Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action
Abstract
:1. Introduction
| Assay | Source of Free Radical | Reaction Mechanisms | Method of Quantification of the Targeted Free Radical | Application to Measure Antioxidant Activity of Coffee | ||
|---|---|---|---|---|---|---|
| Species of the Beans | Roasting Degree of the Beans | Ref. | ||||
| DPPH | Dissolve DPPH in ethanol | SET or HAT |
| Blend of different varieties | Green | [8] |
| Blend of different varieties | Light, medium and dark | [9] | ||||
| Arabica and Robusta | Green | [10] | ||||
| Arabica and Robusta | Arabica (219 °C for 905 s); Robusta (228 °C for 859 s) | [11] | ||||
| ABTS | Oxidize ABTS with potassium persulfate | HAT |
| Blend of 80% Arabica and 20% Robusta | Green, light, medium and dark | [12] |
| Arabica, Robusta and a blend of these two | Green, medium and dark | [13] | ||||
| Arabica | Light (225 °C for 3 min); medium (233 °C for 3 min); dark (240 °C for 3 min) | [14] | ||||
| Arabica and Robusta | Arabica (219 °C for 905 s); Robusta (228 °C for 859 s) | [11] | ||||
| FRAP | Fe3+/tripyridyltriazine complex | SET | Measure the absorption of ferrous at 593 nm | Blend of different species | Green | [8] |
| Arabica | Medium, dark and blend of medium (70%) and dark (30%) | [15] | ||||
| Arabica and Robusta | Green | [10] | ||||
| ORAC | Dissolve AAPH in buffer to form peroxyl radicals | HAT |
| Arabica | Not declared | [16] |
| Arabica and Robusta | Light, medium, and dark | [17] | ||||
| Arabica | Green | [18] | ||||
| HO− Scavenging Assay |
| Not defined |
| Arabica and Robusta | Green | [10] |
| Not declared | Not declared | [19] | ||||
| Arabica | Green | [18] | ||||
| Arabica and Robusta | 190 ± 3 °C for 18–20 min | [20] | ||||
| O2− Scavenging Capacity Assay |
| Not defined |
| Arabica | Green | [18] |

2. In Vitro Assays Commonly Used to Evaluate Antioxidant Activity of Coffee and Mechanisms of Action
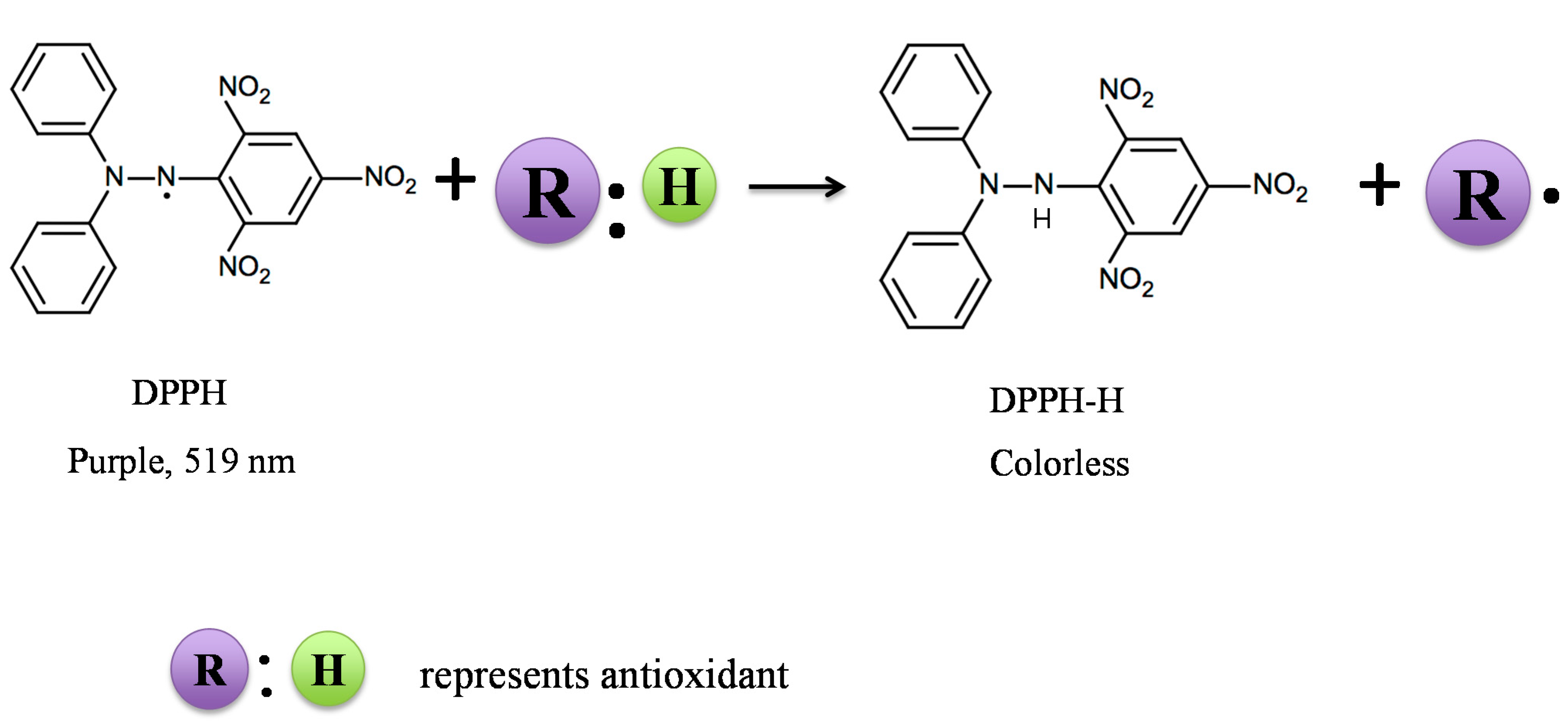
2.1. DPPH Assay
2.2. ABTS Assay
2.3. FRAP and TRAP Assays
2.4. ORAC Assay
2.5. Hydroxyl Radical Scavenging Assay
2.6. O2− Scavenging Capacity Assay
3. Coffee Components with Antioxidant Activity (Chemical Assays)
3.1. Caffeine
3.2. Chlorogenic Acids
3.3. Coffee Maillard Reaction Products
4. Coffee Components with Antioxidant Capacity (Cellular Antioxidant Activity Assays and Animal Studies)
4.1. Caffeine
4.2. Chlorogenic Acid
| Coffee Components | Cell-Based Studies | Animal-Based Studies | ||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | Oxidative Stress Stimulator | Description of Oxidative Stress after Coffee Component Treatment, Compared to the Negative Control | Ref. | Animal | Oxidative Stress Stimulator | Description of Oxidative Status after Coffee Component Treatment Compared to the Negative Control | Ref. | |
| Caffeine | Pulmonary epithelial A549 cell | hyperoxia | ↓ROS level | [86] | Rat | None | ↑GR, ↑GSH, ↑SOD, (No change) in GPx in brain | [88] |
| MLE 12 | hyperoxia | ↓ROS level | [86] | Mice | 5% ethanol in diet | ↓ROS, ↓TNF-α,↓ proinflammatory cytokines and chemokines in liver | [89] | |
| Human skin fibroblast WS-1 cell | H2O2 | ↓ROS level; ↓4-hydroxy-2-nonenal | [87] | Rabbit | Cholesterol-enriched diet | ↑GSH, ↓ROS, ↓8-Isoprostaglandin F2α | [90] | |
| Chlorogenic acid | Human HaCaT keratinocyte | UVB irradiation | ↓DNA damage, ↓Apoptotic bodies, ↓Apoptosis-related proteins, ↑Cell viability | [91] | Rat | Paraquat | ↑Liver triacylglycerol, ↑Phospholipid | [96] |
