Inhibition of Cancer Derived Cell Lines Proliferation by Synthesized Hydroxylated Stilbenes and New Ferrocenyl-Stilbene Analogs. Comparison with Resveratrol
Abstract
:1. Introduction
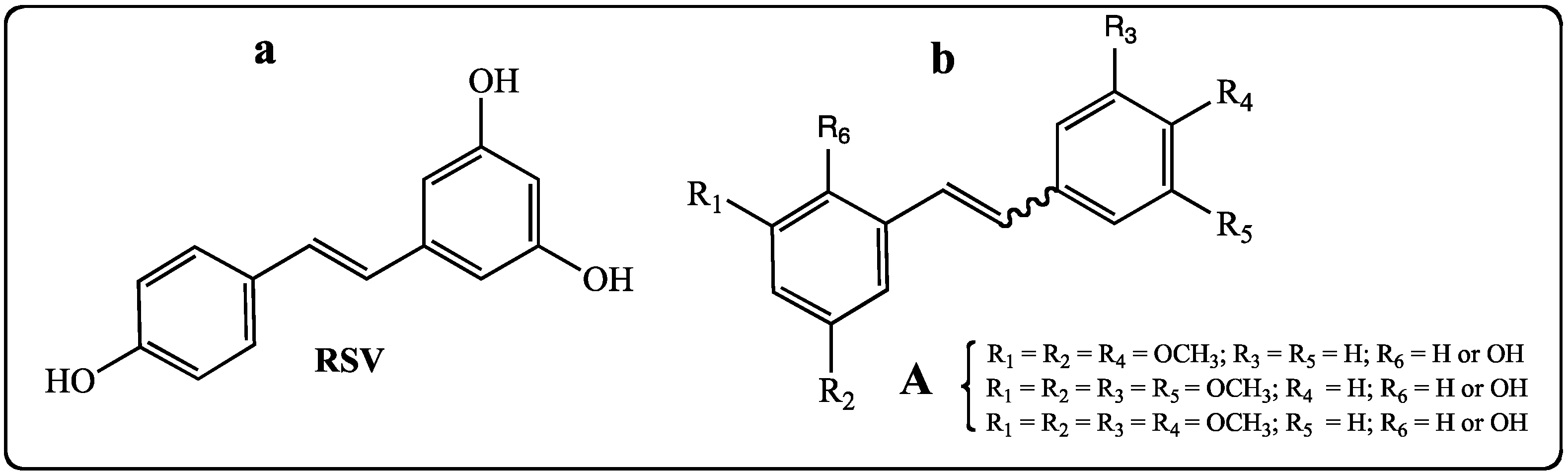
2. Results and Discussion
2.1. Chemical Results
2.1.1. Synthesis of E-4-Hydroxystilbenes
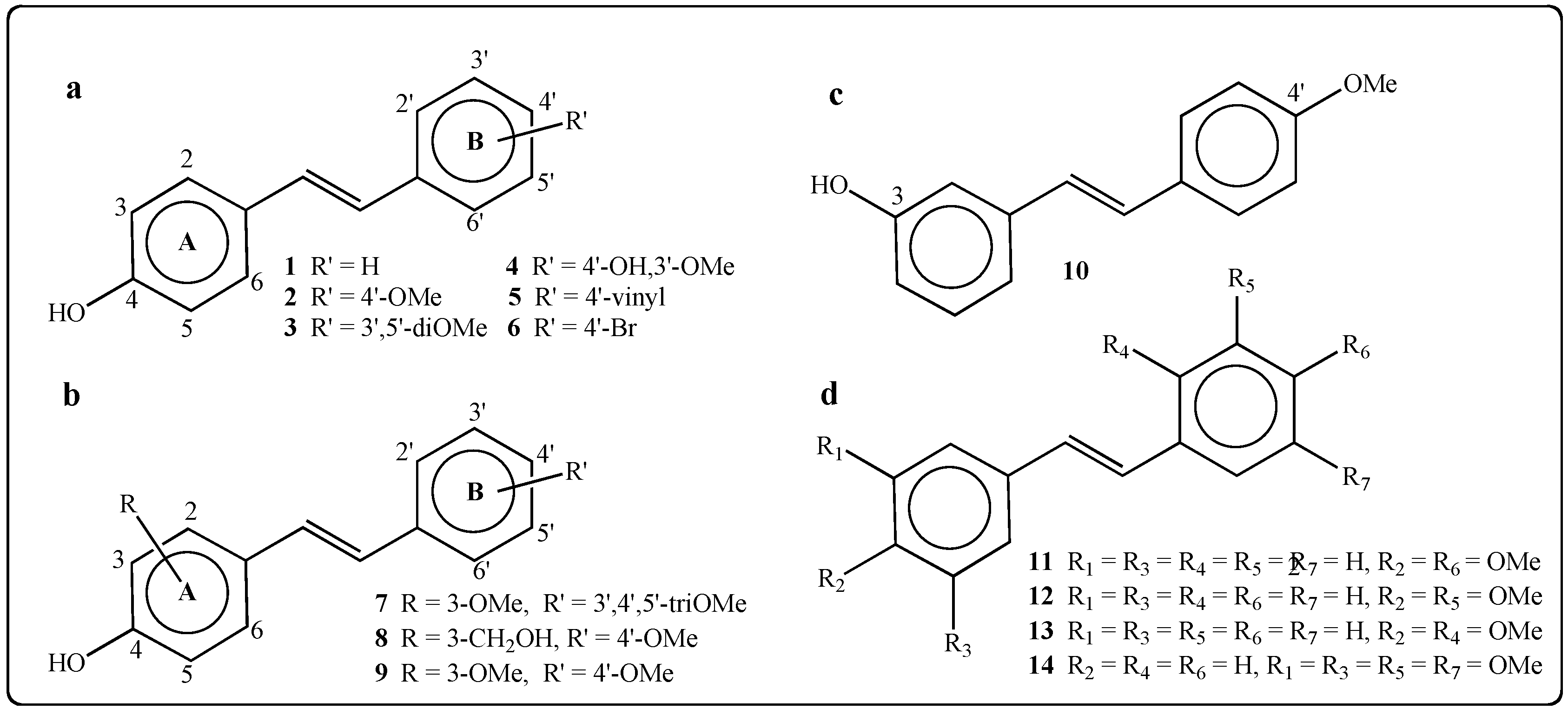
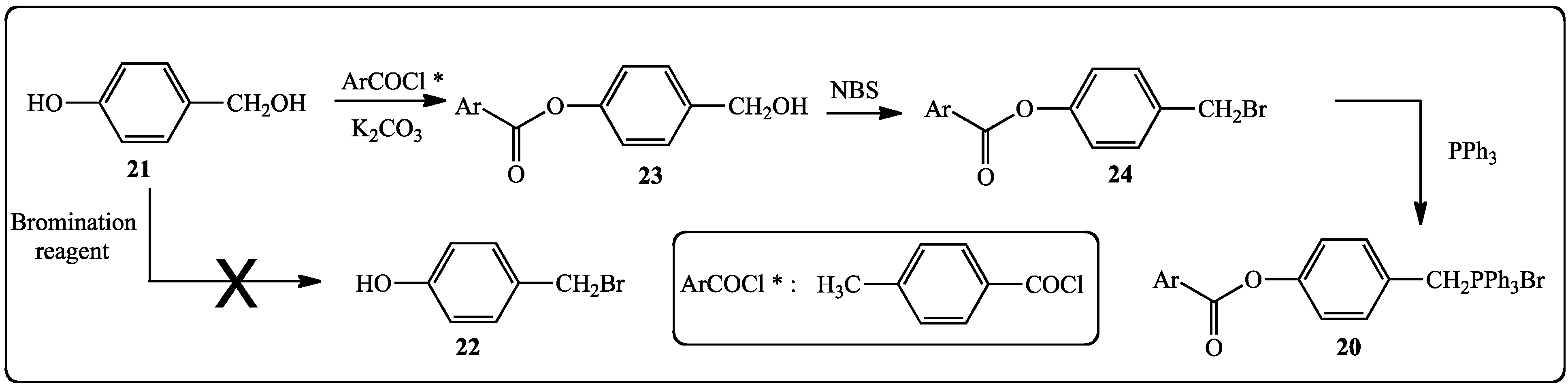
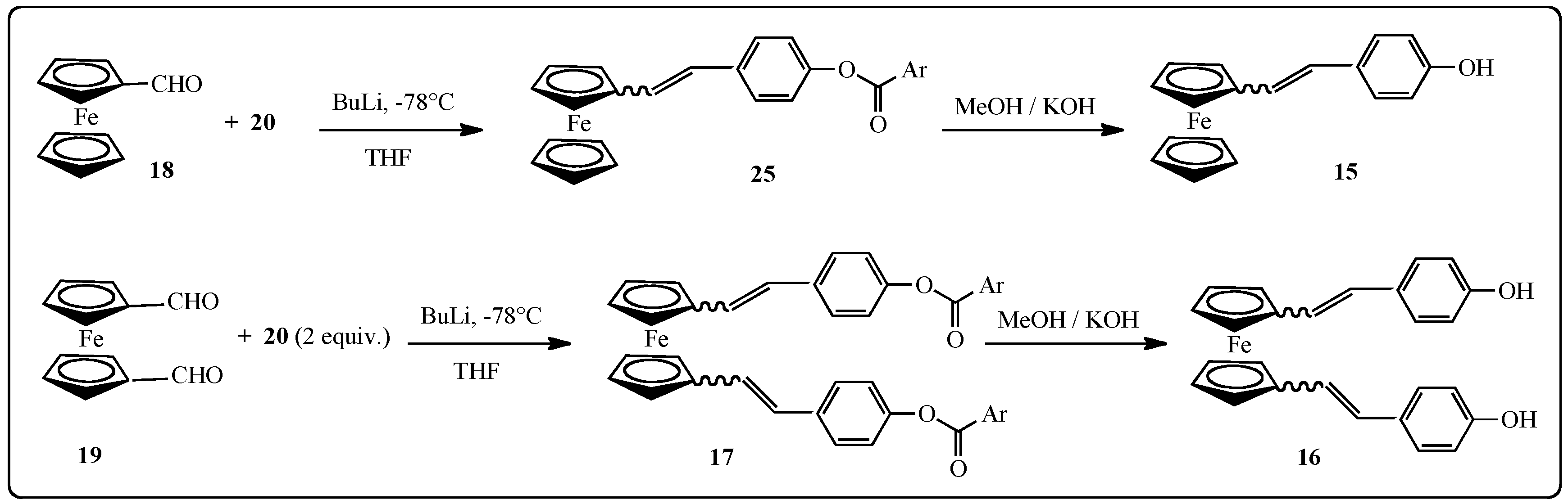
2.1.2. Synthesis of Stilbenes Bearing Ferrocenylstilbene Analogs
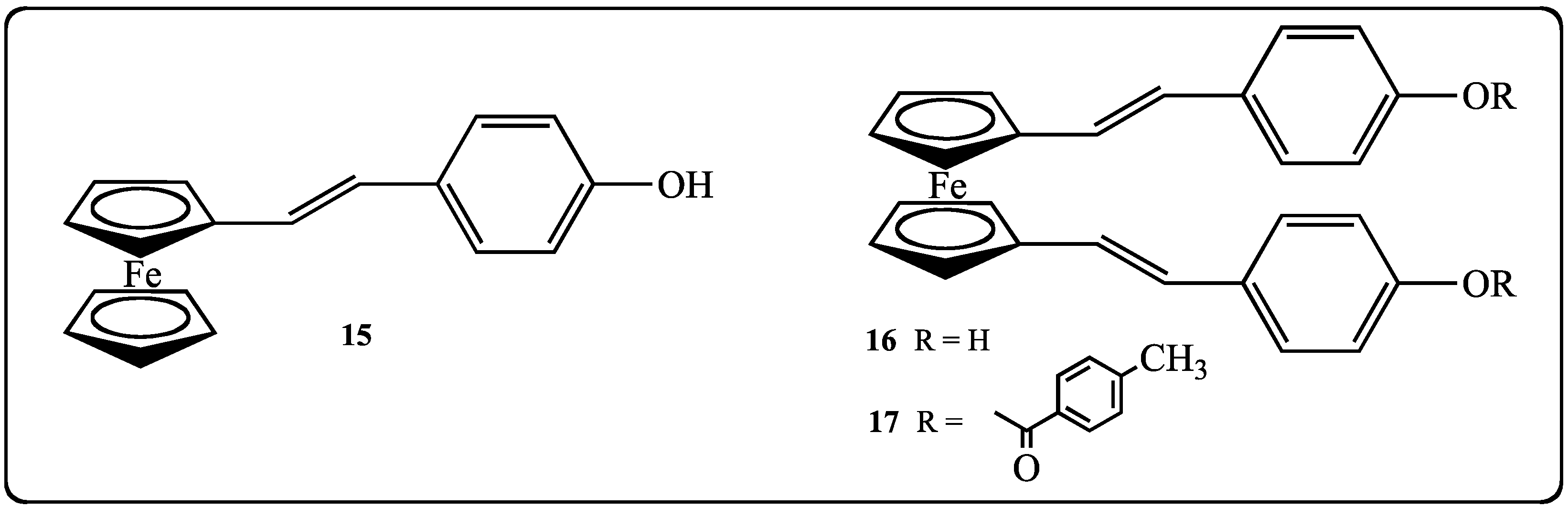
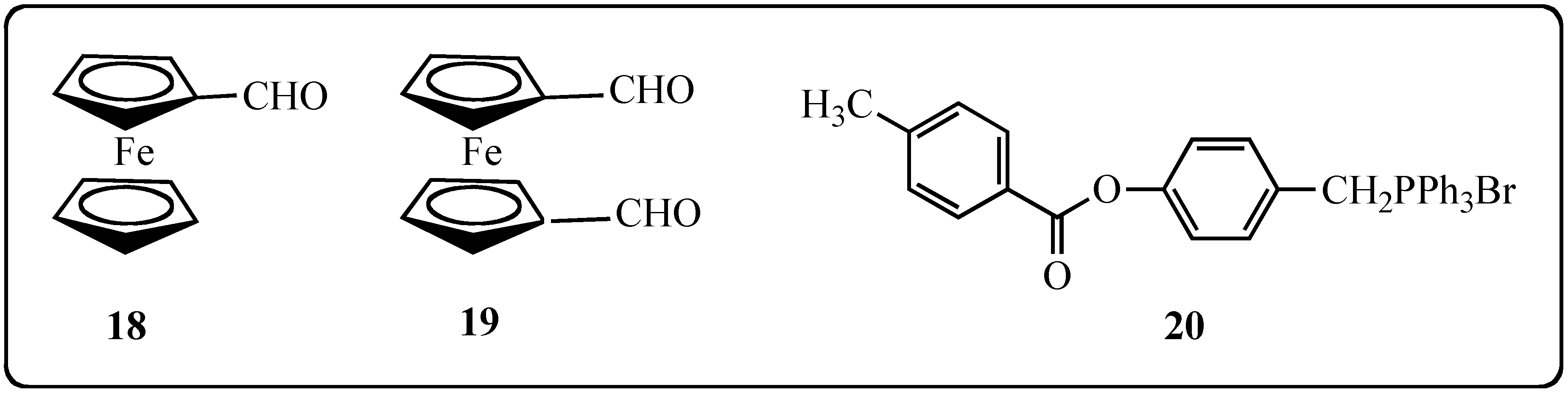
2.2. Biological Effects
2.2.1. Effect of Stilbene Derivatives on Human Colorectal Tumor SW 480 Cell Line Proliferation
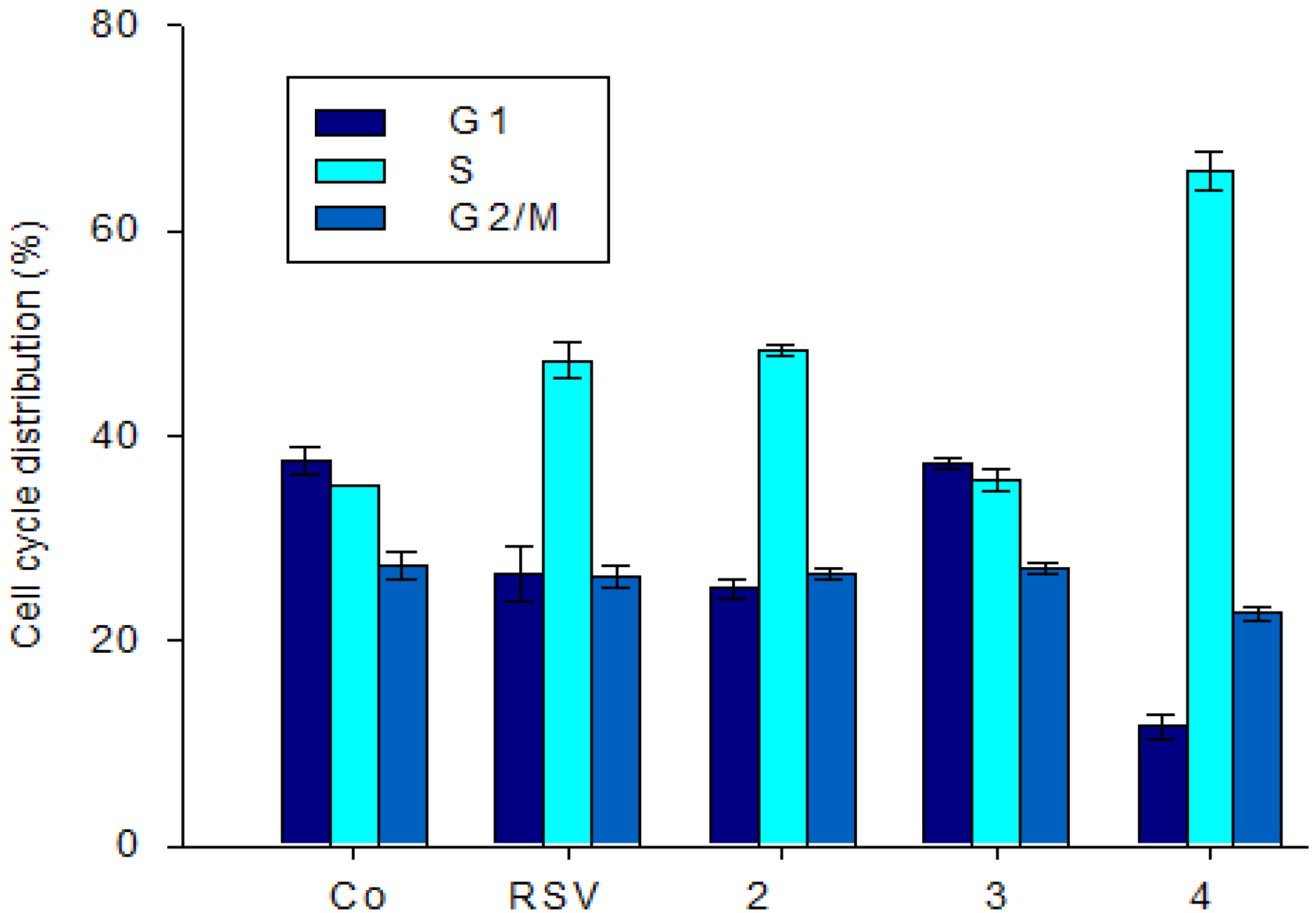
2.2.2. Effect of Stilbene Derivatives on the Cell Cycle Phase of the SW480 Cell Line
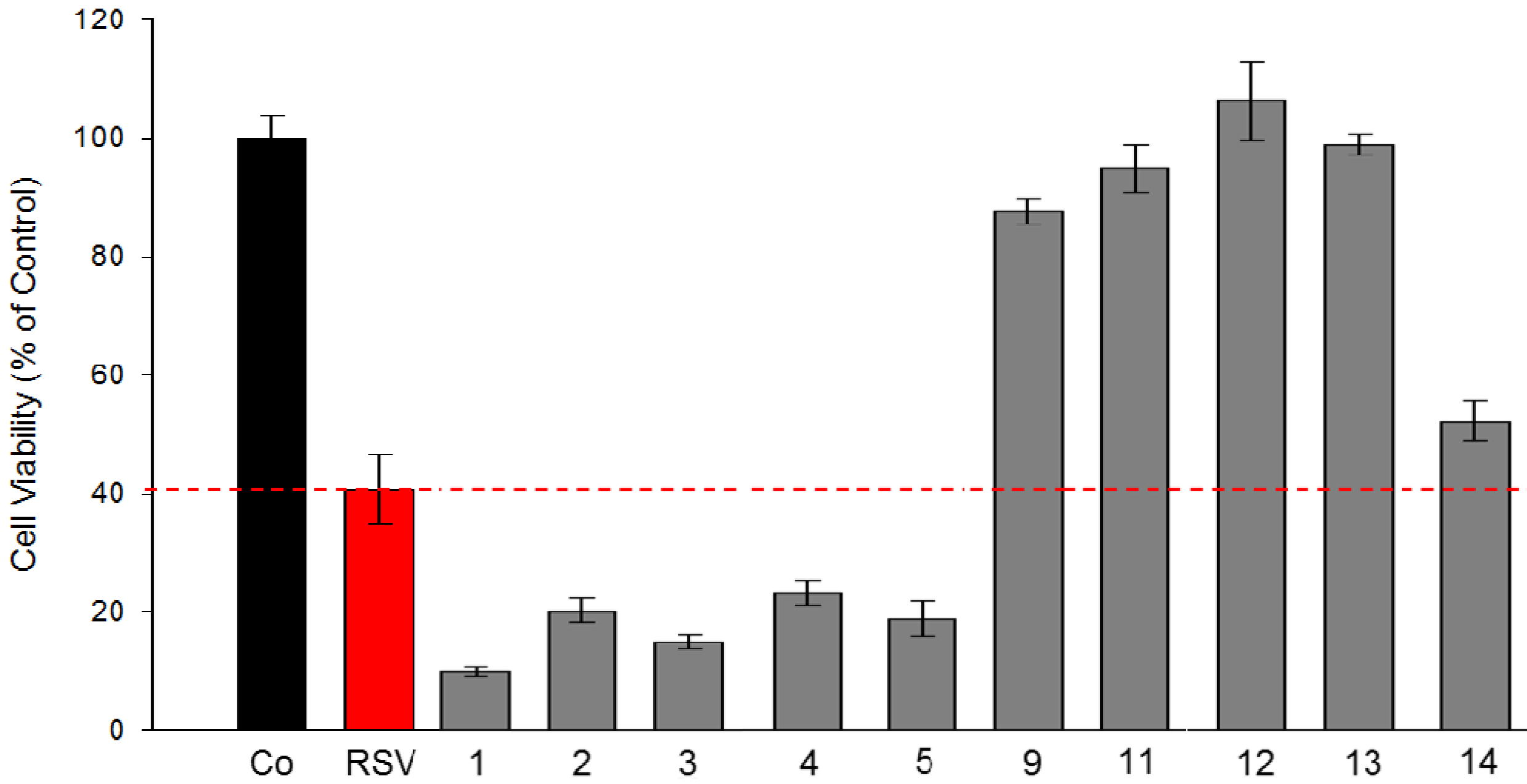
2.2.3. Evaluation of Toxicity Level of Stilbene Derivatives Towards Non-Cancerous Intestinal Epithelial Cells
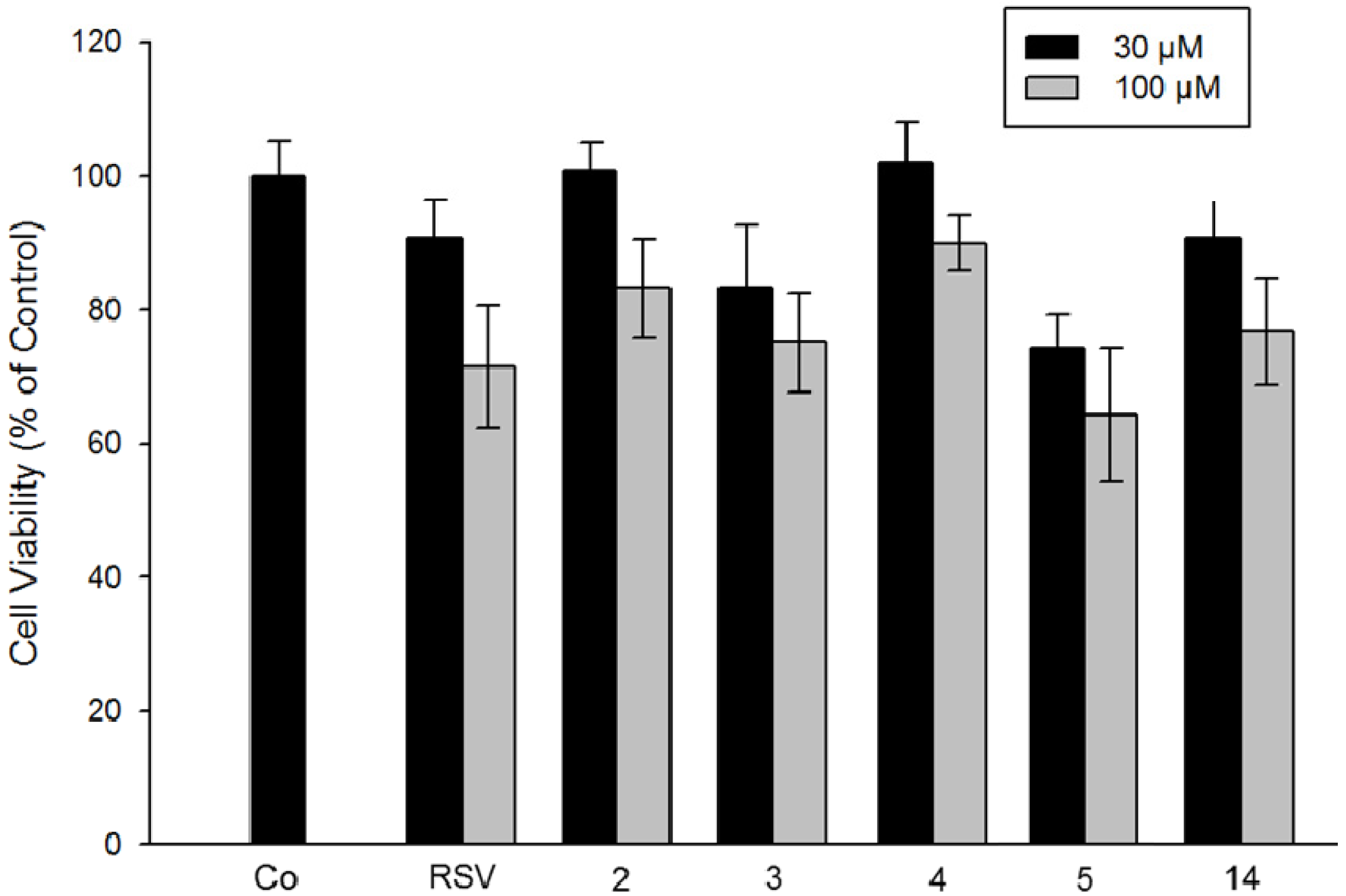
2.2.4. Comparison of Resveratrol Analogs on Cytotoxicity of Colorectal Tumor Cells and on Hepatoblastoma Cells
| Compound Number | Compound Name | SW480 IC50 (μM) | HepG2 IC50 (μM) |
|---|---|---|---|
| E-resveratrol | 68.1 ± 5.5 | 57.3 ± 8.1 | |
| 1 | E-4-hydroxystilbene | 18.6 ± 3.2 | 27.6 ± 5.0 |
| 2 | E-4-hydroxy-4'-methoxystilbene | 14.7 ± 2.1 | 26.3 ± 3.2 |
| 3 | E-4-hydroxy-3',5'-dimethoxystilbene | 16.1(± 2.9 | Not Tested |
| 4 | E-4,4'-dihydroxy-4'-methoxystilbene | 15.0 ± 0.9 | Not Tested |
| 5 | E-4-hydroxy-4'-vinylstilbene | 21.4 ± 0.3 | 33.2 ± 6.2 |
| 6 | E-4-bromo-4'-hydroxystilbene | 25.3 ± 2.4 | 18.6 ± 0.2 |
| 7 | E-4-hydroxy-3,3',4',5'-tetramethoxystilbene | 38.2 ± 0.7 | Not Tested |
| 8 | E-3-carbinol-4-hydroxy-4'methoxystilbene | 25.7 ± 2.1 | 77.7 ± 4.1 |
| 10 | E-3-hydroxy-4'-methoxystilbene | 81.7 ± 3.7 | Not Tested |
| — | Ferrocene | >100 | >100 |
| 15 | E-(4-vinylphenol)-ferrocene | 25.5 ± 1.6 | 40.2 ± 4.3 |
| 16 | (E,E)-1,1'-bis(4-vinylphenol)-ferrocene | >100 | >100 |
| 17 | (E,E)-1,1'-bis[(1-p-toluoyloxy-4-vinyl)benzene]-ferrocene | 5.9 ± 0.1 | 5.1 ± 0.2 |
2.2.5. Effect of Resveratrol Isosteres Bearing a Ferrocenyl Moiety. Determination of IC50 Values
3. Experimental
3.1. General Experimental Procedures
