Evaluation of a Triple-Helical Peptide with Quenched Fluorophores for Optical Imaging of MMP-2 and MMP-9 Proteolytic Activity
Abstract
:1. Introduction
2. Results and Discussion
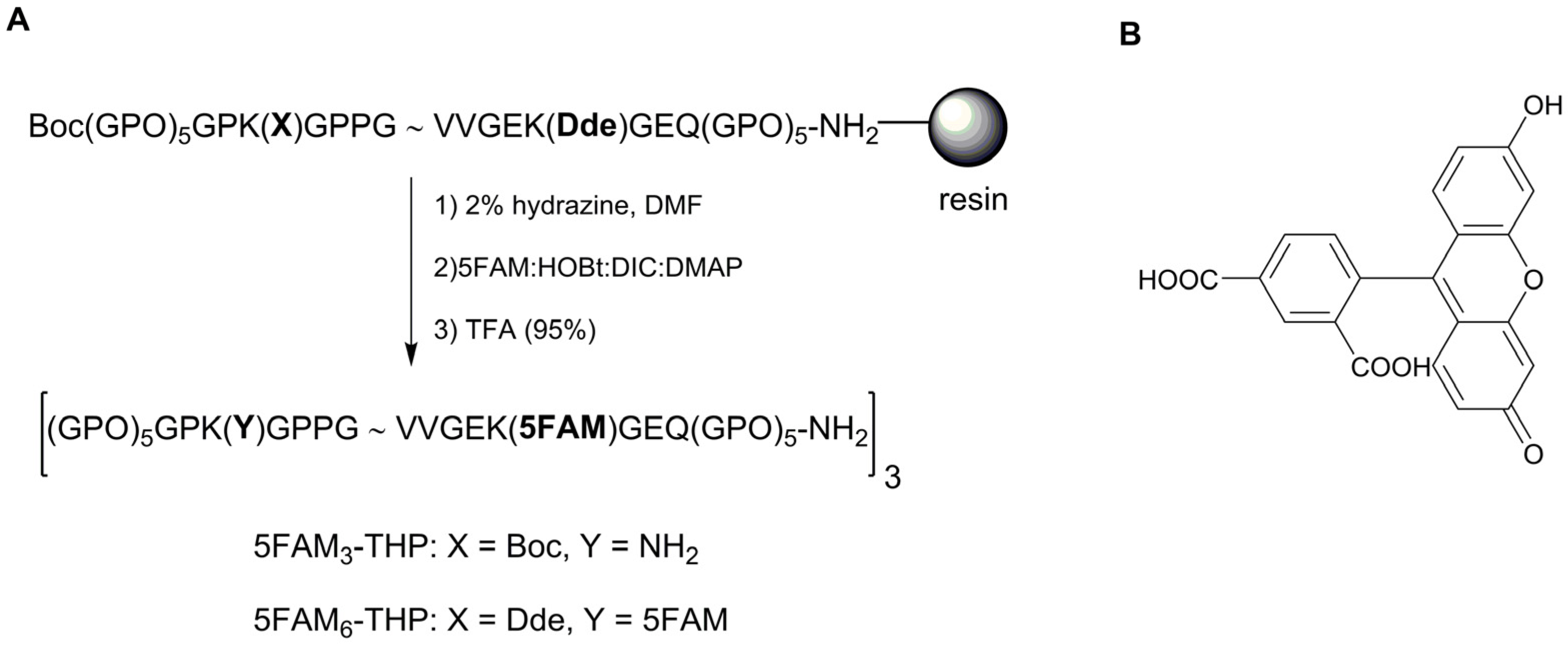
2.1. 5FAM-THP Design and Synthesis
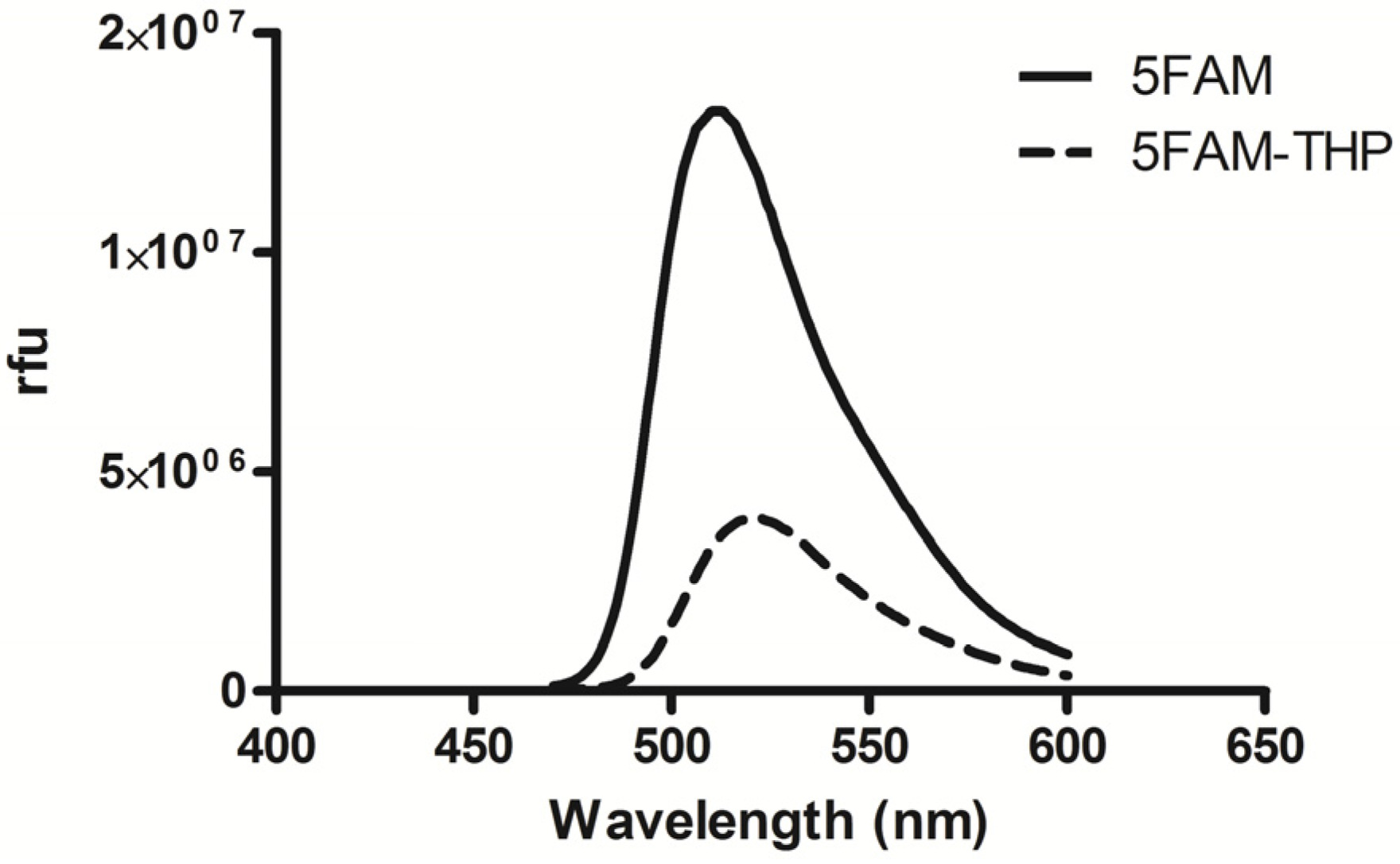
2.2. 5FAM-THP Quenching
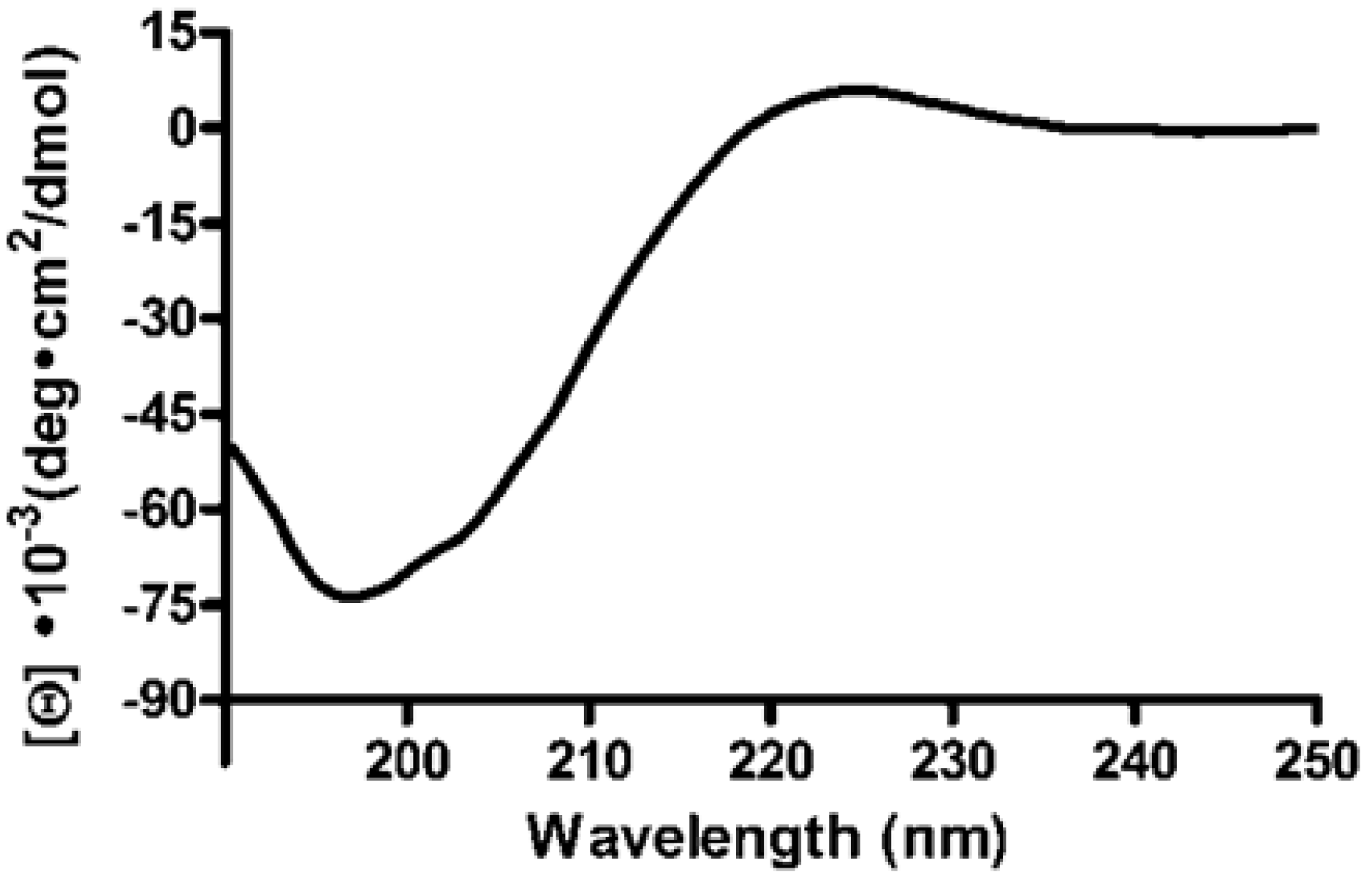
2.3. 5FAM-THP Kinetics
| Substrate | KM (μM) | kcat (s−1) | kcat/KM (M−1 s−1) | MMPs |
|---|---|---|---|---|
| fTHP a | 4.4 | 0.061 | 14,002 | MMP-2 |
| fTHP a | 8.1 | 0.044 | 5,400 | MMP-9 |
| 5FAM3-THP | 8.9 ± 0.7 b | 0.092 | 10,337 | MMP-2 |
| 5FAM3-THP | 10.2 ± 0.4 b | 0.047 | 4,628 | MMP-9 |
| 5FAM3-THP | 3.3 ± 0.3 b | 0.051 | 15,421 | MMP-2 (Rat) |
| 5FAM3-THP | 8.9 ± 3.3 b | 0.040 | 4,507 | MMP-9 (Rat) |
| 5FAM6-THP | 4.3 ± 0.3 b | 0.065 | 15,116 | MMP-2 |
| 5FAM6-THP | 6.6 ± 0.2 b | 0.036 | 5,400 | MMP-9 |


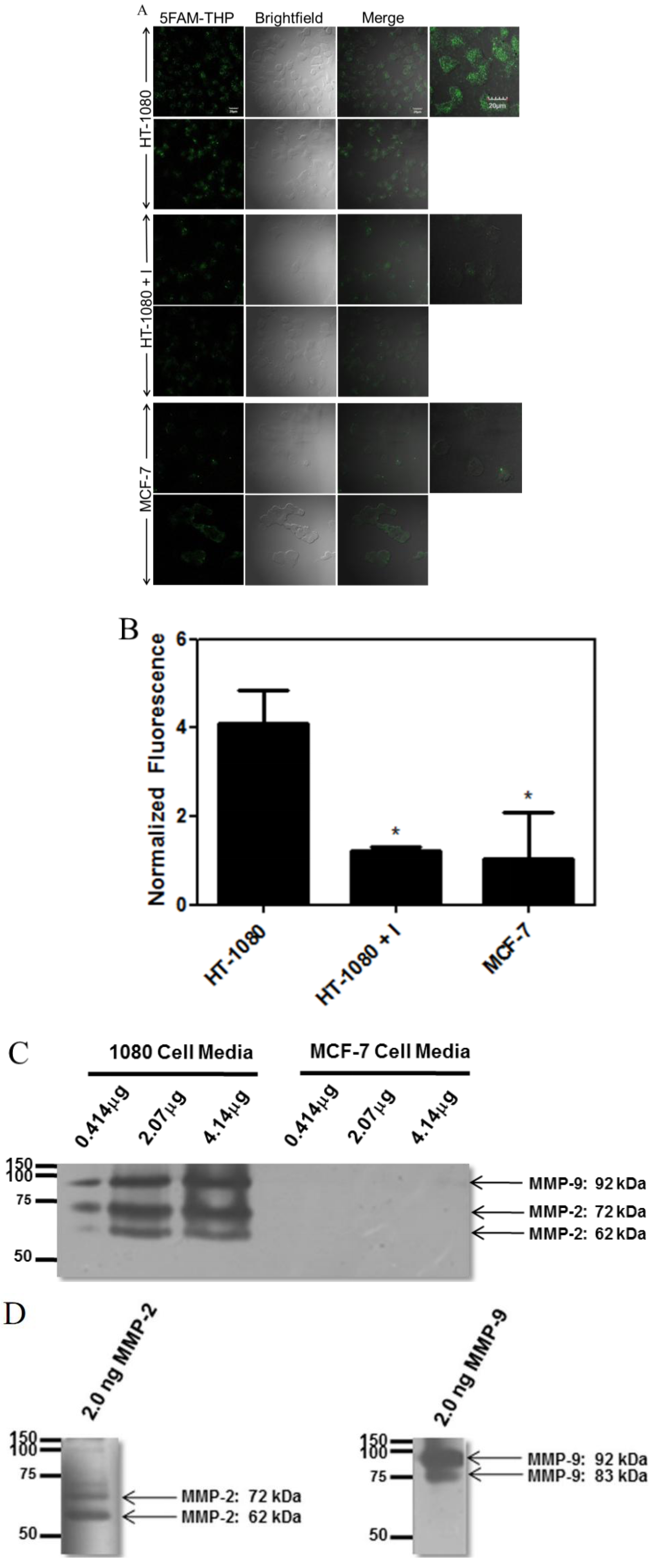
2.4. Microscopy of 5FAM-THP Hydrolysis and MMP Zymography
3. Experimental
3.1. Chemicals
3.2. Solid Phase Peptide Synthesis
3.3. THP Quenching
3.4. Calculation of Förster Distance
3.5. Enzyme Assays
3.6. Circular Dichroism Spectroscopy and Melting Curves
3.7. MMP Zymography
3.8. Confocal Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Cauwe, B.; van den Steen, P.E.; Opdenakker, G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–185. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: Rationale and therapeutic approaches. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 211–242. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Viappiani, S.; Nicolescu, A.C.; Holt, A.; Sawicki, G.; Crawford, B.D.; Leon, H.; van Mulligen, T.; Schulz, R. Activation and modulation of 72kda matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef]