| Mesenchymal stem cell | H2O2 | ↑Expression of FOXO family genes, ↓Apoptosis | [92] | Rat | High fat diet/streptozotocin treated | ↓Thiobarbituric acid, ↑SOD, ↑Catalase | [97] | |
| PC12 cell | t-BOOH, or H2O2, or FeSO4 | ↑GSH, ↓MDA | [93] | |||||
| Human hepatoma HepG2 cell | t-BOOH | ↑GSH | [94] | |||||
| Melanoidins | Human hepatoma HepG2 cell | t-BOOH | ↑GSH, ↓MDA | [98] | Rat | High-fat, high-calorie solid diet | ↓Pro-inflammatory cytokines, ↑Anti-inflammatory cytokines | [99] |
| Human neuroblastoma cell IMR32 | H2O2 | ↑Cell viability | [100] | |||||
| Trigonelline | Not available | Rat | Streptozotocin treated | ↑SOD, ↑Catalase, ↑GSH, ↓MDA, ↓NO | [101] | |||
| Cafestol and kahweol | Neuronal cell line SH-SY5Y | 6-Hydroxydopamine | ↑Nrf2 nuclear translocation, | [102] | Mice | CCl4 | ↑GSH, ↓MDA | [103] |
| NIH3T3 cell | H2O2 | ↓ TBARS, ↓ ROS, ↓ DNA damage | [104] | |||||
4.3. Coffee Maillard Reaction Products
4.4. Trigonelline
4.5. Cafestol and Kahweol
5. Antioxidant Activity of Coffee in Human Studies
| Participants and Exclusion Criteria | Experiment Design and Treatment Conditions | Indicators/Biomarkers of Oxidative Status | Results and Conclusion | Ref. | |
|---|---|---|---|---|---|
| N = not given; Gender: not given; Age: not given; Eligibility: healthy, non-smoking, moderate coffee drinker; avoid antioxidant supplements and have a diet low in “coffee, wine, chocolate, tea, fruit and vegetable” in the two days before the experiment. | Experiment group: 200 mL of coffee (60 g of roasted ground coffee beans were prepared by a 5-min infusion in 1 L of boiling water); Control: 200 mL of tea. | Plasma antioxidant activity; plasma SH groups | Results: Experiment group: significantly ↑ plasma antioxidant capacity measured by TRAP method; the plasma SH group did not change significantly. Control: Non-significant change in both parameters. Conclusions: Coffee consumption improves antioxidant capacity in vivo. | [108] | |
| N = 36; Gender: both; Age: 27 ± 8; Eligibility: healthy, non-smoking adults with BMI 20–25, no intake of drugs and supplements four week prior the study, no pregnancy and no blood withdrawal three weeks before the study. | Experiment group: instant coffee (800 mL/day for five days) co-extracted from green and roasted beans Control: 800 mL water/day for five days | 8-Isoprostaglandin F2α; DNA migration; MDA; GPx; GST; SOD; intracellular ROS | Results: Experiment group showed a 15.3% 8-isoprostaglandin F2α decrease in urine and 16.1% 3-nitrotyrosine compared to the control group. Other parameters did not change significantly compared to the control. Conclusion: Coffee consumption protects humans against oxidative damage. | [109] | |
| N = 38; Gender: 14 males and 24 females Age: 27.6 ± 8.0 Eligibility: healthy, non-smokers, no intake of pharmaceutical drugs, no intake of food supplements four weeks prior and during the study, no pregnancy, compliance with the protocol, no blood withdrawal three weeks before the study. | Cross-over design: participants were allocated into two groups (18 coffee/water and 20 water/coffee); the coffee/water group drank 800 mL coffee/day for five days, after a five-week washout phase and a one-week restriction (800 mL water/day); the water/coffee group followed the reverse order | Oxidized purines, MDA, 3-nitrotyrosine, glutathione, intracellular ROS, SOD and GPx, 8-isoprostaglandin F2α | Results: Coffee intake decreased DNA-damage (oxidized purines) by 12.3%; coffee intake did not markedly alter other redox status parameters. Conclusion: Coffee consumption prevents endogenous formation of oxidative DNA-damage in humans. | [110] | |