3.2. Precursors of Ferrocenyl-Stilbene Analogs
3.3. Ferrocenyl-Stilbene Analogs 15–17 and 25
3.4. Biological Methods
3.4.1. Cell Culture
3.4.2. Cell Viability Assays
3.4.3. Cell Proliferation Assays
3.4.4. Cell Cycle Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Arichi, H.; Kimura, Y.; Okuda, H.; Baba, M.; Kozowa, K.; Arichi, S. Effects of stilbene compounds of the roots of Polygonum cuspidatum Sieb et Zucc on lipid metabolism. Chem. Pharm. Bull. 1982, 30, 1766–1770. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. Medicinal and poisonous plants. Nature 1962, 196, 609–610. [Google Scholar]
- Langcake, P.; Pryce, R. The production of Resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant. Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.A. New class of phytoalexins from grapevines. Experientia 1977, 33, 151–152. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Sahli, R.; Bisson, J.; Rivière, C.; Delaunay, J.C.; Richard, T.; Gomes, E.; Bordenave, L.; Waffo-Teguo, P.; Mérillon, J.M. Stilbenoid profiless of canes from Vitis and Muscadinia species. J. Agric. Food Chem. 2013, 61, 501–511. [Google Scholar] [CrossRef]
- Siemann, E.H.; Creasy, L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar]
- Boutegrabet, L.; Fekete, A.; Hertkorn, N.; Papastamoulis, Y.; Waffo-Téguo, P.; Mérillon, J.M.; Jeandet, P.; Gougeon, R.D.; Schmitt-Koplin, P. Determination of stilbene derivatives in Burgundy red wines by ultra-high pressure liquid chromatography. Anal. Bioanal. Chem. 2011, 401, 1517–1525. [Google Scholar]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Ingham, J.L. 3,5,4'-Trihydroxystilbene as a phytoalexin from groundnuts (Arachis hypogaea). Phytochemistry 1976, 15, 1791–1793. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Park, J.Y.; Harima, S.; Yoshikawa, M. Antioxidant constituents from rhubarb: Structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg. Med. Chem. 2001, 9, 41–50. [Google Scholar] [CrossRef]
