C: Additional antibiotic compounds from Hypericum japonicu. Planta Med. 1987, 5, 415–417. [Google Scholar]
- Ishiguro, K.; Yamaki, M.; Kashihara, M.; Takagi, S.; Isoi, K. Sarothralin G: A new antimicrobial compound from Hypericum japonicum. Planta Med. 1990, 56, 274–276. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yamaki, M.; Takagi, S.; Yamagata, Y.; Tomita, K. X-ray crystal structure of sarothralin, a novel antibiotic compound from Hypericum japonicum. J. Chem. Soc. Chem. Commun. 1985, 1, 26–27. [Google Scholar]
- Wang, X.W.; Mao, Y.; Wang, N.L.; Yao, X.S. A new phloroglucinol diglycoside derivative from Hypericum japonicum Thunb. Molecules 2008, 13, 2796–2803. [Google Scholar] [CrossRef]
- Fu, P.; Li, T.Z.; Liu, R.H.; Zhang, W.; Zhang, W.D.; Chen, H.S. Xanthones from the whole plant of Hypericum japonicum. Nat. Product Res. Dev. 2004, 16, 51–513. [Google Scholar]
- Ishiguro, K.; Yamaki, M.; Kashihara, M.; Takagi, S.; Isoi, K. Studies on antimicrobial compounds from Hypericum japonicum. Part 4: A chromene from Hypericum japonicum. Phytochemistry 1990, 29, 1010–1011. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, W.D.; Li, T.Z.; Liu, R.H.; Zhang, W.; Chen, H.S. Study on chemical constituents of Hypericum japonicum Thunb. ex Murray. Acad. J. Sec. Mil. Med. Univ. 2004, 25, 1274–1275. [Google Scholar]
- Lv, J.; Kong, L.Y. Studies on the constituents of Hypericum japonicum Thunb. ex Murray. Mod. Chin. Med. 2007, 9, 12–14. [Google Scholar]
- Hu, L.H.; Khoo, C.W.; Vittal, J.J.; Sim, K.Y. Phloroglucinol derivatives from Hypericum japonicum. Phytochemistry 2000, 53, 705–709. [Google Scholar]
- Li, X.F.; Zhang, Z.Z.; Ouyang, Y.Z.; Xu, Y.W.; Sun, Y.L. Analyses on the chemical composition of the volatile oil from the Hypericum japonicum by GC-MS. Guangdong Chem. Ind. 2013, 40, 94–95. [Google Scholar]
- Wei, Z.L.; Rui, Y.K.; Tian, Z.H. Content of rare earth elements in wild Hypericum japonicum Thunb. Spectrosc. Spectr. Anal. 2009, 29, 1696–1697. [Google Scholar]
- Ouyang, Y.Z.; Wei, Y.; Wu, D.H.; Sun, Y.L. Determination of heavy metal in Hypericum japonicum Thunb by microwave digestion and flame atomic absorption spectrophotmetry (FAAS). Appl. Chem. Ind. 2012, 41, 1820–1822. [Google Scholar]
- Samaga, P.V.; Rai, V.R. Evaluation of pharmacological properties and phenolic profile of Hypericum japonicum Thunb. from Western Ghats of India. J. Pharm. Res. 2013, 7, 626–632. [Google Scholar] [CrossRef]
- Liang, S.; Su, W.W.; Wang, Y.G.; Peng, W.; Nie, Y.C.; Li, P.B. Effect of quercetin7-rhamnoside on glycochenodeoxycholic acid-inducedL-02 human normal liver cell apoptosis. Int. J. Mol. Med. 2013, 32, 323–330. [Google Scholar]
- Su, J.; Fu, P.; Zhang, W.D.; Liu, R.H.; Xu, X.K.; Zhang, C. Experimental study on extracts of Hypericum japonicum in liver-protective effect. J. Pharm. Pract. 2005, 23, 342–344. [Google Scholar]
- Wang, N.; Li, P.B.; Wang, Y.G.; Peng, W.; Wu, Z.; Tan, S.Y.; Liang, S.L.; Shen, X.; Su, W.W. Hepatoprotective effect of Hypericum japonicum extract and its fractions. J. Ethnopharmacol. 2008, 116, 1–6. [Google Scholar]