- Pacheco, M.M.; Mourao, M.; Mantovani, E.B.; Nishimoto, I.N.; Brentani, M.M. Expression of gelatinases a and b, stromelysin-3 and matrilysin genes in breast carcinomas: Clinico-pathological correlations. Clin. Exp. Metastasis 1998, 16, 577–585. [Google Scholar] [CrossRef]
- Remacle, A.G.; Noel, A.; Duggan, C.; McDermott, E.; O’Higgins, N.; Foidart, J.M.; Duffy, M.J. Assay of matrix metalloproteinases types 1, 2, 3 and 9 in breast cancer. Br. J. Cancer 1998, 77, 926–931. [Google Scholar] [CrossRef]
- Baker, E.A.; Bergin, F.G.; Leaper, D.J. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br. J. Surg. 2000, 87, 1215–1221. [Google Scholar] [CrossRef]
- Baker, E.A.; Leaper, D.J. Measuring gelatinase activity in colorectal cancer. Eur. J. Surg. Oncol. 2002, 28, 24–29. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Troncoso, P.; Johnston, D.; Bucana, C.D.; Tahara, E.; Fidler, I.J.; Pettaway, C.A. Relative expression of type iv collagenase, e-cadherin, and vascular endothelial growth factor/vascular permeability factor in prostatectomy specimens distinguishes organ-confined from pathologically advanced prostate cancers. Clin. Cancer Res. 2000, 6, 2295–2308. [Google Scholar]
- Upadhyay, J.; Shekarriz, B.; Nemeth, J.A.; Dong, Z.; Cummings, G.D.; Fridman, R.; Sakr, W.; Grignon, D.J.; Cher, M.L. Membrane type 1-matrix metalloproteinase (mt1-mmp) and mmp-2 immunolocalization in human prostate: Change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin. Cancer Res. 1999, 5, 4105–4110. [Google Scholar]
- Nomura, H.; Fujimoto, N.; Seiki, M.; Mai, M.; Okada, Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase a) in human gastric carcinomas. Int. J. Cancer 1996, 69, 9–16. [Google Scholar] [CrossRef]