- Andrus, M.B.; Liu, J.; Meredith, E.L.; Nartey, E. Synthesis of resveratrol using a direct decarbonylative Heck approach from resorcylic acid. Tetrahedron Lett. 2003, 44, 4819–4822. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. 2008, 658, 68–94. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Gonzales-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vivo studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer, chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Boyer, J.Z.; Jandova, J.; Janda, J.; Vleugels, F.R.; Elliott, D.A.; Sligh, J.E. Resveratrol-sensitized UVA induced apoptosis in human keratinocytes through mitochondrial oxidative stress and pore opening. J. Phytochem. Photobiol. B 2012, 113, 42–50. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef]
- Docherty, J.J.; Fu, M.M.H.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; de Lucia, A.L. Resveratrol inhibition of herpes simplex virus replication. Antiviral. Res. 1999, 43, 145–155. [Google Scholar] [CrossRef]
- Yim, N.; Ha, D.T.; Trung, T.N.; Kim, J.P.; Lee, S.; Na, M.; Jung, H.; Kim, H.S.; Kim, Y.H.; Bae, K. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 1165–1168. [Google Scholar]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Lambert, C.; Bisson, J.; Waffo-Téguo, P.; Papastamoulis, Y.; Richard, T.; Corio-Costet, M.-F.; Mérillon, J.-M.; Cluzet, S. Phenolics and their antifungal role in grapevine wood decay: Focus on the Botryosphaeriaceae family. J. Agric. Food Chem. 2012, 60, 11859–11868. [Google Scholar] [CrossRef]
- Rivière, C.; Richard, T.; Quentin, L.; Krisa, S.; Mérillon, J.M.; Monti, J.P. Inhibitory activity of stilbenes on Alzeimer’s β-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007, 15, 1160–1167. [Google Scholar] [CrossRef]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. NeuroSci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef]
- Stef, G.; Csiszar, A.; Lerea, K.; Ungvari, Z.; Veress, G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J. Card. Pharm. 2006, 48, 1–5. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef]
- Mohar, D.S.; Malik, S. The sirtuin system: The holy grail of resveratrol? J. Clin. Exp. Cardiolog. 2012, 3, 1000216/1–1000216/4. [Google Scholar]
- Wang, M.; Jin, Y.; Ho, C.T. Evaluation of resveratrol derivatives as potential antioxidants and identification of a reaction product of resveratrol and 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 1999, 47, 3974–3977. [Google Scholar]
