Synthesis and Anti-hypertensive Effects of the Twin Drug of Nicotinic Acid and Quercetin Tetramethyl Ether
Abstract
:1. Introduction
2. Results and Discussion
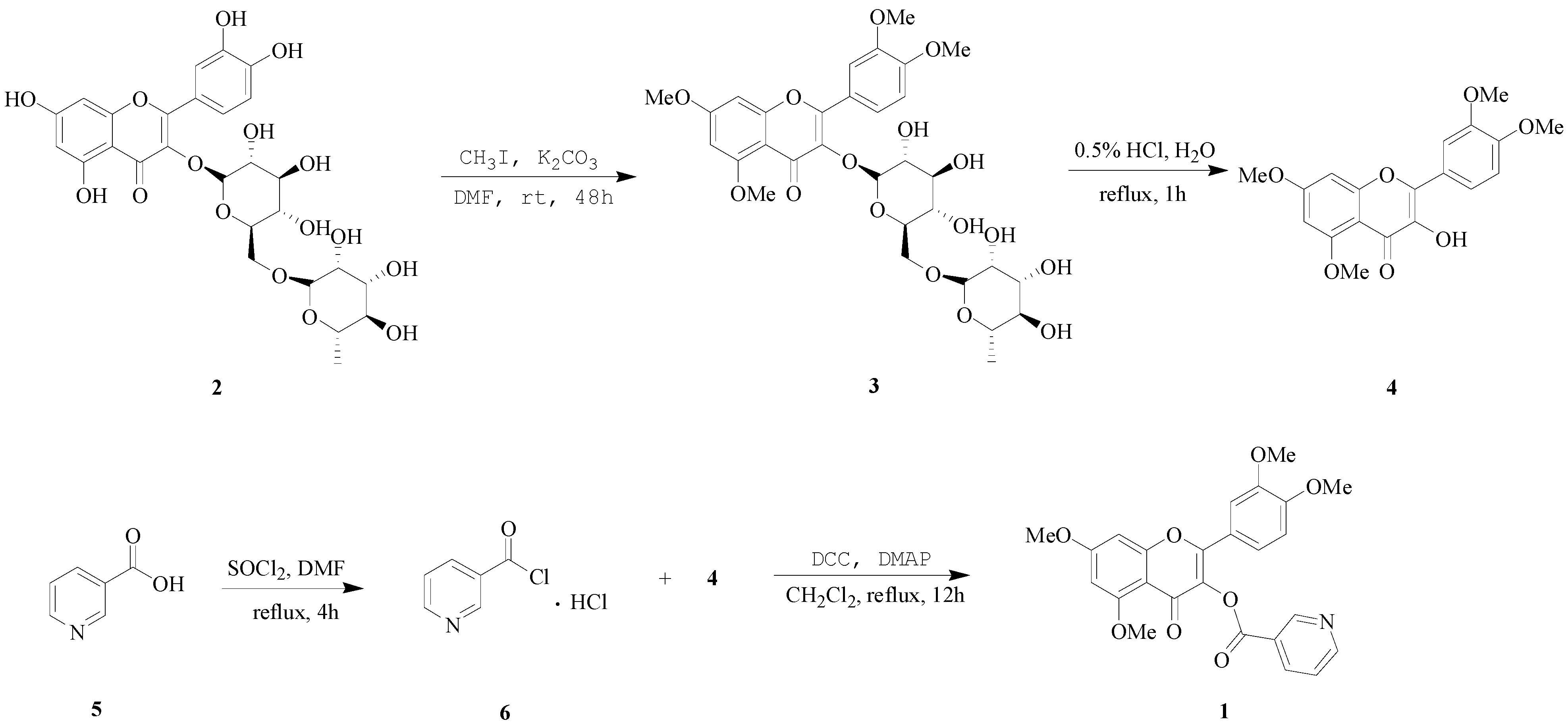
3. Experimental
3.1. General Information
3.2. Chemistry Synthesis
3.2.1. Preparation of 5,7,3',4'-O-tetramethylrutin (3)
3.2.2. Preparation of Quercetin 5,7,3',4'-tetramethyl Ether (QTME, 4)
3.2.3. Preparation of Nicotinoyl Chloride Hydrochloride (6)
3.2.4. Synthesis of VB3-QTME (1)
3.3. Animal Experiments
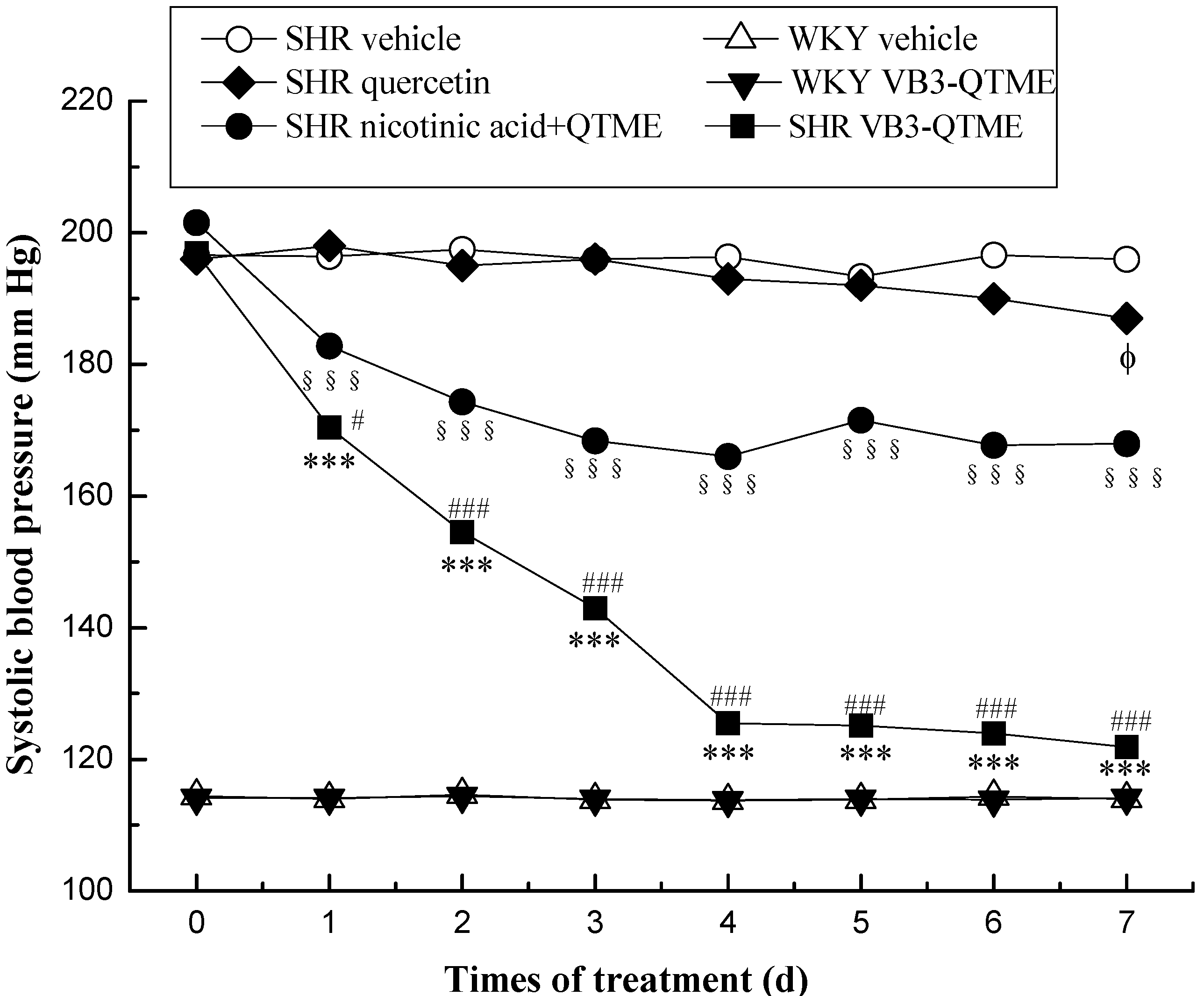
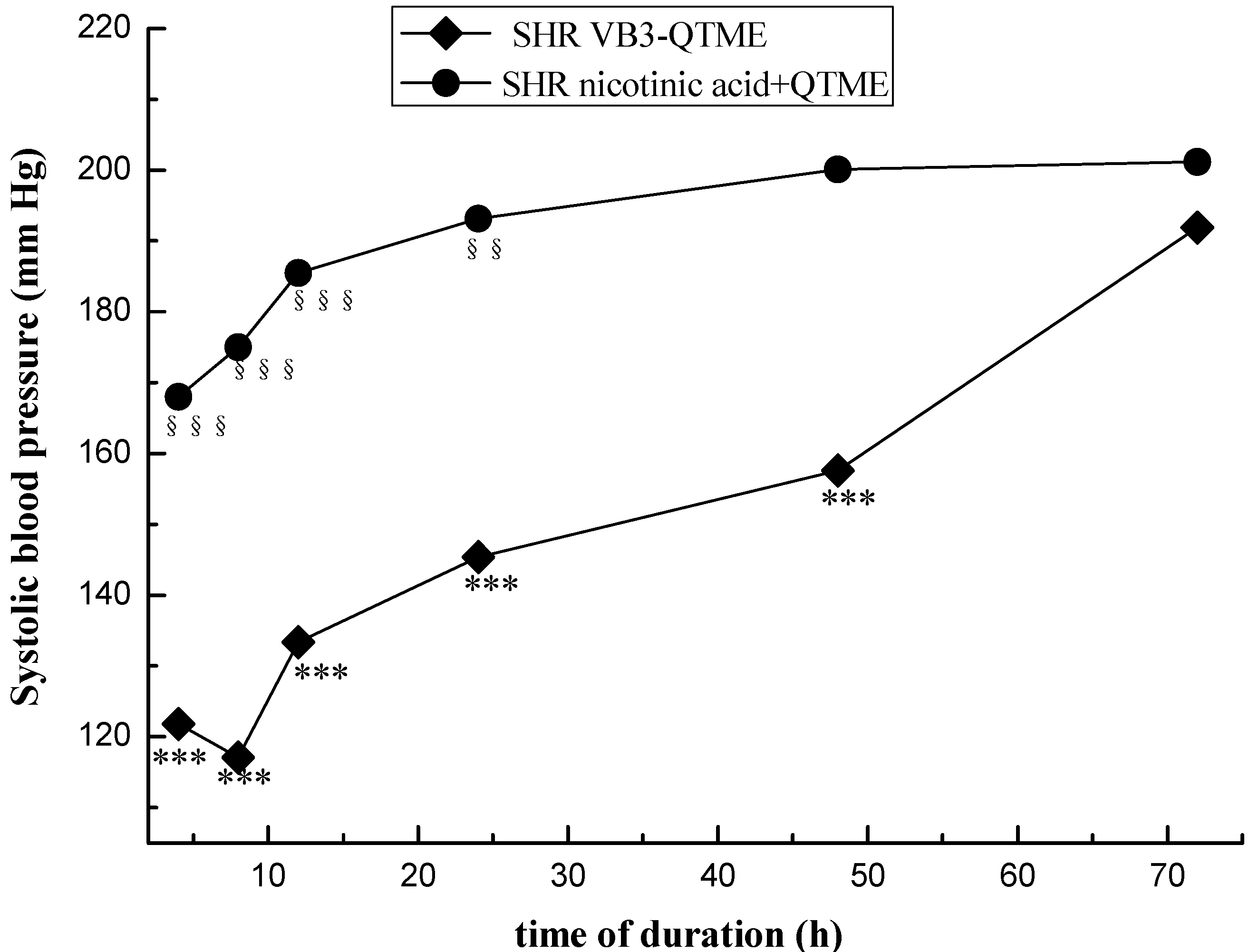
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gammon, K. Drugs: Blood battles. Nature 2013, 493, S14–S15. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Brown, M.J.; Palmer, C.R.; Castaigne, A.; de Leeuw, P.W.; Mancia, G.; Rosenthal, T.; Ruilope, L.M. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000, 356, 366–372. [Google Scholar]
- Wing, L.M.; Reid, C.M.; Ryan, P.; Beilin, L.; Brown, M.; Jennings, G.; Johnston, C.; McNeil, J.; Macdonald, G.; Marley, J.; et al. A comparison of outcomes with angiotensin-converting–enzyme inhibitors and diuretics for hypertension in the elderly. N. Engl. J. Med. 2003, 348, 583–592. [Google Scholar] [CrossRef]
- Staessen, J.A.; Fagard, R.; Thijs, L.; Celis, H.; Arabidze, G.G.; Birkenhäger, W.H.; Bulpitt, C.J.; de Leeuw, P.W.; Dollery, C.T.; Fletcher, A.E.; et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997, 350, 757–764. [Google Scholar] [CrossRef]
- Jamerson, K.; Weber, M.A.; Bakris, G.L.; Dahlöf, B.; Pitt, B.; Shi, V.; Hester, A.; Gupte, J.; Gatlin, M.; Velazquez, E.J. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N. Engl. J. Med. 2008, 359, 2417–2428. [Google Scholar] [CrossRef]