- Li, P.B.; Wang, Y.G.; Wu, D.H.; Wu, Z.; Su, W.W. Experimental study of three flavonoids isolated from Hypericum japonicum Thunb. on hepatoprotective and jaundice-relieving effects. J. Sun Yat.-Sen. Univ. (Med. Sci.) 2007, 28, 40–43. [Google Scholar]
- Li, P.B.; Yang, C.P.; Wang, Y.G.; Peng, W. Study on the effect of quercetin-7-β-d-rhamnosein against liver fibrosis in rats. J. Chin. Med. Mater. 2011, 34, 424–428. [Google Scholar]
- Jin, H.X.; Li, J.R. A study of the cytotoxic effects of Hypericum japonicum Thunb on human tongue cancer cell line TSCCa in vitro. J. Clin. Stomatol. 1997, 13, 19–20. [Google Scholar]
- Xiao, D.J.; Zhu, G.C.; Wang, X.L.; Zhang, Y.S.; Wu, S.H.; Yuan, Y. Effects of Hypericum japonicum Thunb on growth inhibition and apoptosis of human nasopharyngeal carcinoma cell line CNE-2 in vitro. Chin. J. Otorhinolaryngol.-Skull. Base Surg. 2007, 13, 337–339. [Google Scholar]
- Xiao, D.J.; Zhu, G.C.; Wang, Y.P.; Hu, Y.L. Inhibiting effect of Hypericum japonicum Thunb on human nasopharyngeal carcinoma line CNE-2 in vitro. Mod. Oncol. 2008, 16, 15–16. [Google Scholar]
- Lin, J.M.; Wang, R.G.; Chen, X.Z.; Zhao, J.Y. Effect of Hypericum japonicum on proliferation of human hepatoma cell line HepG2. Pharm. Clin. Chin. Mater. Med. 2007, 23, 136–137. [Google Scholar]
- Zhang, H.B.; Lu, P.; Cao, W.B.; Zhang, Z.H.; Meng, X.L. The effect-enhancing and toxicity-reducing activity of Hypericum japonicum Thunb. extract in murine liver cancer chemotherapy. Mol. Clin. Oncol. 2013, 1, 395–399. [Google Scholar]
- Pan, X.J.; Yang, K.; Zeng, J.Q.; Wei, Z.Y.; Chen, C. Experimental study on anti-hepatitis B and anti-hepatoma effect of different extracts in serum of Hypericum japonicum Thunb. in vitro. Lishizhen Med. Mater. Med. Res. 2009, 20, 1076–1078. [Google Scholar]
- Zuo, G.Y.; An, J.; Han, J.; Zhang, Y.L.; Wang, G.C.; Hao, X.Y.; Bian, Z.Q. Isojacareubin from the Chinese herb Hypericum japonicum: Potent antibacterial and synergistic effects on clinical methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2012, 13, 8210–8218. [Google Scholar] [CrossRef]
- Li, P.B.; Yang, C.P.; Wang, Y.G.; Peng, W. Study on the effects of the extract of Hypericum japonicum on duck hepatitis B viral. J. Chin. Med. Mater. 2011, 34, 956–958. [Google Scholar]
- Liu, N.; Hu, X.L.; Meng, Y.R.; Zhu, Y.T.; Huang, B.S.; Lin, P.Z. Effect of anti-influenza virus H3N2 of Hypericum japonicum in vivo. J. Chin. Med. Mater. 2008, 31, 1022–1024. [Google Scholar]
- Hu, X.Y.; Shu, X.C.; Ma, Y. Effect of aqueous extract of Hypericum japonicum on blood fat and hemorheology in restraint and hyperlipidemia rats. J. Chin. Med. Mater. 2011, 34, 1418–1420. [Google Scholar]
- Hu, X.Y.; Ma, Y.; Li, L.L. Effect of aqueous extract of Hypericum japonicum on the progress of atherosclerosis in hyperlipidemia rats. Asia-Pac. Trad. Med. 2011, 7, 5–6. [Google Scholar]
- Zhou, X.L.; Ke, M.Z.; Song, Z.J. Effect of Hypericum japonicum Thunb on the respiratory tract and systemic immune functions of rats. J. Guangxi Med. Univ. 2001, 18, 211–212. [Google Scholar]
- World Health Organization. WHO general guidelines for methodologies on research and evaluation of traditional medicine. Available online: http://whqlibdoc.who.int/hq/2000/WHO_EDM_ TRM_2000.1.pdf (accessed on 22 February 2010).