- Fukuhara, K.; Nagakawa, M.; Nakanishi, I.; Ohkubo, K.; Imai, K.; Urano, S.; Fukuzumi, S.; Ozawa, T.; Ikota, N.; Mochizuki, M.; et al. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu(II). Bioorg. Med. Chem. 2006, 14, 1437–1443. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple targets of resveratrol: Anti-carcenogic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef]
- Pirola, L.; Frödjö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef]
- Latruffe, N.; Delmas, D.; Lizard, G.; Tringali, C.; Spatafora, C.; Vervandier-Fasseur, D.; Meunier, P. Resveratrol against major pathologies: From diet prevention to possible alternative chemotherapies with new structural analogues. In Bioactive Compounds from Natural Sources, 2nd ed; Corrado, T., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 340–378. [Google Scholar]
- St John, S.E.; Jensen, K.C.; Kang, S.; Chen, Y.; Calamini, B.; Mesecar, A.D.; Lipton, M.A. Design, synthesis, biological and structural evaluation of functionalized resveratol analogues as inhibitors of quinone reductase 2. Bioorg. Med. Chem. 2013, 21, 6022–6037. [Google Scholar]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef]
- Cheng, J.C.; Fang, J.G.; Chen, W.-F.; Zhou, B.; Yang, L.; Liu, Z.L. Structure-activity relationship studies of resveratrol and its analogues by the reaction kinetics of low density lipoprotein peroxidation. Bioorg. Chem. 2006, 34, 142–157. [Google Scholar] [CrossRef]
- Cardile, V.; Lombardo, L.; Spatafora, C.; Tringali, C. Chemo-enzymatic synthesis and cell-growth inhibition activity of resveratrol analogues. Bioorg. Chem. 2005, 33, 22–33. [Google Scholar] [CrossRef]
- Horvath, Z.; Marihart-Fazekas, S.; Saiko, P.; Grusch, M.; Ozsüy, M.; Harik, M.; Handler, N.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; et al. Novel resveratrol derivatives induce apoptosis and cause cell cycle arrest in prostate cancer cell lines. Anticancer Res. 2007, 27, 3459–3464. [Google Scholar]
- Pan, M.H.; Lin, C.L.; Tsai, J.H.; Ho, C.T.; Chen, W.J. 3,5,3',4',5'-Pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J. Agric. Food Chem. 2010, 58, 226–234. [Google Scholar]
- Zhang, W.; Go, M.L. Methoxylation of resveratrol: Effects on induction of NAD(P)H quinone oxidoreductase 1 (NQO1) activity and growth inhibitory properties. Bioorg. Med. Chem. Lett. 2011, 21, 1032–1035. [Google Scholar] [CrossRef]