| N = 40; Gender: not given Age: not given Eligibility: biopsy-proven HCV-related chronic hepatitis or cirrhosis, no consumption of other caffeine-containing beverages | Cross-over design: participants were allocated into Groups 1 and 2: Group 1 drank four cups coffee/day for one month and had the first blood sample taken, then continued with no coffee for 30 days and had the second blood sample taken). Group 2 followed the reverse order. | Makers of oxidative damage: 8-hydroxydeoxyguanosine, nitric oxide, advanced oxidation protein products | Results: 8-hydroxydeoxyguanosine was significantly lower than during abstinence. Conclusion: In chronic hepatitis C patients, coffee consumption induces a reduction in oxidative damage. | [112] | |
| N = 18; Gender: male; Age: not given; Eligibility: non-smoking, BMI < 32, not on medication and does not have chronic disease, regular coffee drinker, restricted intake of coffee, caffeinated products, dietary supplements and foods rich in polyphenols. | Intervention trial: Four-week wash out period, four weeks of brewed coffee (prepared from a blend of green and roasted beans; 29.5 g ground coffee in 600 mL water) consumption (750 mL/day); and another eight weeks of wash out. | Nrf2 gene transcription level in blood sample | Results: Thirty six percent of participants displayed a significant >1.5 alteration of Nrf2 transcription after coffee consumption compared to the wash out period; 64% of the participants showed no change. Conclusion : Induction of Nrf2 gene transcription by coffee in humans depends on the genotype of subjects. | [113] | |
| N = 29; Gender: not given; Age: not given; Eligibility: healthy volunteers | Intervention trial: Four-week wash out period, four weeks of brewed coffee (prepared from a blend of green and roasted beans; 29.5 g ground coffee in 600 mL water) consumption (750 mL/day); and another four weeks of wash out. | Nrf2 transcription level in peripheral blood lymphocytes | Results: Coffee consumption increased Nrf2 transcription in peripheral blood lymphocytes on average. Conclusion: Coffee acts as a modulator of Nrf2-depended gene response in humans, but genetic polymorphisms play an important role in the individual response pattern. | [114] | |
| N = 47; Gender: 11 male and 36 female; Age: 54 ± 9; Eligibility: habitual coffee drinkers, free of type 2 diabetes. | Single blind, three-stage clinical trial: One-month wash out period; 600 mL filtered coffee per day for one month, followed by 1,200 mL filtered coffee per day for another month. | IL-18, IL-6, macrophage migration inhibitory factor, leptin, C-reactive protein, serum amyloid A, 8-isoprostane, nitrotyrosine, LDL:HDL cholesterol | Results: Significant ↓ in IL-18, 8-isoprostane, LDL:HDL cholesterol. Conclusion: Coffee consumption appears to have beneficial effects on subclinical inflammation. | [115] | |