- Sier, C.F.; Kubben, F.J.; Ganesh, S.; Heerding, M.M.; Griffioen, G.; Hanemaaijer, R.; van Krieken, J.H.; Lamers, C.B.; Verspaget, H.W. Tissue levels of matrix metalloproteinases mmp-2 and mmp-9 are related to the overall survival of patients with gastric carcinoma. Br. J. Cancer 1996, 74, 413–417. [Google Scholar] [CrossRef]
- Lochter, A.; Sternlicht, M.D.; Werb, Z.; Bissell, M.J. The significance of matrix metalloproteinases during early stages of tumor progression. Ann. N. Y. Acad. Sci. 1998, 857, 180–193. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Wu, Q.; Ke, S.; Houston, J.; Sevick-Muraca, E.; Dong, L.; Chow, D.; Charnsangavej, C.; Gelovani, J.G. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl. Med. Biol. 2006, 33, 349–358. [Google Scholar] [CrossRef]
- Piao, D.; Xie, H.; Zhang, W.; Krasinski, J.S.; Zhang, G.; Dehghani, H.; Pogue, B.W. Endoscopic, rapid near-infrared optical tomography. Opt. Lett. 2006, 31, 2876–2878. [Google Scholar] [CrossRef]
- Rudin, M.; Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2003, 2, 123–131. [Google Scholar] [CrossRef]
- Weissleder, R. Scaling down imaging: Molecular mapping of cancer in mice. Nat Rev Cancer 2002, 2, 11–18. [Google Scholar] [CrossRef]
- Garbett, E.A.; Reed, M.W.; Brown, N.J. Proteolysis in human breast and colorectal cancer. Br. J. Cancer 1999, 81, 287–293. [Google Scholar] [CrossRef]
- Gonzalez, L.O.; Pidal, I.; Junquera, S.; Corte, M.D.; Vazquez, J.; Rodriguez, J.C.; Lamelas, M.L.; Merino, A.M.; Garcia-Muniz, J.L.; Vizoso, F.J. Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br. J. Cancer 2007, 97, 957–963. [Google Scholar]
- Iwata, H.; Kobayashi, S.; Iwase, H.; Masaoka, A.; Fujimoto, N.; Okada, Y. Production of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human breast carcinomas. Jpn. J. Cancer Res. 1996, 87, 602–611. [Google Scholar] [CrossRef]
