Identification of Natural Compound Carnosol as a Novel TRPA1 Receptor Agonist
Abstract
:1. Introduction
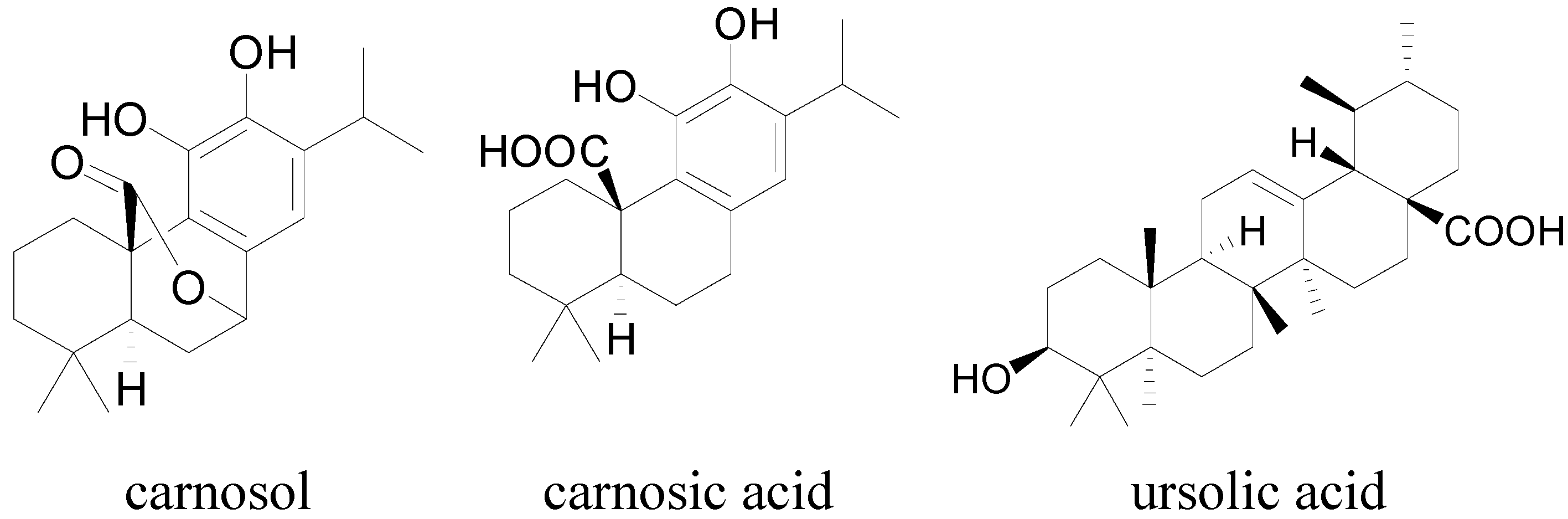
2. Results and Discussion
2.1. HEK293/TRPA1 Cell Line Validation
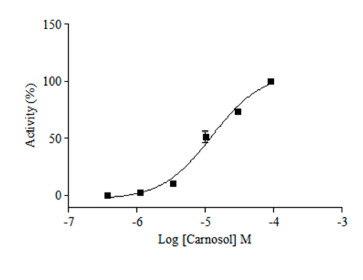
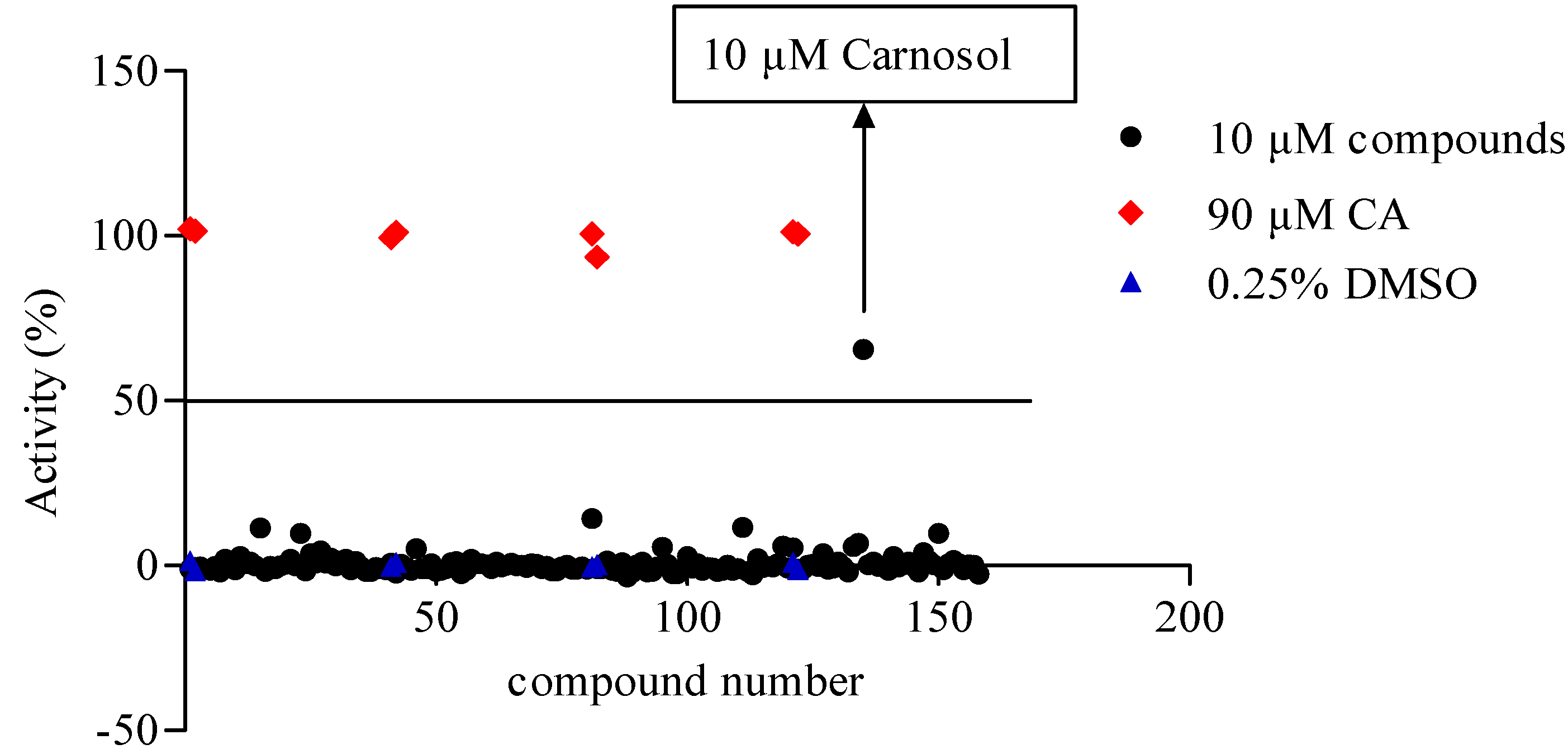
2.2. TRPA1 Agonist Primary Screening

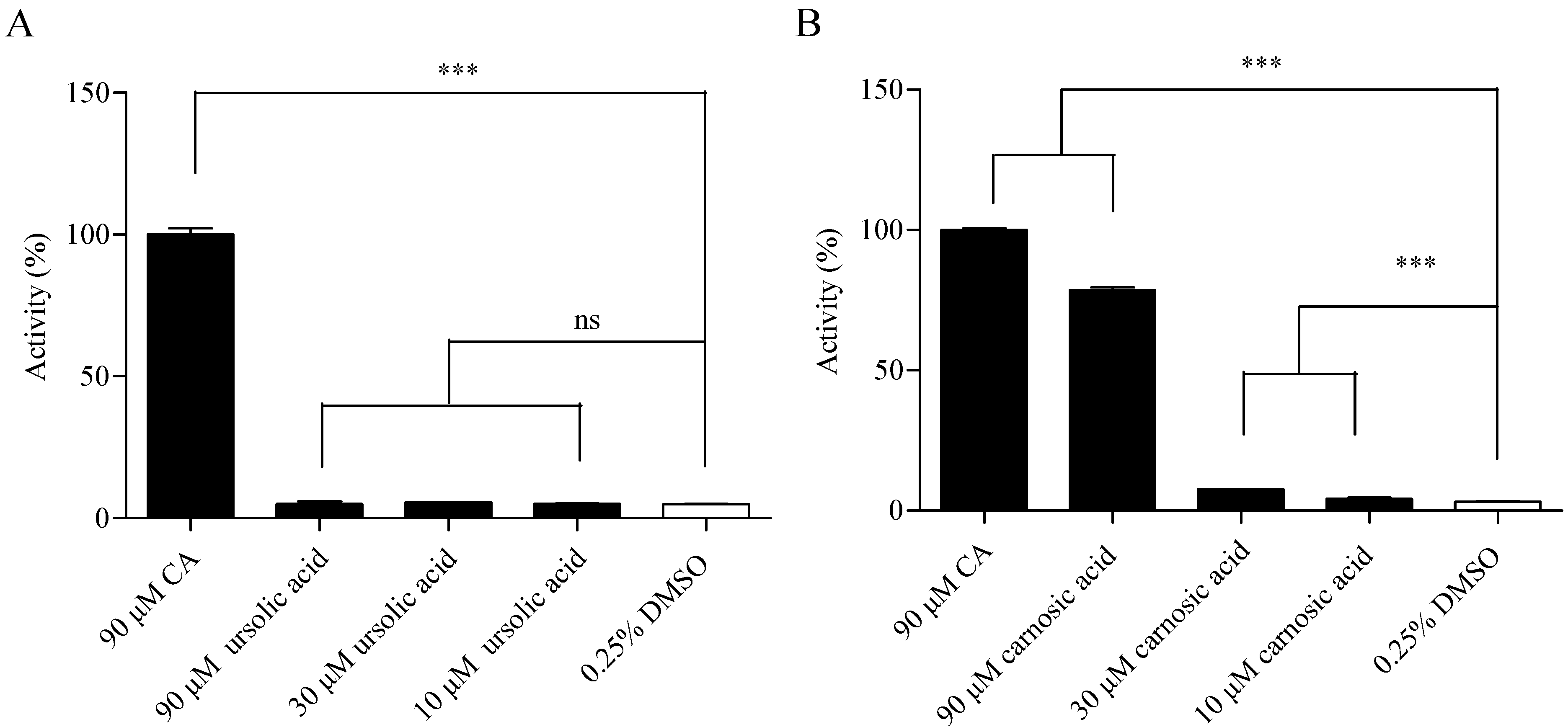
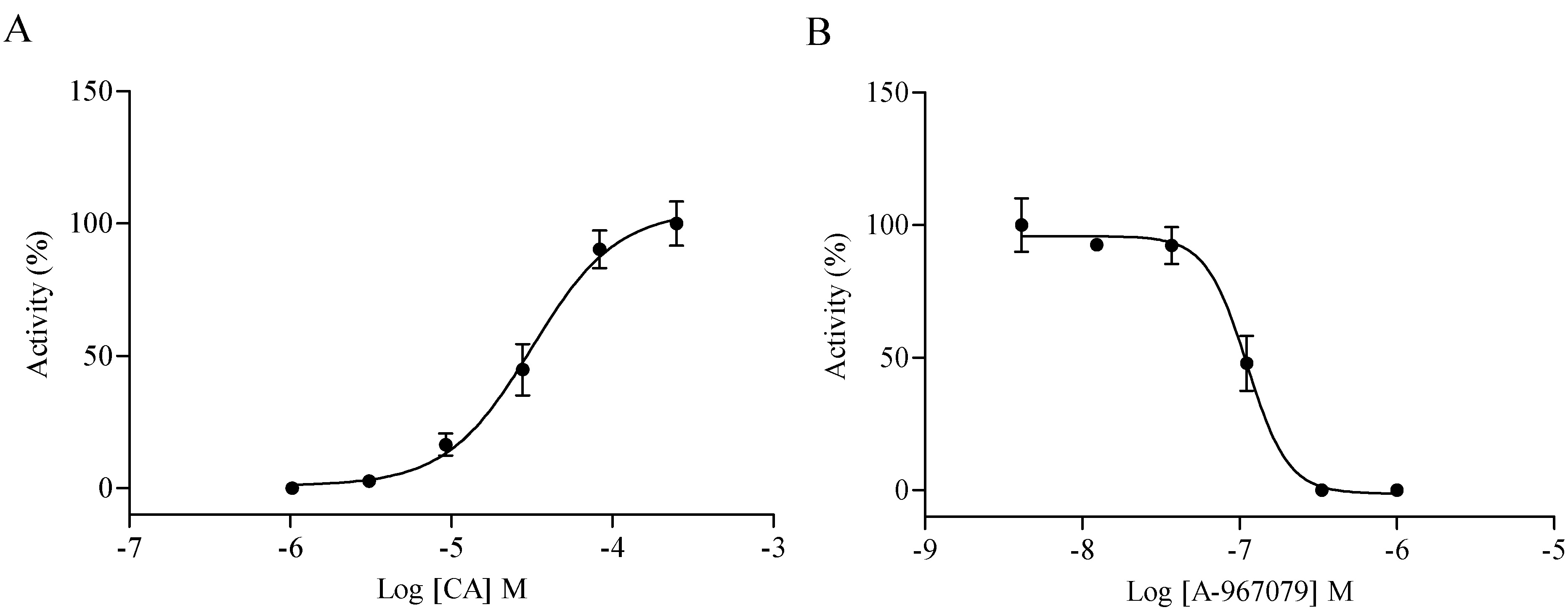
2.3. Compound Specificity Determination
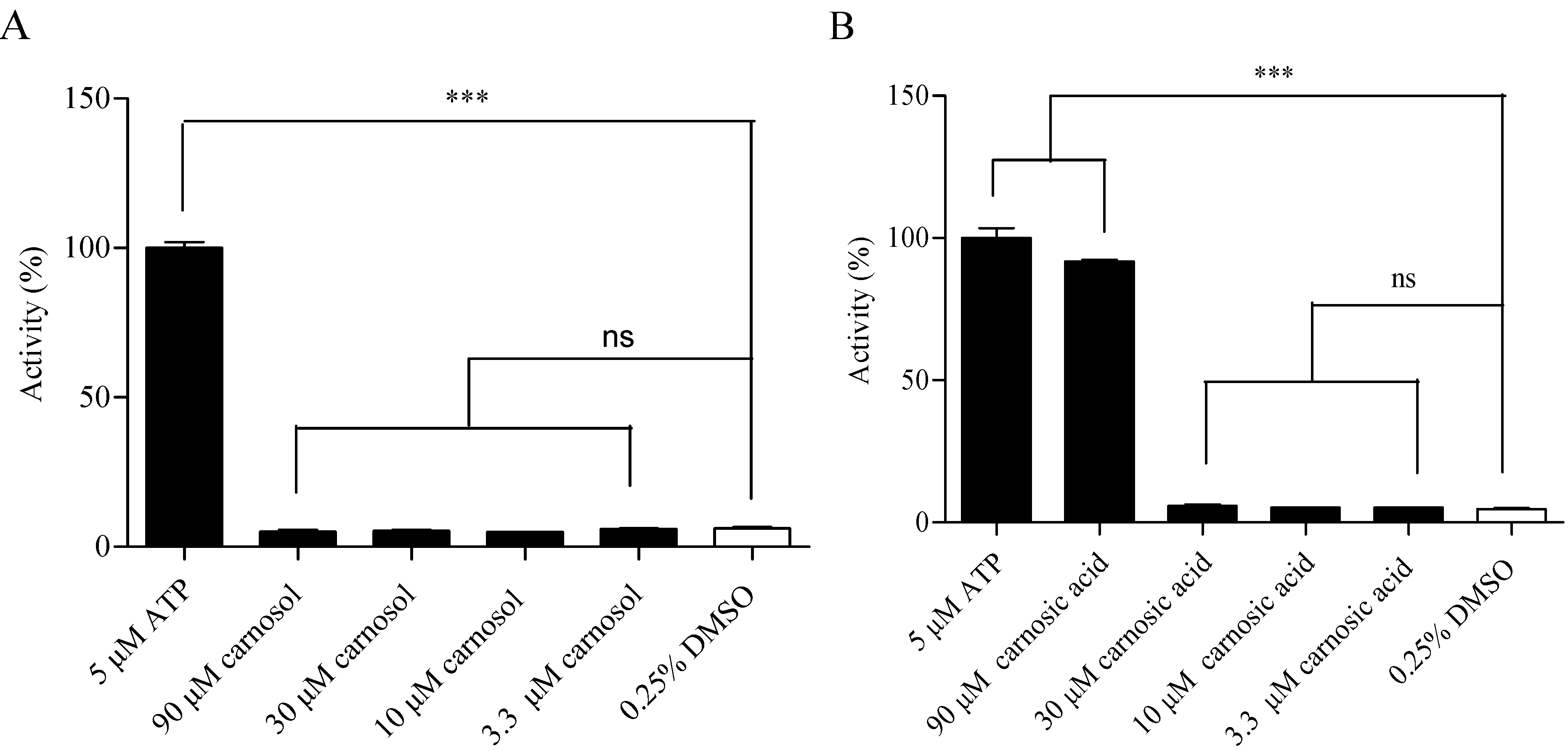
2.4. Hit Verification
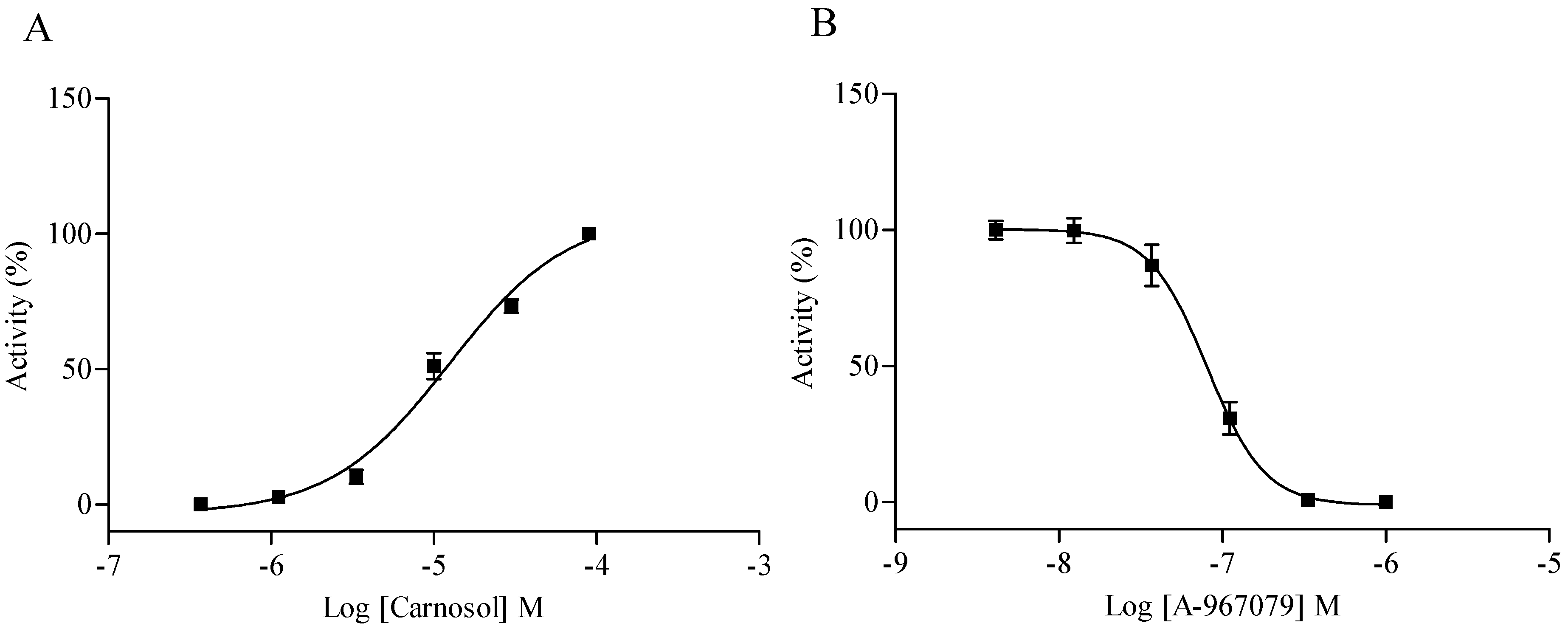
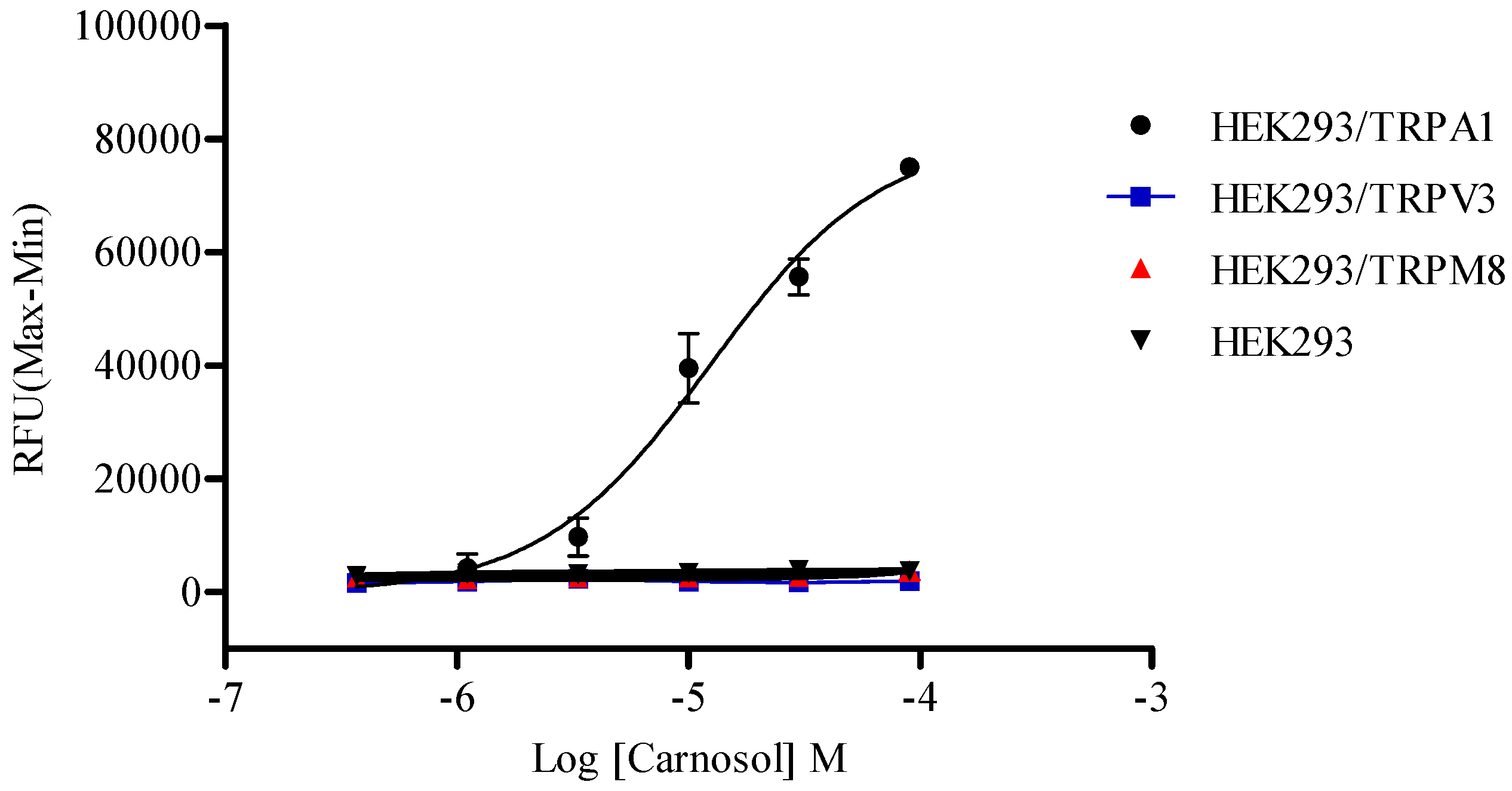
2.5. Compound Selectivity Evaluation
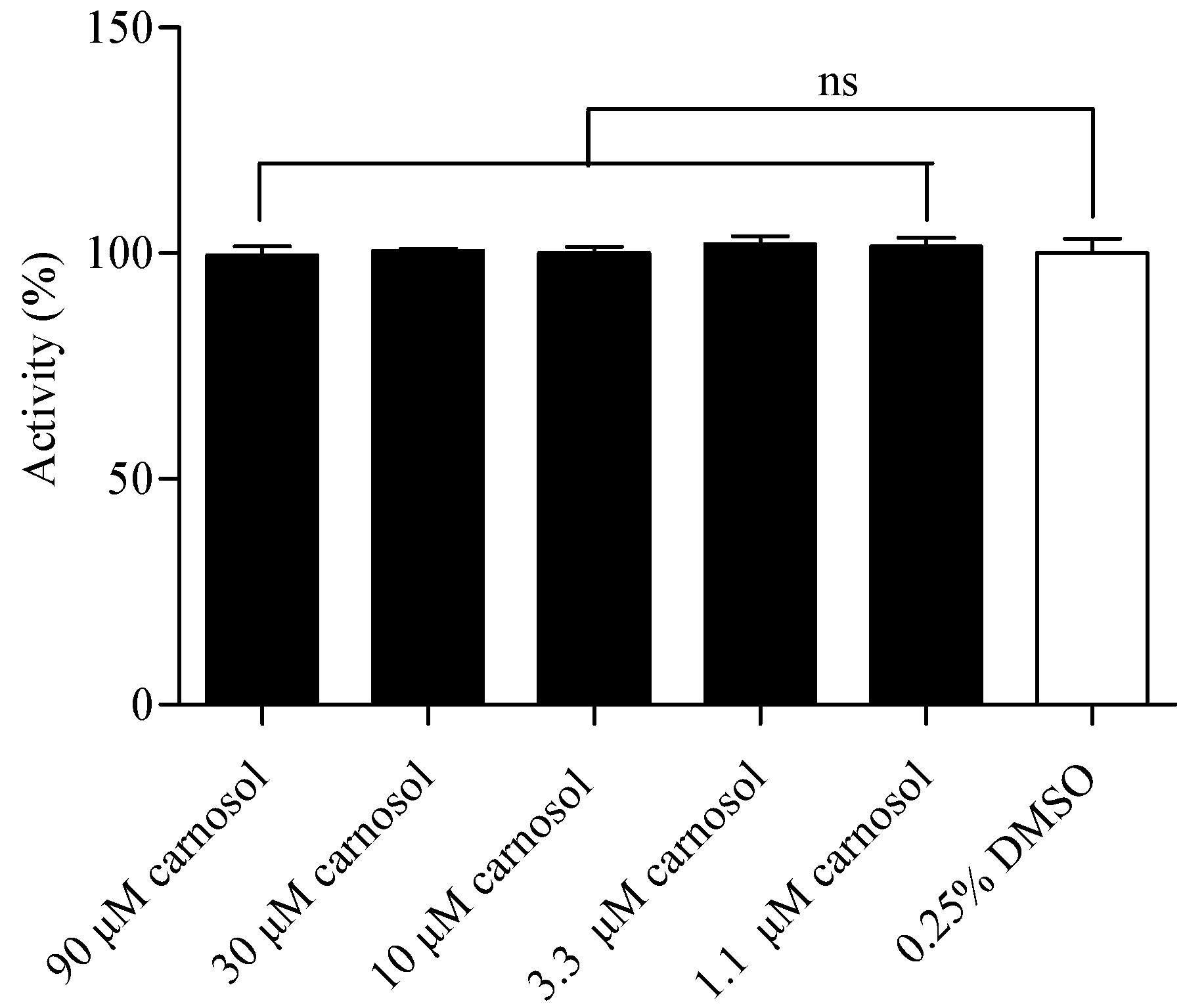
2.6. Compound Cytotoxicity Evaluation
3. Experimental Section
3.1. Materials
3.2. Compounds Preparation
3.3. Cell Culture
3.4. Agonist Screening Assay
3.5. Compound Specificity Assay
3.6. Compound Selectivity Assay
3.7. Compound Cytotoxicity Assay
3.8. Statistics
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaquemar, D.; Schenker, T.; Trueb, B. An Ankyrin-like Protein with Transmembrane Domains is Specifically Lost after Oncogenic Transformation of Human Fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Otto, W.R.; Facer, P.; Zebda, N.; Selmer, I.; Gunthorpe, M.J.; Chessell, I.P.; Sinisi, M.; Birch, R.; Anand, P. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci. Lett. 2008, 438, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, S.; Dhaka, A.; Story, G.M.; Cao, Y.