- Bakris, G.; Sarafidis, P.; Agarwal, R.; Ruilope, L. Review of blood pressure control rates and outcomes. J. Am. Soc. Hypertens. 2014, 8, 127–141. [Google Scholar] [CrossRef]
- Tabassum, N.; Ahmad, F. Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev. 2011, 5, 30–40. [Google Scholar] [CrossRef]
- Xiong, X.J.; Yang, X.C.; Liu, Y.M.; Zhang, Y.; Wang, P.Q.; Wang, J. Chinese herbal formulas for treating hypertension in traditional Chinese medicine: Perspective of modern science. Hypertens. Res. 2013, 36, 570–579. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yang, L.Y.; Yang, X.W.; Zhang, X.H. Advances in the first total synthesis of natural flavonoids. Synthetic. Commun. 2013, 23, 3093–3114. [Google Scholar]
- Bhosle, D.; Bharambe, S.; Gairola, N.; Dhaneshwar, S.S. Mutual prodrug concept: Fundamentals and applications. Indian J. Pharm. Sci. 1994, 56, 69–79. [Google Scholar]
- Wu, D.H.; Xu, X.J.; Zhang, M.Z.; Wang, L. A New Practice: Study on the Molecular Mechanism of Traditional Chinese Medicine by Computational Pharmacology Methods: Part 2: Pharmacodynamic Modeling and Distribution on Ligand-Target Space of Effective Components. Lett. Drug Des. Discov. 2011, 8, 1009–1014. [Google Scholar] [CrossRef]
- Cienfuegos-Jovellanos, E.; Quiñones, M.M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef]
- Fonseca-Silva, F.; Inacio, J.D.F.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive oxygen species production by quercetin causes the death of leishmania amazonensis intracellular amastigotes. J. Nat. Prod. 2013, 76, 1505–1508. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, M.K.; Mok, H.; Choo, H.; Chong, Y. Separation of quercetin’s biological activity from its oxidative property through bioisosteric replacement of the catecholic hydroxyl groups with fluorine atoms. J. Agric. Food Chem. 2012, 60, 6499–6506. [Google Scholar] [CrossRef]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (allium cepa) skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009, 61, 67–75. [Google Scholar]
- Yamamoto, Y.; Oue, E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2006, 70, 933–939. [Google Scholar] [CrossRef]
- Franceschini, G.; Favari, E.; Calabresi, L.; Simonelli, S.; Bondioli, A.; Adorni, M.P.; Zimetti, F.; Gomaraschi, M.; Coutant, K.; Rossomanno, S.; et al. Differential effects of fenofibrate and extended-release niacin on high-density lipoprotein particle size distribution and cholesterol efflux capacity in dyslipidemic patients. J. Clin. Lipidol. 2013, 7, 414–422. [Google Scholar] [CrossRef]
- Boden, W.E.; Sidhu, M.S.; Toth, P.P. The therapeutic role of niacin in dyslipidemia management. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 141–158. [Google Scholar]
- Pang, J.; Chan, D.C.; Hamilton, S.J.; Tenneti, V.S.; Watts, G.F.; Barrett, P.H. Effect of niacin on high-density lipoprotein apolipoprotein A-I kinetics in statin-treated patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 427–432. [Google Scholar] [CrossRef]
- Scoffone, H.M.; Krajewski, M.; Zorca, S.; Bereal-Williams, C.; Little, P.; Seamon, C.; Mendelsohn, L.; Footman, E.; Abi-Jaoudeh, N.; Sachdev, V.; et al. Effect of extended-release niacin on serum lipids and on endothelial function in adults with sickle cell anemia and low high-density lipoprotein cholesterol levels. Am. J. Cardiol. 2013, 112, 1499–1504. [Google Scholar] [CrossRef]