- Littlepage, L.E.; Sternlicht, M.D.; Rougier, N.; Phillips, J.; Gallo, E.; Yu, Y.; Williams, K.; Brenot, A.; Gordon, J.I.; Werb, Z. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010, 70, 2224–2234. [Google Scholar] [CrossRef]
- Morgia, G.; Falsaperla, M.; Malaponte, G.; Madonia, M.; Indelicato, M.; Travali, S.; Mazzarino, M.C. Matrix metalloproteinases as diagnostic (mmp-13) and prognostic (mmp-2, mmp-9) markers of prostate cancer. Urol. Res. 2005, 33, 44–50. [Google Scholar] [CrossRef]
- Scherer, R.L.; McIntyre, J.O.; Matrisian, L.M. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008, 27, 679–690. [Google Scholar] [CrossRef]
- Aguilera, T.A.; Olson, E.S.; Timmers, M.M.; Jiang, T.; Tsien, R.Y. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr. Biol. (Camb) 2009, 1, 371–381. [Google Scholar] [CrossRef]
- Olson, E.S.; Aguilera, T.A.; Jiang, T.; Ellies, L.G.; Nguyen, Q.T.; Wong, E.H.; Gross, L.A.; Tsien, R.Y. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr. Biol. (Camb) 2009, 1, 382–393. [Google Scholar] [CrossRef]
- Van Duijnhoven, S.M.; Robillard, M.S.; Nicolay, K.; Grull, H. Tumor targeting of mmp-2/9 activatable cell-penetrating imaging probes is caused by tumor-independent activation. J. Nucl. Med. 2011, 52, 279–286. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Sritharan, T.; Stack, M.S.; Nagase, H.; Fields, G.B. Selective hydrolysis of triple-helical substrates by matrix metalloproteinase-2 and -9. J. Biol. Chem. 2003, 278, 18140–18145. [Google Scholar]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef]
- Akers, W.J.; Xu, B.; Lee, H.; Sudlow, G.P.; Fields, G.B.; Achilefu, S.; Edwards, W.B. Detection of MMP-2 and MMP-9 activity in vivo with a triple-helical peptide optical probe. Bioconjug. Chem. 2012, 23, 656–663. [Google Scholar] [CrossRef]