-Q. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol. Pain 2012, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, T.; Ishida, Y.; Nakamura, Y.; Yamada, T.; Kitahara, T.; Takimoto, Y.; Horii, A.; Uno, A.; Imai, T.; Okazaki, S.; et al. Functional expression of TRPV1 and TRPA1 in rat vestibular ganglia. Neurosci. Lett. 2013, 552, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Forner, S.; Lima-Garcia, J.F.; Viana, A.F.; Calixto, J.B. Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem. Biol. Interact. 2013, 203, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Weinhold, P.; Reich, O.; Seitz, M.; Schlenker, B.; Stief, C.G.; Andersson, K.E.; Hedlund, P. Transient receptor potential A1 and cannabinoid receptor activity in human normal and hyperplastic prostate: Relation to nerves and interstitial cells. Eur. Urol. 2010, 57, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One 2012, 7, e42454. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Russell, F.A.; Spina, D.; McDougall, J.J.; Graepel, R.; Gentry, C.; Staniland, A.A.; Mountford, D.M.; Keeble, J.E.; Malcangio, M.; et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011, 63, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflug. Arch. Eur. J. Physiol. 2012, 464, 425–458. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- DeBerry, J.J.; Schwartz, E.S.; Davis, B.M. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain 2014, 155, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Dhennin-Duthille, I.; Ay, A.S.; Rybarczyk, P.; Korichneva, I.; Ouadid-Ahidouch, H. New insights into pharmacological tools to TR(i)P cancer up. Br. J. Pharmacol. 2014, 171, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Baxter, M.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Transient receptor potential (TRP) channels in the airway: Role in airway disease. Br. J. Pharmacol. 2014, 171, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.S.; Meotti, F.C.; Andrade, E.L.; Leal, P.C.; Motta, E.M.; Calixto, J.B. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 2010, 148, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Nozawa, K.; Doihara, H.; Keto, Y.; Kaku, H.; Yokoyama, T.; Itou, H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur. J. Pharmacol. 2014, 723, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Hwang, S.W.; Eid, S.R.; Earley, T.J.; Story, G.M.; Viswanath, V.; Petrus, M.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Amura, Y.T.; Wasaki, Y.I.; Arukawa, M.N.; Atanabe, T.W. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. J. Nutr. Sci. Vitaminol. 2012, 58, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Prenen, J.; Meseguer, V.; Owsianik, G.; Voets, T.; Nilius, B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflug. Arch. Eur. J. Physiol. 2008, 457, 77–89. [Google Scholar] [CrossRef]