- Abdulaeva-Panova, M.V. Effect of nicotinic acid on blood pressure and on electrolyte content of the blood; in hypertension; mechanism of hypotensive action of nicotinic acid. Ter. Arkh. 1952, 24, 30–35. [Google Scholar]
- Ruggieri, R. Various nicotinic acid derivatives & their hypotensive & vasodilatation effects. G. Med. Mil. 1957, 107, 460–462. [Google Scholar]
- Bays, H.E.; Maccubbin, D.; Meehan, A.G.; Kuznetsova, O.; Mitchel, Y.B.; Paolini, J.F. Blood pressure-lowering effects of extended-release niacin alone and extended-release niacin/laropiprant combination: A post hoc analysis of a 24-week, placebo-controlled trial in dyslipidemic patients. Clin. Ther. 2009, 31, 115–122. [Google Scholar] [CrossRef]
- Bays, H.E.; Rader, D.J. Does nicotinic acid (niacin) lower blood pressure? Int. J. Clin. Pract. 2009, 63, 151–159. [Google Scholar] [CrossRef]
- Gadegbeku, C.A.; Dhandayuthapani, A.; Shrayyef, M.Z.; Eqan, B.M. Hemodynamic effects of nicotinic acid infusion in normotensive and hypertensive subjects. Am. J. Hypertens. 2003, 16, 67–71. [Google Scholar] [CrossRef]
- Duarte, J.; Galisteo, M.; Ocete, M.A.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Effects of chronic quercetin treatment on hepatic oxidative status of spontaneously hypertensive rats. Mol. Cell. Biochem. 2001, 221, 155–160. [Google Scholar] [CrossRef]
- Mackraj, I.; Govender, T.; Ramesar, S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J. Cardiovasc. Pharmacol. 2008, 51, 239–245. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin Ameliorates Cardiovascular, Hepatic, and Metabolic Changes in Diet-Induced Metabolic Syndrome in Rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef]