- Bremer, C.; Tung, C.H.; Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001, 7, 743–748. [Google Scholar] [CrossRef]
- Giambernardi, T.A.; Grant, A.M.; Taylor, G.P.; Hay, R.J.; Maher, V.M.; McCormick, J.; Klebe, R.J. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998, 16, 483–496. [Google Scholar] [CrossRef]
- Cheney, P.P.; Fields, G.B.; Achilefu, S.; Edwards, W.B. Characterization of quenched fluorescent triple helical peptides for mmp-2 and mmp-9 optical imaging. Proceed. SPIE 2009, 7190, 719011–719018. [Google Scholar]
- Fischer, R.; Mader, O.; Jung, G.; Brock, R. Extending the applicability of carboxyfluorescein in solid-phase synthesis. Bioconjug. Chem. 2003, 14, 653–660. [Google Scholar] [CrossRef]
- Hӧfle, G.; Steglich, W.; Vorbrüggen, H. 4-Dialkylaminopyridines as highly active acylation catalysts. [New synthetic method (25)]. Angew. Chem. Int. Ed. Engl. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Xu, S.; Held, I.; Kempf, B.; Mayr, H.; Steglich, W.; Zipse, H. The DMsAP-catalyzed acetylation of alcohols—a mechanistic study (DMAP=4-(dimethylamino)pyridine). Chemistrys 2005, 11, 4751–4757. [Google Scholar]
- Rainey, J.K.; Goh, M.C. A statistically derived parameterization for the collagen triple-helix. Protein Sci. 2002, 11, 2748–2754. [Google Scholar]
- Pham, W.; Choi, Y.; Weissleder, R.; Tung, C.H. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjug. Chem. 2004, 15, 1403–1407. [Google Scholar] [CrossRef]
- Packard, B.Z.; Toptygin, D.D.; Komoriya, A.; Brand, L. Profluorescent protease substrates: Intramolecular dimers described by the exciton model. Proc. Natl. Acad. Sci. USA 1996, 93, 11640–11645. [Google Scholar] [CrossRef]