- Balasubramani, S.P.; Goraya, G.S.; Venkatasubramanian, P. Development of ITS sequence-based markers to distinguish Berberis. aristata DC. from B. lycium Royle and B. asiatica Roxb. 3 Biotech 2011, 1, 11–19. [Google Scholar]
- Peng, W.; Wu, D.H.; Yang, L.W.; Wang, Y.G.; Li, P.B.; Su, W.W. Quality study of Hypericum japonicum Thunb. (Tianjihuang). Cent. South. Pharm. 2006, 4, 340–342. [Google Scholar]
- Qin, S.Y.; Chen, X.H.; Mi, D.; Bi, K.S. RP-HPLC determination of isojacareubin in Hypericum japonicum Thunb. Chin. Pharm. Anal. 2007, 27, 718–720. [Google Scholar]
- Xiong, L.; Liang, J.; Chen, X.H.; Ma, A.L.; Shen, Z.D.; Bi, K.S. HPLC determination of isojacareubin in Hypericum japonicum. Chin. Prac. Med. 2008, 3, 8–9. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, X.H.; Cao, X.; Mao, Z.Y.; Bi, K.S. Content determination of quercetin-7-O-α-l-rhamnoside in Hypericum japonicum by HPLC. J. Shenyang Pharm. Univ. 2008, 25, 806–809. [Google Scholar]
- Wang, X.X.; Zhang, Y.P.; Feng, Y.Z.; Liang, G.Y. Content determination of quercetinin Hypericum japonicum by HPLC. J. Guiyang Coll. Trad. Chin. Med. 2009, 31, 18–20. [Google Scholar]
- Yu, J.P.; Chen, Y.; Zheng, J.B. Determination of quercitrin in Hypericum japonicum Thunb. by HPLC. Chin. J. Mod. Appl. Pharm. 2009, 26, 499–500. [Google Scholar]
- Xiong, L.; Chen, X.H.; Liang, J.; Bi, K.S. Simultaneous RP-HPLC determination of isoquercitrin, quercitrin and quercetin in Hypericum japonicum Thunb. Chin. Pharm. Anal. 2008, 28, 1619–1622. [Google Scholar]
- Teng, J.L.; Ou, L.Y.; Tian, J.T. Simultaneous determination of quercetin, rutoside and isorhamnetin in Hyericum japonicum Thunb. by HPLC. Cent. South. Pharm. 2010, 8, 41–44. [Google Scholar]
- Xiong, L.; Chen, X.H.; Liang, J. Simultaneous determination of contents of four flavonoids in Herba Hyperici Japonici by RP-HPLC. J. Shenyang Pharm. Univ. 2010, 27, 639–642. [Google Scholar]
- Peng, W.; Wang, Y.G.; Su, W.W. Simultaneous quatitation of four flavonoids from Hypericum japonicum Thunb by HPLC. J. Chin. Med. Mater. 2011, 34, 1229–1231. [Google Scholar]
- Li, J.; Jiang, B.; Liu, X.; Zhang, J.; Chen, X.H.; Bi, K.S. Simultaneous determination of five bioactive flavonoids in Hypericum japonicum Thunb by high-performance liquid chromatography. Asian J. Trad. Med. 2007, 2, 75–81. [Google Scholar]
- Su, J.; Fu, P.; Shen, Y.H.; Zhang, C.; Liang, M.J.; Liu, R.H.; Li, H.L.; Zhang, W.D. Simultaneous analysis of flavonoids from Hypericum japonicum Thunb. ex Murra (Hypericaceae) by HPLC-DAD-ESI/MS. J. Pharm. Biomed. Anal. 2008, 46, 342–348. [Google Scholar] [CrossRef]
- Lu, L.; Han, L.; Zou, L.S.; Liu, X.H. Analysis study on the flavonoids constituents in herba hyperici japonica by UPLC-MS. J. Nanjing Univ. Trad. Chin. Med. 2013, 29, 482–485. [Google Scholar]