- Kim, W.J.; Kang, H.; Kim, J.E.; Choi, G.J.; Shin, H.Y.; Baek, C.W.; Jung, Y.H.; Woo, Y.C.; Kim, S.H.; Lee, J.H. Effect of intraperitoneal administered ginseng total saponins on hyperalgesia induced by repeated intramuscular injection of acidic saline in rats. J. Med. Food 2014, 17, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Lee, H.J.; Choi, J.K.; Kim, H.J.; Kang, J.H.; Lee, E.J.; Kim, Y.R.; Kang, J.H.; Yoo, J.K.; Cho, H.Y.; et al. Novel anti-nociceptive effects of cardamonin via blocking expression of cyclooxygenase-2 and transglutaminase-2. Pharmacol. Biochem. Behav. 2014, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jung, I.; Hur, J.; Kim, S.H.; Lee, J.H.; Kang, J.Y.; Jung, K.C.; Kim, K.S.; Yoo, M.C.; Park, D.S.; et al. The analgesic and anti-inflammatory effect of WIN-34B, a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J. Ethnopharmacol. 2010, 131, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, P.J.; Jeong, H.J.; Vaughan, C.W. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br. J. Pharmacol. 2009, 157, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Kundu, J.; Chae, I.G.; Kundu, J.K. Carnosol: A phenolic diterpene with cancer chemopreventive potential. J. Cancer Prev. 