- Das, J.; Pany, S.; Mahji, A. Chemical modification of resveratrol for improved protein kinase C alpha activity. Bioorg. Med. Chem. 2011, 19, 5321–5333. [Google Scholar]
- Biasutto, L.; Mattarei, A.; Marotta, E.; Bradaschia, A.; Sassi, N.; Garbisa, S.; Zoratti, M.; Paradisi, C. Development of mitochondria-targeted derivatives of resveratrol. Bioorg. Med. Chem. Lett. 2008, 18, 5594–5597. [Google Scholar] [CrossRef]
- Huang, X.F.; Ruan, B.F.; Wang, X.T.; Xu, C.; Ge, H.M.; Zhu, H.L.; Tan, R.X. Synthesis and cytotoxic evaluation of a series of resveratrol derivatives modified in C2 position. Eur. J. Med. Chem. 2007, 42, 263–267. [Google Scholar] [CrossRef]
- Mazué, F.; Colin, D.; Gobbo, J.; Wegner, M.; Rescifina, A.; Spatafora, C.; Fasseur, D.; Delmas, D.; Meunier, P.; Tringali, C.; et al. Structural determinants of resveratrol for cell proliferation inhibition potency: Experimental and docking studies of new analogs. Eur. J. Med. Chem. 2010, 45, 2972–2980. [Google Scholar] [CrossRef]
- Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Cattey, H.; Hierso, J.C. Syntheses of polyfunctionalized resveratrol derivatives using Wittig and Heck protocols. Tetrahedron 2012, 68, 3899–3907. [Google Scholar] [CrossRef]
- Heynekamp, J.J.; Weber, W.M.; Hunsaker, L.A.; Gonzales, A.M.; Orlando, R.A.; Deck, L.M.; Vander Jagt, D.L. Substituted trans-stilbenes, including analogues of the natural product resveratrol, inhibit the human tumor necrosis factor alpha-induced activation of transcription factor nuclease factor kappaB. J. Med. Chem. 2006, 49, 7182–7189. [Google Scholar] [CrossRef]
- Shang, Y.J.; Qian, Y.P.; Liu, X.D.; Dai, F.; Shang, X.L.; Jia, W.Q.; Liu, Q.; Fang, J.G.; Zhou, B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical, and substitution. J. Org. Chem. 2009, 74, 5025–5031. [Google Scholar]
- Chandra, V.; Srivastava, V.B. Condensation of p-bromophenylacetic acid with aldehydes. J. Indian Chem. Soc. 1967, 44, 675–678. [Google Scholar]
- Sinha, A.K.; Kumar, V.; Sharma, A.; Sharma, A.; Kumar, R. An unusual, mild and convenient one-pot two-step access to E.-stilbenes from hydroxy-substituted benzaldehydes and phenylacetic acids under microwave activation: A new facet of the classical Perkin reaction. Tetrahedron 2007, 63, 11070–11077. [Google Scholar] [CrossRef]