| N = 16; Gender: 8 male and 8 female; Age: 29.2 ± 14.4; Eligibility: non-smoking, healthy, without a history of cardiovascular or metabolic disease, did not subject use medications or dietary supplements throughout the study period. | Random, crossover design: Each subject has three visits (one visit every day); at each visit, the subject firstly consumes a milk shake (containing 12.2 kCal/kg of body weight) followed by consuming either 480 mL of freshly brewed caffeinated coffee, or decaffeinated coffee, or water. The order of assignment for the three days of testing was random; each subject received all three conditions over the course of the study. | MDA, H2O2, triglycerides | Results: Coffee had no impact on MDA, H2O2 or triglyceride level in blood. Conclusion: Acute coffee consumption following a high-fat milk shake has no impact on postprandial oxidative stress. | [116] |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gunalan, G.; Myla, N.; Balabhaskar, R. In vitro antioxidant analysis of selected coffee bean varieities. J. Chem. Pharm. Res. 2012, 4, 2126–2132. [Google Scholar]
- Fogliano, V.; Morales, F.J. Estimation of dietary intake of melanoidins from coffee and bread. Food Funct. 2011, 2, 117–123. [Google Scholar]
- Reichardt, N.; Gniechwitz, D.; Steinhart, H.; Bunzel, M.; Blaut, M. Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol. Nutr. Food Res. 2009, 53, 287–299. [Google Scholar]
- Morales, F.J.; Jiménez-pérez, S. Peroxyl radical scavenging activity of melanoidins in aqueous systems. Eur. Food Res. Technol. 2004, 218, 515–520. [Google Scholar]
- Mader, E.A.; Davidson, E.R.; Mayer, J.M. Large ground-state entropy changes for hydrogen atom transfer reactions of iron complexes. J. Am. Chem. Soc. 2007, 129, 5153–5166. [Google Scholar]
- Ashby, E.C. Single-electron transfer, a major reaction pathway in organic chemistry. An answer to recent criticisms. Acc. Chem. Res. 1988, 21, 414–421. [Google Scholar]
- Shalaby, E.A.; Shanab, S.M.M. Antioxidant compounds, assays of determination and mode of action. Afr. J. Pharm. Pharmacol. 2013, 7, 528–539. [Google Scholar]
- Ramalakshmi, K.; Rahath Kubra, I.; Jagan Mohan Rao, L. Antioxidant potential of low-grade coffee beans. Food Res. Int. 2008, 41, 96–103. [Google Scholar]
- Anese, M.; Nicoli, M.C. Antioxidant properties of ready-to-drink coffee brews. J. Agric. Food Chem. 2003, 51, 942–946. [Google Scholar]
- Madhava Naidu, M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; de Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. Int. 2012, 48, 57–64. [Google Scholar]
- Cammerer, B.; Kroh, L.W. Antioxidant activity of coffee brew. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar]
- Sacchetti, G.; di Mattia, C.; Pittia, P.; Mastrocola, D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar]
- Del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar]
- Sánchez-González, I.; Jiménez-Escrig, A.; Saura-Calixto, F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter). Food Chem. 2005, 90, 133–139. [Google Scholar]
- Chu, Y.-F.; Brown, P.H.; Lyle, B.J.; Chen, Y.; Black, R.M.; Williams, C.E.; Lin, Y.-C.; Hsu, C.-W.; Cheng, I.H. Roasted Coffees High in Lipophilic Antioxidants and Chlorogenic Acid Lactones Are More Neuroprotective than Green Coffees. J. Agric. Food Chem. 2009, 57, 9801–9808. [Google Scholar]
- Kwon, D.Y.; Choi, K.H.; Kim, S.J.; Choi, D.W.; Kim, Y.S.; Kim, Y.C. Peroxyl radical-scavenging activity of coffee brews. Eur. Food Res. Technol. 2005, 221, 471–477. [Google Scholar]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.N.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762. [Google Scholar]
- Brezová, V.; Šlebodová, A.; Staško, A. Coffee as a source of antioxidants: An EPR study. Food Chem 2009, 114, 859–868. [Google Scholar]