2014, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Baggio, C.H.; Freitas, C.S.; dos Santos, A.C.; Twardowschy, A.; Horst, H.; Pizzolatti, M.G.; Micke, G.A.; Heller, M.; dos Santos, E.P.; et al. Gastroprotective constituents of Salvia officinalis L. Fitoterapia 2009, 80, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Bouajaj, S.; Benyamna, A.; Bouamama, H.; Romane, A.; Falconieri, D.; Piras, A.; Marongiu, B. Antibacterial, allelopathic and antioxidant activities of essential oil of Salvia officinalis L. growing wild in the Atlas Mountains of Morocco. Nat. Prod. Res. 2013, 27, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Greiner, C.; Verhoff, M.; Rau, O.; Tausch, L.; Hornig, C.; Steinhilber, D.; Schubert-Zsilavecz, M.; Werz, O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008, 76, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Jorgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the nuclear receptor PPARgamma by metabolites isolated from sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Abad, A.N.A.; Nour, M.H.K.; Tavakkoli, F. Effect of Salvia officinalis Hydroalcoholic Extract on Vincristine-induced Neuropathy in Mice. Chin. J. Nat. Med. 2011, 9, 354–358. [Google Scholar]
- Qnais, E.Y.; Abu-Dieyeh, M.; Abdulla, F.A.; Abdalla, S.S. The antinociceptive and anti-inflammatory effects of Salvia officinalis leaf aqueous and butanol extracts. Pharm. Biol. 2010, 48, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.R.; Kanazawa, L.K.; das Neves, T.L.; da Silva, C.F.; Horst, H.; Pizzolatti, M.G.; Santos, A.R.; Baggio, C.H.; Werner, M.F. