Antimicrobial Activity of Resveratrol Analogues
Abstract
:1. Introduction
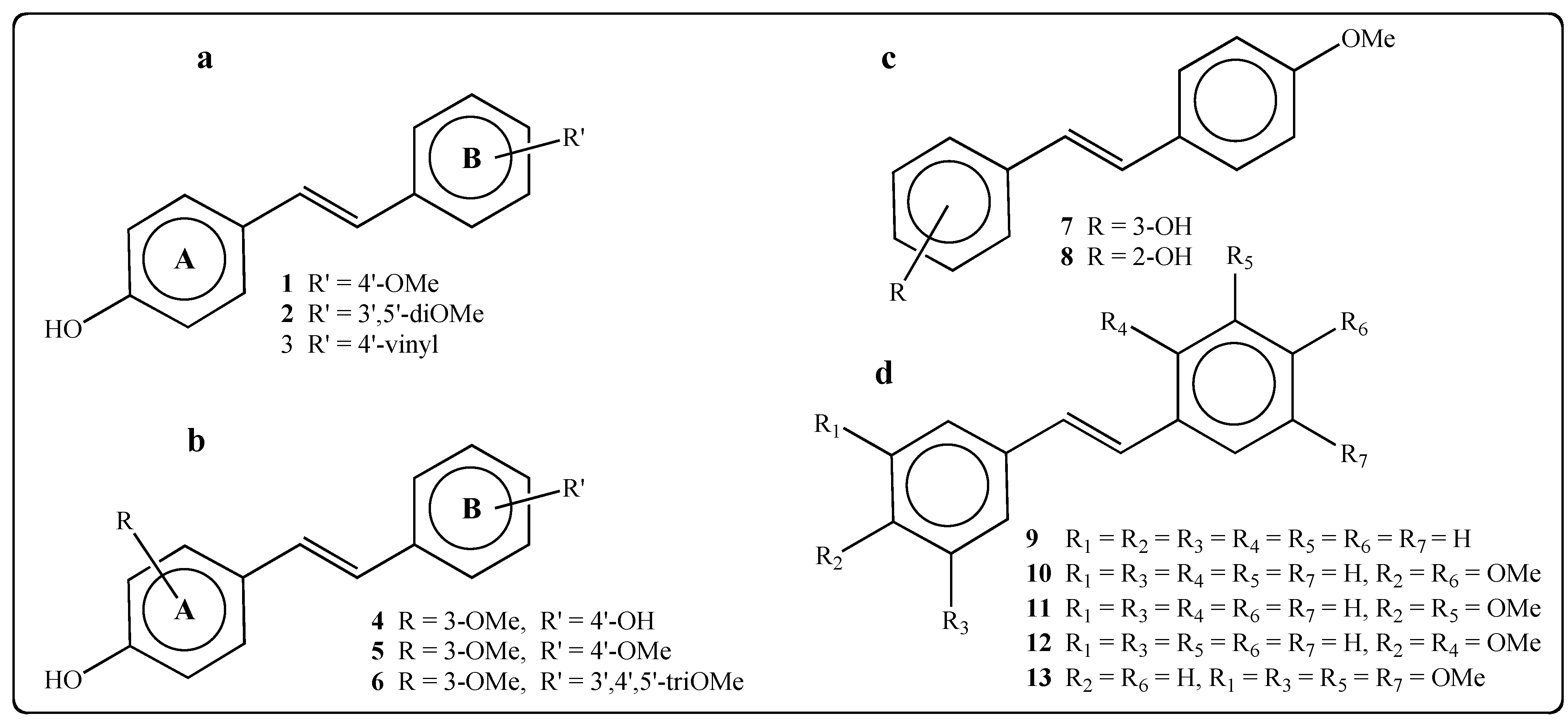
2. Results and Discussion
2.1. Activity of Stilbenes on P. viticola

| Compound | 0.25 mM | 0.5 mM | 0.75 mM |
|---|---|---|---|
| 2 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 |
| RSV | 38 | 0 | 0 |
| 46 | 3846 | 2315 | 00 |
2.2. Activity of Stilbenes on B. cinerea
| Compound | IC50 ± SE (µM) |
|---|---|
| 7 | 28 ± 3 |
| 8 | 30 ± 5 |
| 2 | 52 ± 4 |
| 4 | 55 ± 11 |
| 1 | >100 |
| 6 | >100 |

3. Experimental
3.1. Synthesis
3.2. Plant Material
3.3. P. viticola Bioassays
3.3.1. Assessment of Stilbene Effects on Sporulation
3.3.2. Assessment of Stilbene Effects on Zoospore Mobility
3.4. B. cinerea Bioassays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nürnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar]
- Kuc, J. Phytoalexins, stress metabolism, and disease resistance in plants. Annu. Rev. Phytopathol. 1995, 33, 275–297. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Mol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Chong, J.L.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Pont, V.; Pezet, R. Relation between the chemical structure and the biological activity of hydroxystilbenes against Botrytis cinerea. J. Phytopathol. 1990, 130, 1–8. [Google Scholar] [CrossRef]
- Orsini, F.; Pellizzoni, F.; Verotta, L.; Aburjai, T.; Rogers, C.B. Isolation, synthesis, and antiplatelet aggregation activity of resveratrol 3-o-beta-d-glucopyranoside and related compounds. J. Nat. Prod. 1997, 60, 1082–1087. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar]
- Roupe, K.A.; Remsberg, C.M.; Yanez, J.A.; Davies, N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K.; Ann, N.Y. Clinical trials of resveratrol. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Pezzuto, J.M. The phenomenon of resveratrol: Redefining the virtues of promiscuity. Ann. N. Y. Acad. Sci. 2011, 1215, 123–130. [Google Scholar] [CrossRef]
- Hart, J.H.; Shrimpton, D.M. Role of stilbenes in resistance of wood to decay. Phytopathology 1979, 69, 1138–1143. [Google Scholar] [CrossRef]
- Hart, H. Role of phytostilbenes in decay and disease resistance. Annu. Rev. Phytopathol. 1981, 19, 437–458. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Adrian, M.; de Rosso, M.; Bavaresco, L.; Poinssot, B.; Héloir, M.C. Resveratrol from vine to wine. In Resveratrol Sources, Production and Health benefits; Delmas, D., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2013; pp. 3–19. [Google Scholar]