- Parras, P.; Martínez-Tomé, M.; Jiménez, A.M.; Murcia, M.A. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem. 2007, 102, 582–592. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar]
- Antonio, C. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem. Anal. 1998, 9, 196–202. [Google Scholar]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determing antioxidant potential of water and methanol extracts of spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar]
- Bravo, J.; Monente, C.; Juániz, I.; de Peña, M.P.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar]
- Svilaas, A.; Sakhi, A.K.; Andersen, L.F.; Svilaas, T.; Strom, E.C.; Jacobs, D.R., Jr.; Ose, L.; Blomhoff, R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J. Nutr. 2004, 134, 562–567. [Google Scholar]
- Yanagimoto, K.; Lee, K.-G.; Ochi, H.; Shibamoto, T. Antioxidative activity of heterocyclic compounds found in coffee volatiles produced by maillard reaction. J. Agric. Food Chem. 2002, 50, 5480–5484. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Valadao, V.S.J.; Queiroz, Y.S.; Gotlieb, S.L.D.; da Silva Torres, E.A.F. Stability of phenolic compounds and antioxidant capacity of regular and decaffeinated coffees. Braz. Arch. Biol. Technol. 2014, 57, 110–118. [Google Scholar]
- Wayner, D.D.; Burton, G.W.; Ingold, K.U.; Barclay, L.R.; Locke, S.J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim. Biophys. Acta 1987, 924, 408–419. [Google Scholar]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar]
- Kitts, D.D.; Hu, C. Biological and chemical assessment of antioxidant activity of sugar-lysine model maillard reaction products. Ann. N. Y. Acad. Sci. 2005, 1043, 501–512. [Google Scholar]
- Liu, Y.; Kitts, D.D. Activation of antioxidant response element (ARE)-dependent genes by roasted coffee extracts. Food Funct. 2012, 3, 950–954. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar]
- Gómez-Ruiz, J.Á.; Leake, D.S.; Ames, J.M. In Vitro Antioxidant Activity of Coffee Compounds and Their Metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar]
- Klein, S.M.; Cohen, G.; Cederbaum, A.I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry 1981, 20, 6006–6012. [Google Scholar]
- Gutteridge, J.M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar]
- Althaus, J.; Andrus, P.; Williams, C.; VonVoigtlander, P.; Cazers, A.; Hall, E. The use of salicylate hydroxylation to detect hydroxyl radical generation in ischemic and traumatic brain injury. Mol. Chem. Neuropathol. 1993, 20, 147–162. [Google Scholar]
- Wijewickreme, A.N.; Kitts, D.D. Modulation of metal-induced genotoxicity by Maillard reaction products isolated from coffee. Food Chem. Toxicol. 1998, 36, 543–553. [Google Scholar]
- Li, L.; Abe, Y.; Kanagawa, K.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. Distinguishing the 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-OH radical quenching effect from the hydroxyl radical scavenging effect in the ESR spin-trapping method. Anal. Chim. Acta 2004, 512, 121–124. [Google Scholar]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140. [Google Scholar]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar]
- Fox, G.P.; Wu, A.; Yiran, L.; Force, L. Variation in caffeine concentration in single coffee beans. J. Agric. Food Chem. 2013, 61, 10772–10778. [Google Scholar]
- Casal, S.; Beatriz Oliveira, M.; Ferreira, M.A. HPLC/diode-array applied to the thermal degradation of trigonelline, nicotinic acid and caffeine in coffee. Food Chem. 2000, 68, 481–485. [Google Scholar]