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol. 2012, 139, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Rittiner, J.E.; Korboukh, I.; Hull-Ryde, E.A.; Jin, J.; Janzen, W.P.; Frye, S.V.; Zylka, M.J. AMP is an adenosine A1 receptor agonist. J. Biol. Chem. 2012, 287, 5301–5309. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Bettini, S.; Tinelli, A.; Valli, L.; Storelli, C.; Leo, S.; Santino, A.; Maffia, M. Antitumor activity of the dietary diterpene carnosol against a panel of human cancer cell lines. Food Funct. 2014, 5, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.W.; Lee, D.Y.; Ma, Z.Z.; Huang, L.S.; Sun, L.P.; Li, Y.D.; Chen, J.X.; Niu, J.Z. The antioxidant effect of carnosol in bovine aortic endothelial cells is mainly mediated via estrogen receptor α pathway. Biol. Pharm. Bull. 2012, 35, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, A.; Garcia-Caballero, M.; Medina, M.A.; Quesada, A.R. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur. J. Nutr. 2013, 52, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jin, Y.R.; Lim, Y.; Hong, J.T.; Kim, T.J.; Chung, J.H.; Yun, Y.P. Antiplatelet activity of carnosol is mediated by the inhibition of TXA2 receptor and cytosolic calcium mobilization. Vasc. Pharmacol. 