- Larson, A.; Witman, M.A.; Guo, Y.; Ives, S.; Richardson, R.S.; Bruno, R.S.; Jalili, T.; Symons, J.D. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr. Res. 2012, 32, 557–564. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, J.D.; Wang, B.; Lv, Y.J.; Jiang, H.; Liu, G.L.; Qiao, Y.; Ren, M.; Guo, X.F. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity. PLoS One 2013, 8, e72548. [Google Scholar]
- Jalili, T.; Carlstrom, J.; Kim, S.; Freeman, D.; Jin, H.; Wu, T.C.; Litwin, S.E.; Symons, J.D. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J. Cardiovasc. Pharmacol. 2006, 47, 531–541. [Google Scholar] [CrossRef]
- Rangaswamy, S.; Sambamurthy, K. Chemical examination of the leaves of Rhododendron nilagiricum Zenk. Proc. Indian Acad. Sci. 1959, 50, 366–373. [Google Scholar]
- Peng, W.J.; Han, X.W.; Yu, B. Synthesis of C-aryl-flavonoid derivatives via Suzuki-Miyaura coupling reaction. Chin. J. Chem. 2006, 24, 1154–1162. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; Vekemans, J.A.; Bast, A. Peroxynitrite scavengingof flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Chu, H.W.; Wu, H.T.; Lee, Y.J. Regioselective hydroxylationof 2-hydroxychalcones by dimethyldioxirane towards polymethoxylated flavonoids. Tetrahedron 2004, 60, 2647–2655. [Google Scholar] [CrossRef]
- Monteiro, M.M.O.; França-Silva, M.S.; Alves, N.F.B.; Porpino, S.K.P.; Braga, V.A. Quercetin improves baroreflex sensitivity in spontaneously hypertensive rats. Molecules 2012, 17, 12997–13008. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Galindo, P.; González-Manzano, S.; Zarzuelo, M.J.; Gómez-Guzmán, M.; Quintela, A.M.; González-Paramás, A.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; Duarte, J.; Jiménez, R. Different cardiovascular protective effects of quercetin administered orally or intraperitoneally in spontaneously hypertensive rats. Food Funct. 2012, 3, 643–650. [Google Scholar] [CrossRef]