- Tfouni, S.A.V.; Carreiro, L.B.; Teles, C.R.A.; Furlani, R.P.Z.; Cipolli, K.M.V.A.B.; Camargo, M.C.R. Caffeine and chlorogenic acids intake from coffee brew: Influence of roasting degree and brewing procedure. Int. J. Food Sci. Technol. 2014, 49, 747–752. [Google Scholar]
- McCusker, R.R.; Fuehrlein, B.; Goldberger, B.A.; Gold, M.S.; Cone, E.J. Caffeine content of decaffeinated coffee. J. Anal. Toxicol. 2006, 30, 611–613. [Google Scholar]
- León-Carmona, J.R.; Galano, A. Is Caffeine a Good Scavenger of Oxygenated Free Radicals? J.Phys. Chem. B 2011, 115, 4538–4546. [Google Scholar]
- Shi, X.; Dalal, N.S.; Jain, A.C. Antioxidant behaviour of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem. Toxicol. 1991, 29, 1–6. [Google Scholar]
- Kumar, S.S.; Devasagayam, T.P.; Jayashree, B.; Kesavan, P.C. Mechanism of protection against radiation-induced DNA damage in plasmid pBR322 by caffeine. Int. J. Radiat. Biol. 2001, 77, 617–623. [Google Scholar]
- Lee, C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin. Chim. Acta 2000, 295, 141–154. [Google Scholar]
- Devasagayam, T.P.A.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys. Acta 1282, 63–70. [Google Scholar]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar]
- Clifford, M.N. Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar]
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J. Agric. Food Chem. 2001, 49, 4646–4655. [Google Scholar]
- Magadula, J.J.; Tewtrakul, S.; Gatto, J.; Richomme, P. In vitro antioxidant and anti-HIV-1 protease (PR) activities of two Clusiaceae plants endemic to Tanzania. Int. J. Biol. Chem. Sci. 2011, 5, 1096–1104. [Google Scholar]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar]
- Namiki, M. Chemistry of Maillard reactions: Recent studies on the browning reaction mechanism and the development of antioxidants and mutagens. Adv. Food Res. 1988, 32, 115–184. [Google Scholar]
- Rufián-Henares, J.A.; Morales, F.J. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res. Int. 2007, 40, 995–1002. [Google Scholar]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical characterization and antioxidant properties of coffee melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar]
- Gniechwitz, D.; Reichardt, N.; Ralph, J.; Blaut, M.; Steinhart, H.; Bunzel, M. Isolation and characterisation of a coffee melanoidin fraction. J. Sci. Food Agric. 2008, 88, 2153–2160. [Google Scholar]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar]
- Delgado-Andrade, C.; Rufian-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar]
- Morales, F.J. Assessing the non-specific hydroxyl radical scavenging properties of melanoidins in a Fenton-type reaction system. Anal. Chim. Acta 2005, 534, 171–176. [Google Scholar]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar]
- Rufian-Henares, J.A.; Morales, F.J. Effect of in vitro enzymatic digestion on antioxidant activity of coffee melanoidins and fractions. J. Agric. Food Chem. 2007, 55, 10016–10021. [Google Scholar]
- Samunl, A.; Neta, P. Electron spin resonance study of the reaction of hydroxyl radicals with pyrrole, imidazole, and related compound. J. Phys. Chem. 1973, 77, 1629–1635. [Google Scholar]
- Zhang, D.; Xie, L.; Wei, Y.; Liu, Y.; Jia, G.; Zhou, F.; Ji, B. Development of a cell-based antioxidant activity assay using dietary fatty acid as oxidative stressor. Food Chem. 2013, 141, 347–356. [Google Scholar]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsi, F. Evaluation of the stability and antioxidant activity of nanoencapsulated resveratrol during in vitro digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar]