2006, 45, 148–153. [Google Scholar] [CrossRef]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Bettio, L.E.; Oliveira, A.; Pazini, F.L.; Dalmarco, J.B.; Simionatto, E.L.; et al. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013, 136, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Leamy, A.W.; Shukla, P.; McAlexander, M.A.; Carr, M.J.; Ghatta, S. Curcumin ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci. Lett. 2011, 503, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Dubin, A.E.; Bursulaya, B.; Viswanath, V.; Jegla, T.J.; Patapoutian, A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 9640–9651. [Google Scholar] [CrossRef]
- Liu, K.; Samuel, M.; Ho, M.; Harrison, R.K.; Paslay, J.W. NPPB structure-specifically activates TRPA1 channels. Biochem. Pharmacol. 2010, 80, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Brederson, J.D.; Kym, P.R.; Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013, 716, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, F.; Owsianik, G.; Verbert, L.; Janssens, A.; de Smedt, H.; Nilius, B.; Voets, T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J. Biol. Chem. 2007, 282, 3325–3336. [Google Scholar] [PubMed]
- Hu, H.Z.; Gu, Q.; Wang, C.; Colton, C.K.; Tang, J.; Kinoshita-Kawada, M.; Lee, L.Y.; Wood, J.D.; Zhu, M.X. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 2004, 279, 35741–35748. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.; Im, S.T.; Kim, Y.H.; Jung, S.J.; Rhyu, M.R.; Oh, S.B. Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience 2014, 261, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, J.; Guo, H.; Sun, S.; Wang, S.; Zhang, Y.; Li, S.; Qiao, Y. [6]-gingerol: A novel AT1 antagonist for the treatment of cardiovascular disease. Planta Med. 2013, 79, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.H.; Deng, Y.; Zhang, B.; Bai, Y.Q.; Cao, J.; Li, S.Y.; Liu, J.F. Pinacidil, a Katp channel opener, identified as a novel agonist for TRPA1. Chin. Sci. Bull. 2012, 57, 1810–1817. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; Liu, Q.; Zhang, Y.; Wang, S.; Zhang, Y.; Li, S.; Qiao, Y. Identification of Natural Compound Carnosol as a Novel TRPA1 Receptor Agonist. Molecules 2014, 19, 18733-18746. https://doi.org/10.3390/molecules191118733
Zhai C, Liu Q, Zhang Y, Wang S, Zhang Y, Li S, Qiao Y. Identification of Natural Compound Carnosol as a Novel TRPA1 Receptor Agonist. Molecules. 2014; 19(11):18733-18746. https://doi.org/10.3390/molecules191118733
Chicago/Turabian StyleZhai, Chenxi, Qing Liu, Yuxin Zhang, Shifeng Wang, Yanling Zhang, Shiyou Li, and Yanjiang Qiao. 2014. "Identification of Natural Compound Carnosol as a Novel TRPA1 Receptor Agonist" Molecules 19, no. 11: 18733-18746. https://doi.org/10.3390/molecules191118733