- Häckl, L.P.; Cuttle, G.; Dovichi, S.S.; Lima-Landman, M.T.; Nicolau, M. Inhibition of angiotensin-converting enzyme by quercetin alters the vascular response to bradykinin and angiotensin I. Pharmacology 2002, 65, 182–186. [Google Scholar]
- Duarte, J.; Jimenez, R.; O’Valle, F.; Galisteo, M.; Perez-Palencia, R.; Vargas, F.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J. Hypertens. 2002, 20, 1843–1854. [Google Scholar] [CrossRef]
- Pérez-Vizcaino, F.; Ibarra, M.; Cogolludo, A.L.; Duarte, J.; Zaragoza-Arnaez, F.; Moreno, L.; Lopez-Lopez, G.; Tamargo, J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Exp. Ther. 2002, 302, 66–72. [Google Scholar] [CrossRef]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.; Yang, L.; Cui, S.; Liang, Y.; Zhang, X. Synthesis and Anti-hypertensive Effects of the Twin Drug of Nicotinic Acid and Quercetin Tetramethyl Ether. Molecules 2014, 19, 4791-4801. https://doi.org/10.3390/molecules19044791
Wang Z, Yang L, Cui S, Liang Y, Zhang X. Synthesis and Anti-hypertensive Effects of the Twin Drug of Nicotinic Acid and Quercetin Tetramethyl Ether. Molecules. 2014; 19(4):4791-4801. https://doi.org/10.3390/molecules19044791
Chicago/Turabian StyleWang, Zhonglei, Liyan Yang, Shuai Cui, Yingxi Liang, and Xiaohua Zhang. 2014. "Synthesis and Anti-hypertensive Effects of the Twin Drug of Nicotinic Acid and Quercetin Tetramethyl Ether" Molecules 19, no. 4: 4791-4801. https://doi.org/10.3390/molecules19044791