- Sinh, A.K.; Kumar, V.; Sharma, A. A Single Step Microwave Induced Process for the Preparation of Substituted Stilbenes and Analogs from Arylaldehydes and Phenylacetic Acids. WO2007/110883, 2007. [Google Scholar]
- Albert, S.; Horbach, R.; Deising, H.B.; Siewert, B.; Csuk, R. Synthesis and antimicrobial activity of E.-stilbene derivatives. Bioorg. Med. Chem. 2011, 19, 5155–5166. [Google Scholar] [CrossRef]
- Rosenberg, B.; van Camp, L.; Trosko, J.E.; Mansour, V.H. Platinium compounds: A new class of potent antitumor agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Metzler-Note, N.; Dyson, P.J. Challenges and opportunities in the development of organometallic anticancer drugs. Organometallics 2012, 31, 5677–5685. [Google Scholar] [CrossRef]
- Fouda, M.F.R.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625. [Google Scholar] [CrossRef]
- Navarro, M.; Castro, W.; Biot, C. Bioorganometallic compounds with antimalarial targets: inhibiting hemozoin formation. Organometallics 2012, 31, 5715–5727. [Google Scholar] [CrossRef]
- Top, S.; Vessières, A.; Cabestaing, C.; Laios, I.; Leclercq, G.; Provot, C.; Jaouen, G.J. Studies on organometallic selective estrogen receptor modulators. (SERMs) dual activity in the hydroxy-ferrocifen series. Organomet. Chem. 2001, 637, 500–506. [Google Scholar]
- Messina, P.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Hamels, D.; Top, S.; Jaouen, G.; Frapart, Y.M.; Mansuy, D.; et al. Deciphering the activation sequence of ferrociphenol anticancer drug candidates. Chem. Eur. J. 2012, 18, 6581–6587. [Google Scholar] [CrossRef]
- Balavoine, G.G.A.; Doisneau, G.; Fillebeen-Khan, T. An improved synthesis of ferrocene-1,1'-dicarbaldehyde. J. Organomet. Chem. 1991, 412, 381–382. [Google Scholar] [CrossRef]
- We reacted 3,5-dihydroxybenzylic alcohol or 4-hydroxybenzylic alcohol with different brominating reagents: PBr3, CBr4 or HBr/AcOH but attempts to isolate by chromatography the corresponding bromides failed and the starting alcohols were recovered