- Mazue, F.; Colin, D.; Gobbo, J.; Wegner, M.; Rescifina, A.; Spatafora, C.; Fasseur, D.; Delmas, D.; Meunier, P.; Tringali, C.; et al. Structural determinants of resveratrol for cell proliferation inhibition potency: Experimental and docking studies of new analogs. Eur. J. Med. Chem. 2010, 45, 2972–2980. [Google Scholar] [CrossRef]
- Albert, S.; Horbach, R.; Deising, H.B.; Siewert, B.; Csuk, R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorg. Med. Chem. 2011, 19, 5155–5166. [Google Scholar] [CrossRef]
- Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Cattey, H.; Hierso, J.C. Syntheses of polyfunctionalized resveratrol derivatives using Wittig and Heck protocols. Tetrahedron Lett. 2012, 68, 3899–3907. [Google Scholar] [CrossRef]
- Bhusainahalli, V.M.; Spatafora, C.; Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Latruffe, N.; Tringali, C. Resveratrol-related dehydrofimers: Laccase mediated biomimetic synthesis and antiproliferative activity. Eur. J. Org. Chem. 2012, 27, 5217–5224. [Google Scholar]
- Jeandet, P.; Vasserot, Y.; Chastang, T.; Courot, E. Engineering microbial cells for the biosynthesis of natural compounds of pharmaceutical significance. BioMed Res. Int. 2013, 2013, 780145:1–780145:13. [Google Scholar]
- Trouvelot, S.; Varnier, A.L.; Allègre, M.; Mercier, L.; Baillieul, F.; Arnould, C.; Gianinazzi-Pearson, V.; Klarzynski, O.; Joubert, J.M.; Pugin, A.; et al. A beta-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol. Plant Microbe Int. 2008, 21, 232–243. [Google Scholar] [CrossRef]
- Langcake, P. Disease resistance of Vitis spp. and the production of the stress metabolites resveratrol, ε-viniferin, α-viniferin and pterostilbene. Physiol. Plant Pathol. 1981, 18, 213–226. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Richter, H. Effect of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis 2004, 43, 145–148. [Google Scholar]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Allègre, M.; Héloir, M.C.; Trouvelot, S.; Daire, X.; Pugin, A.; Wendehenne, D.; Adrian, M. Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol. Plant Microbe Int. 2009, 22, 977–986. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial Activity of Resveratrol Analogues. Molecules 2014, 19, 7679-7688. https://doi.org/10.3390/molecules19067679
Chalal M, Klinguer A, Echairi A, Meunier P, Vervandier-Fasseur D, Adrian M. Antimicrobial Activity of Resveratrol Analogues. Molecules. 2014; 19(6):7679-7688. https://doi.org/10.3390/molecules19067679
Chicago/Turabian StyleChalal, Malik, Agnès Klinguer, Abdelwahad Echairi, Philippe Meunier, Dominique Vervandier-Fasseur, and Marielle Adrian. 2014. "Antimicrobial Activity of Resveratrol Analogues" Molecules 19, no. 6: 7679-7688. https://doi.org/10.3390/molecules19067679