- Gao, W.N.; Luo, J.G.; Kong, L.Y. Quality evaluation of Hpericum japomicum by using high-performance liquid chromatography coupled with photodiode array detector and electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2009, 23, 1022–1030. [Google Scholar] [CrossRef]
- Yang, L.W.; Wu, D.H.; Tang, X.; Peng, W.; Wang, X.R.; Ma, Y.; Su, W.W. Fingerprint quality control of Tianjihuang by high-performance liquid chromatography-photodiode array detection. J. Chromatogr. A 2005, 1070, 35–42. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Food and Drug Administration Guidance for Industry-Botanical Drug Products (Draft Guidance); US Food and Drug Administration: Rockville, MD, USA, 2000; p. 4. [Google Scholar]
- European Medicines Agency. Note for Guidance on Quality of Herbal Medicinal Products; European Medicines Agency: London, UK, 2001; p. 6. [Google Scholar]
- State Food Drug Administration of China. Technical Requirements for theDevelopment of Fingerprints of TCM Injections; State Food Drug Administration of China: Beijing, China, 2000. [Google Scholar]
- Han, L.; Liu, X.H.; Wang, L.J.; Fu, X.S. Study on HSGC-MS fingerprint of Hyperici Japonici Herba. Chin. Med. J. Res. Prac. 2011, 25, 22–24. [Google Scholar]
- Han, L.; Song, J.P.; Liu, X.H.; Li, J.S.; Cai, B.C.; Fu, X.S.; Lu, A.P. Studies on HPCE fingerprint of Herba Hyperici Japonici. Chin. J. Exp. Trad. Med. Form. 2011, 17, 104–107. [Google Scholar]
- Li, J.; Wang, Z.W.; Zhang, L.; Liu, X.; Chen, X.H.; Bi, K.S. HPLC analysis and pharmacokinetic study of quercitrin and isoquercitrin in rat plasma after administration of Hypericum japonicum thunb. extract. Biomed. Chromatogr. 2008, 22, 374–378. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, L.-S.; Liu, M.-H.; He, J.-Y. Hypericum japonicum Thunb. ex Murray: Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics of an Important Herbal Medicine. Molecules 2014, 19, 10733-10754. https://doi.org/10.3390/molecules190810733
Liu L-S, Liu M-H, He J-Y. Hypericum japonicum Thunb. ex Murray: Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics of an Important Herbal Medicine. Molecules. 2014; 19(8):10733-10754. https://doi.org/10.3390/molecules190810733
Chicago/Turabian StyleLiu, Lin-Sheng, Meng-Hua Liu, and Jing-Yu He. 2014. "Hypericum japonicum Thunb. ex Murray: Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics of an Important Herbal Medicine" Molecules 19, no. 8: 10733-10754. https://doi.org/10.3390/molecules190810733
APA StyleLiu, L.-S., Liu, M.-H., & He, J.-Y. (2014). Hypericum japonicum Thunb. ex Murray: Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics of an Important Herbal Medicine. Molecules, 19(8), 10733-10754. https://doi.org/10.3390/molecules190810733