- Vervandier-Fasseur, D.; Chalal, M.; Meunier, P. Method for the Production of trans-resveratrol and its Analogs via Wittig Reaction and Their Pharmaceutical and Cosmetic Compositions. PCT Int. Appl. WO 2013008175, 2013. [Google Scholar]
- Firouzabadi, H.; Iranpoor, N.; Ebrahimzadeh, F. Facile conversion of alcohols into their bromides and iodides by N-bromo and N-iodosaccharins/triphenylphosphine under neutral conditions. Tetrahedron Lett. 2006, 47, 1771–1775. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chauhan, S.; Bansal, G. Facile hydrolysis of esters with KOH-Methanol at ambient temperature. Monatsch. Chem. 2004, 135, 83–87. [Google Scholar] [CrossRef]
- Simoni, D.; Roberti, M.; Invidiata, F.P.; Aiello, E.; Aiello, S.; Marchetti, P.; Baruchello, R.; Eleopra, M.; di Cristina, A.; Grimaudo, S.; et al. Stilbene-based anticancer agents: Resveratrol analogues active toward HL60 leukemic cells with a non-specific phase mechanism. Bioorg. Med. Chem. Lett. 2006, 16, 3245–3248. [Google Scholar] [CrossRef]
- Pan, M.H.; Chang, Y.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007, 55, 7777–7785. [Google Scholar] [CrossRef]
- Lee, S.K.; Zhang, W.; Sanderson, B.J.S. Selective growth inhibition of human leukemia and human lymphoblastoid cells by Resveratrol via cell cycle arrest and apoptosis induction. J. Agric. Food Chem. 2008, 56, 7572–7577. [Google Scholar]
- Delmas, D.; Rébé, C.; Lacour, S.; Filomenko, R.; Athias, A.; Gambert, P.; Cherkaoui-Malki, M.; Jannin, B.; Dubrez-Daloz, L.; Latruffe, N.; et al. Resveratrol-induced apoptosis is associated with fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J. Biol. Chem. 2003, 278, 41482–41490. [Google Scholar] [CrossRef]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5-fluorouracil sensitivity in human derived colon cancer cells. Int. J. Cancer 2009, 124, 2780–2788. [Google Scholar]
- Larrosa, M.; Tomas-Barberan, F.; Espin, J.C. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest, and upregulation of cyclins A, E and B1 in human SK-MeI-28 melanoma cells. J. Agric. Food Chem. 2003, 51, 4576–4584. [Google Scholar] [CrossRef]
- Fan, G.J.; Liu, X.D.; Qian, Y.P.; Shang, Y.J.; Li, X.Z.; Dai, F.; Fang, J.G.; Jin, X.L.; Zhou, B. 4,4'-Dihydroxy-trans-stilbene, a resveratrol analogue, exhibited enhanced antioxidant activity and cytotoxicity. Bioorg. Med. Chem. 2009, 17, 2360–2365. [Google Scholar] [CrossRef]