- McIntyre, J.O.; Fingleton, B.; Wells, K.S.; Piston, D.W.; Lynch, C.C.; Gautam, S.; Matrisian, L.M. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem. J. 2004, 377, 617–628. [Google Scholar] [CrossRef]
- Mahmood, U.; Weissleder, R. Near-infrared optical imaging of proteases in cancer. Mol. Cancer Ther. 2003, 2, 489–496. [Google Scholar]
- Tung, C.H.; Mahmood, U.; Bredow, S.; Weissleder, R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000, 60, 4953–4958. [Google Scholar]
- Venugopal, M.G.; Ramshaw, J.A.; Braswell, E.; Zhu, D.; Brodsky, B. Electrostatic interactions in collagen-like triple-helical peptides. Biochemistry 1994, 33, 7948–7956. [Google Scholar] [CrossRef]
- Bremer, C.; Bredow, S.; Mahmood, U.; Weissleder, R.; Tung, C.H. Optical imaging of matrix metalloproteinase-2 activity in tumors: Feasibility study in a mouse model. Radiology 2001, 221, 523–529. [Google Scholar] [CrossRef]
- Faust, A.; Waschkau, B.; Waldeck, J.; Holtke, C.; Breyholz, H.J.; Wagner, S.; Kopka, K.; Heindel, W.; Schafers, M.; Bremer, C. Synthesis and evaluation of a novel fluorescent photoprobe for imaging matrix metalloproteinases. Bioconjug. Chem. 2008, 19, 1001–1008. [Google Scholar] [CrossRef]
- Oliver, G.W.; Leferson, J.D.; Stetler-Stevenson, W.G.; Kleiner, D.E. Quantitative reverse zymography: Analysis of picogram amounts of metalloproteinase inhibitors using gelatinase a and b reverse zymograms. Anal. Biochem. 1997, 244, 161–166. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal. Biochem. 1994, 218, 325–329. [Google Scholar] [CrossRef]
- Ikejiri, M.; Bernardo, M.M.; Bonfil, R.D.; Toth, M.; Chang, M.; Fridman, R.; Mobashery, S. Potent mechanism-based inhibitors for matrix metalloproteinases. J. Biol. Chem. 2005, 280, 33992–34002. [Google Scholar]