- Ziberna, L.; Lunder, M.; Moze, S.; Vanzo, A.; Tramer, F.; Passamonti, S.; Drevensek, G. Acute cardioprotective and cardiotoxic effects of bilberry anthocyanins in ischemia-reperfusion injury: Beyond concentration-dependent antioxidant activity. Cardiovasc. Toxicol. 2010, 10, 283–294. [Google Scholar]
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009, 114, 263–269. [Google Scholar]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar]
- Boettler, U.; Sommerfeld, K.; Volz, N.; Pahlke, G.; Teller, N.; Somoza, V.; Lang, R.; Hofmann, T.; Marko, D. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J. Nutr. Biochem. 2011, 22, 426–440. [Google Scholar]
- Tiwari, K.K.; Chu, C.; Couroucli, X.; Moorthy, B.; Lingappan, K. Differential concentration-specific effects of caffeine on cell viability, oxidative stress, and cell cycle in pulmonary oxygen toxicity in vitro. Biochem. Biophys. Res. Commun. 2014, 450, 1345–1350. [Google Scholar]
- Jagdeo, J.; Brody, N. Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. J. Drugs Dermatol. 2011, 10, 753–761. [Google Scholar]
- Abreu, R.V.; Silva-Oliveira, E.M.; Moraes, M.F.; Pereira, G.S.; Moraes-Santos, T. Chronic coffee and caffeine ingestion effects on the cognitive function and antioxidant system of rat brains. Pharmacol. Biochem. Behav. 2011, 99, 659–664. [Google Scholar]
- Lv, X.; Chen, Z.; Li, J.; Zhang, L.; Liu, H.; Huang, C.; Zhu, P. Caffeine protects against alcoholic liver injury by attenuating inflammatory response and oxidative stress. Inflamm. Res. 2010, 59, 635–645. [Google Scholar]
- Prasanthi, J.R.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The Polyphenol Chlorogenic Acid Attenuates UVB-mediated Oxidative Stress in Human HaCaT Keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar]
- Li, S.; Bian, H.; Liu, Z.; Wang, Y.; Dai, J.; He, W.; Liao, X.; Liu, R.; Luo, J. Chlorogenic acid protects MSCs against oxidative stress by altering FOXO family genes and activating intrinsic pathway. Eur. J. Pharmacol. 2012, 674, 65–72. [Google Scholar]
- Pavlica, S.; Gebhardt, R. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic. Res. 2005, 39, 1377–1390. [Google Scholar]
- Baeza, G.; Amigo-Benavent, M.; Sarriá, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res. Int. 2014, 62, 1038–1046. [Google Scholar]
- Boettler, U.; Volz, N.; Pahlke, G.; Teller, N.; Kotyczka, C.; Somoza, V.; Stiebitz, H.; Bytof, G.; Lantz, I.; Lang, R.; et al. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol. Nutr. Food Res. 2011, 55, 798–802. [Google Scholar]
- Tsuchiya, T.; Suzuki, O.; Igarashi, K. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 1996, 60, 765–768. [Google Scholar]
- Amjid, A.; Pawan, K. Protective effect of chlorogenic acid against diabetic nephropathy in high fat diet/streptozotocin induced type-2 diabetic rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 489–495. [Google Scholar]
- Goya, L.; Delgado-Andrade, C.; Rufian-Henares, J.A.; Bravo, L.; Morales, F.J. Effect of coffee melanoidin on human hepatoma HepG2 cells. Protection against oxidative stress induced by tert-butylhydroperoxide. Mol. Nutr. Food Res. 2007, 51, 536–545. [Google Scholar]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar]