- Horvath, Z.; Murias, M.; Saiko, P.; Erker, T.; Handler, N.; Madlener, S.; Jaeger, W.; Grusch, M.; Fritzer-Szekeres, M.; Krupitza, G.; et al. Cytotoxic and biochemical effects of 3,3',4,4',5,5'-hexahydroxystilbenes, a novel resveratrol analog in HL-60 human promyelocytic leukemia cells. Exp. Hematol. 2006, 34, 1377–1384. [Google Scholar] [CrossRef]
- She, Q.B.; Ma, W.Y.; Wang, M.; Mingfu, K.; Kaji, A.; Ho, C.T.; Dong, Z. Inhibition of cell transformation by resveratrol and its derivatives: Differential effects and mechanism involved. Oncogene. 2003, 22, 2143–2150. [Google Scholar] [CrossRef]
- Nargi, F.E.; Yang, T.J. Optimization of the L-M cell bioassay for quantitating tumor necrosis factor alpha in serum and plasma. J. Immunol. Methods 1993, 159, 81–91. [Google Scholar] [CrossRef]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef]
- Marel, A.K.; Lizard, G.; Izard, J.C.; Latruffe, N.; Delmas, D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef]
- Delmas, D.; Lançon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemoprotective agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–17 are available from D. Vervandier-Fasseur (D.V.-F.).
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chalal, M.; Delmas, D.; Meunier, P.; Latruffe, N.; Vervandier-Fasseur, D. Inhibition of Cancer Derived Cell Lines Proliferation by Synthesized Hydroxylated Stilbenes and New Ferrocenyl-Stilbene Analogs. Comparison with Resveratrol. Molecules 2014, 19, 7850-7868. https://doi.org/10.3390/molecules19067850
Chalal M, Delmas D, Meunier P, Latruffe N, Vervandier-Fasseur D. Inhibition of Cancer Derived Cell Lines Proliferation by Synthesized Hydroxylated Stilbenes and New Ferrocenyl-Stilbene Analogs. Comparison with Resveratrol. Molecules. 2014; 19(6):7850-7868. https://doi.org/10.3390/molecules19067850
Chicago/Turabian StyleChalal, Malik, Dominique Delmas, Philippe Meunier, Norbert Latruffe, and Dominique Vervandier-Fasseur. 2014. "Inhibition of Cancer Derived Cell Lines Proliferation by Synthesized Hydroxylated Stilbenes and New Ferrocenyl-Stilbene Analogs. Comparison with Resveratrol" Molecules 19, no. 6: 7850-7868. https://doi.org/10.3390/molecules19067850