- Fields, C.G.; Lovdahl, C.M.; Miles, A.J.; Hagen, V.L.; Fields, G.B. Solid-phase synthesis and stability of triple-helical peptides incorporating native collagen sequences. Biopolymers 1993, 33, 1695–1707. [Google Scholar] [CrossRef]
- Brooks, P.C.; Stromblad, S.; Sanders, L.C.; von Schalscha, T.L.; Aimes, R.T.; Stetler-Stevenson, W.G.; Quigley, J.P.; Cheresh, D.A. Localization of matrix metalloproteinase mmp-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 1996, 85, 683–693. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Westphal, J.R.; van Kraats, A.A.; Ruiter, D.J.; van Muijen, G.N. Expression of integrin alpha(v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (mt1-mmp) and matrix metalloproteinase-2 (mmp-2) in human melanoma cells in vitro and in vivo. Int. J. Cancer 2000, 87, 12–19. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Westphal, J.R.; van Muijen, G.N.; Ruiter, D.J. Matrix metalloproteinases in human melanoma. J. Investig. Dermatol. 2000, 115, 337–344. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Westphal, J.R.; Waas, E.T.; Becker, J.C.; Ruiter, D.J.; van Muijen, G.N. Coexpression of integrin alpha(v)beta3 and matrix metalloproteinase-2 (mmp-2) coincides with mmp-2 activation: Correlation with melanoma progression. J. Investig. Dermatol. 2000, 115, 625–632. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Gunja-Smith, Z.; Iida, N.; Zhu, H.B.; Young, L.J.; Muller, W.J.; Cardiff, R.D. Cd44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (mmp-9) association in metastatic breast cancer cells. J. Cell Physiol. 1998, 176, 206–215. [Google Scholar] [CrossRef]
- Desai, B.; Rogers, M.J.; Chellaiah, M.A. Mechanisms of osteopontin and cd44 as metastatic principles in prostate cancer cells. Mol. Cancer 2007, 6, 18. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates tgf-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar]