- Daglia, M.; Racchi, M.; Papetti, A.; Lanni, C.; Govoni, S.; Gazzani, G. In vitro and ex vivo antihydroxyl radical activity of green and roasted coffee. J. Agric. Food Chem. 2004, 52, 1700–1704. [Google Scholar]
- Zhou, J.; Zhou, S.; Zeng, S. Experimental diabetes treated with trigonelline: Effect on beta cell and pancreatic oxidative parameters. Fundam. Clin. Pharmacol. 2013, 27, 279–287. [Google Scholar]
- Hwang, Y.P.; Jeong, H.G. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008, 582, 2655–2662. [Google Scholar]
- Lee, K.J.; Choi, J.H.; Jeong, H.G. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem. Toxicol. 2007, 45, 2118–2125. [Google Scholar]
- Lee, K.J.; Jeong, H.G. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol. Lett. 2007, 173, 80–87. [Google Scholar]
- Lang, R.; Wahl, A.; Stark, T.; Hofmann, T. Urinary N-methylpyridinium and trigonelline as candidate dietary biomarkers of coffee consumption. Mol. Nutr. Food Res. 2011, 55, 1613–1623. [Google Scholar]
- Somoza, V.; Lindenmeier, M.; Wenzel, E.; Frank, O.; Erbersdobler, H.F.; Hofmann, T. Activity-Guided Identification of a Chemopreventive Compound in Coffee Beverage Using in Vitro and in Vivo Techniques. J. Agric. Food Chem. 2003, 51, 6861–6869. [Google Scholar]
- Higgins, L.G.; Cavin, C.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol. Appl. Pharmacol. 2008, 226, 328–337. [Google Scholar]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar]
- Hoelzl, C.; Knasmuller, S.; Wagner, K.H.; Elbling, L.; Huber, W.; Kager, N.; Ferk, F.; Ehrlich, V.; Nersesyan, A.; Neubauer, O.; et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol. Nutr. Food Res. 2010, 54, 1722–1733. [Google Scholar]
- Mišík, M.; Hoelzl, C.; Wagner, K.-H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat. Res. 2010, 692, 42–48. [Google Scholar]
- Hori, A.; Kasai, H.; Kawai, K.; Nanri, A.; Sato, M.; Ohta, M.; Mizoue, T. Coffee Intake is Associated With Lower Levels of Oxidative DNA Damage and Decreasing Body Iron Storage in Healthy Women. Nutr. Cancer 2014, 66, 964–969. [Google Scholar]
- Cardin, R.; Piciocchi, M.; Martines, D.; Scribano, L.; Petracco, M.; Farinati, F. Effects of coffee consumption in chronic hepatitis C: A randomized controlled trial. Dig. Liver Dis. 2013, 45, 499–504. [Google Scholar]
- Boettler, U.; Volz, N.; Teller, N.; Haupt, L.M.; Bakuradze, T.; Eisenbrand, G.; Bytof, G.; Lantz, I.; Griffiths, L.R.; Marko, D. Induction of antioxidative Nrf2 gene transcription by coffee in humans: Depending on genotype? Mol. Biol. Rep. 2012, 39, 7155–7162. [Google Scholar]
- Volz, N.; Boettler, U.; Winkler, S.; Teller, N.; Schwarz, C.; Bakuradze, T.; Eisenbrand, G.; Haupt, L.; Griffiths, L.R.; Stiebitz, H.; et al. Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 9631–9641. [Google Scholar]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar]
- Bloomer, R.J.; Trepanowski, J.F.; Farney, T.M. Influence of acute coffee consumption on postprandial oxidative stress. Nutr. Metab. Insights 2013, 6, 35–42. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180-19208. https://doi.org/10.3390/molecules191119180
Liang N, Kitts DD. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules. 2014; 19(11):19180-19208. https://doi.org/10.3390/molecules191119180
Chicago/Turabian StyleLiang, Ningjian, and David D. Kitts. 2014. "Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action" Molecules 19, no. 11: 19180-19208. https://doi.org/10.3390/molecules191119180