- Yu, Q.; Stamenkovic, I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for cd44-mediated tumor invasion. Genes Dev. 1999, 13, 35–48. [Google Scholar] [CrossRef]
- Yu, Y.; Berdnt, P.; Tirrell, M.; Fields, G.B. Self assembling amphiphiles for construction of protein molecular architecture. J. Am. Chem. Soc. 1996, 118, 12515–12520. [Google Scholar] [CrossRef]
- Okada, Y.; Morodomi, T.; Enghild, J.J.; Suzuki, K.; Yasui, A.; Nakanishi, I.; Salvesen, G.; Nagase, H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur. J. Biochem. 1990, 194, 721–730. [Google Scholar] [CrossRef]
- Vempati, P.; Karagiannis, E.D.; Popel, A.S. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J. Biol. Chem. 2007, 282, 37585–37596. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, X.; Bresee, J.; Cheney, P.P.; Xu, B.; Bhowmick, M.; Cudic, M.; Fields, G.B.; Edwards, W.B. Evaluation of a Triple-Helical Peptide with Quenched Fluorophores for Optical Imaging of MMP-2 and MMP-9 Proteolytic Activity. Molecules 2014, 19, 8571-8588. https://doi.org/10.3390/molecules19068571
Zhang X, Bresee J, Cheney PP, Xu B, Bhowmick M, Cudic M, Fields GB, Edwards WB. Evaluation of a Triple-Helical Peptide with Quenched Fluorophores for Optical Imaging of MMP-2 and MMP-9 Proteolytic Activity. Molecules. 2014; 19(6):8571-8588. https://doi.org/10.3390/molecules19068571
Chicago/Turabian StyleZhang, Xuan, Jamee Bresee, Philip P. Cheney, Baogang Xu, Manishabrata Bhowmick, Mare Cudic, Gregg B. Fields, and Wilson Barry Edwards. 2014. "Evaluation of a Triple-Helical Peptide with Quenched Fluorophores for Optical Imaging of MMP-2 and MMP-9 Proteolytic Activity" Molecules 19, no. 6: 8571-8588. https://doi.org/10.3390/molecules19068571




