Novel Polycarbo-Substituted Alkyl (Thieno[3,2-c]quinoline)-2-Carboxylates: Synthesis and Cytotoxicity Studies
Abstract
:1. Introduction
2. Results and Discussion
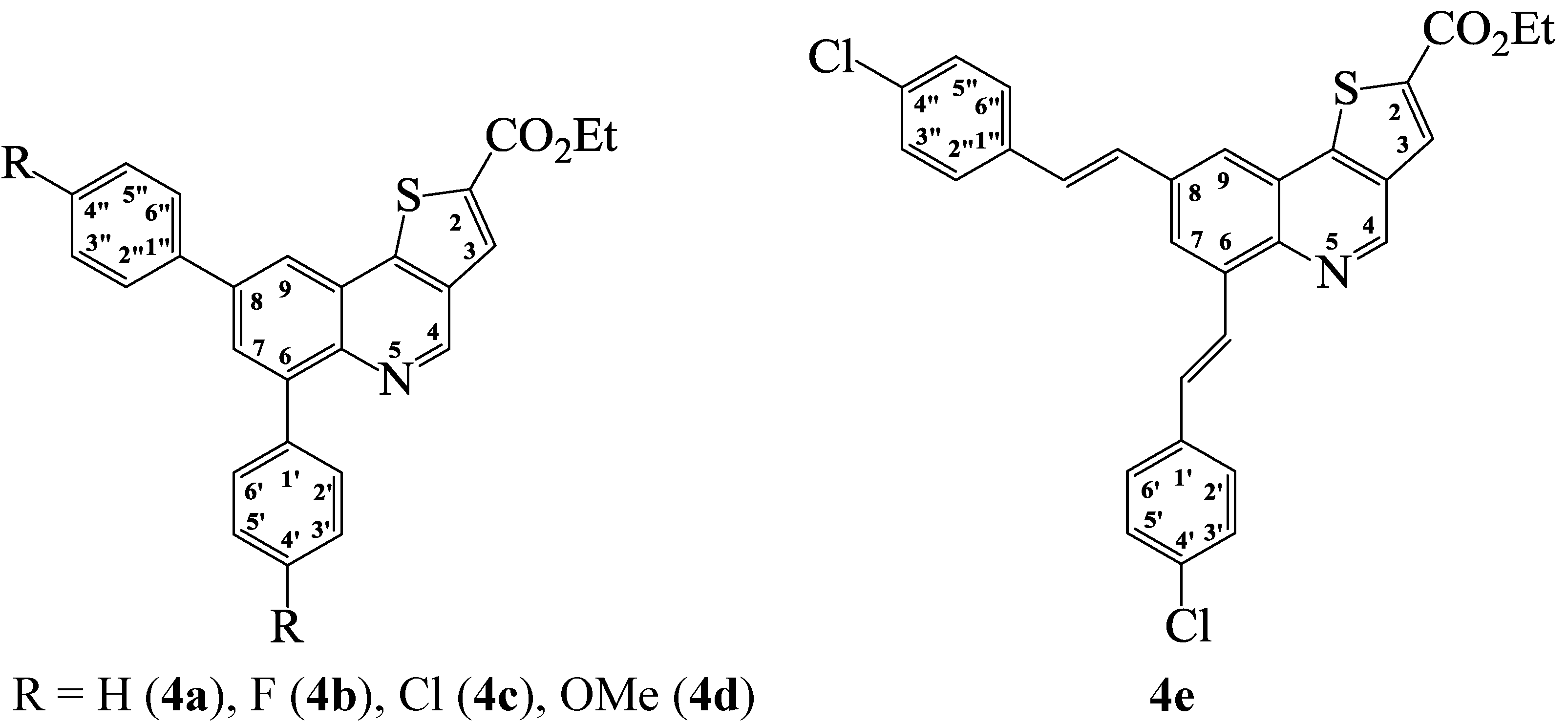
2.1. Chemistry
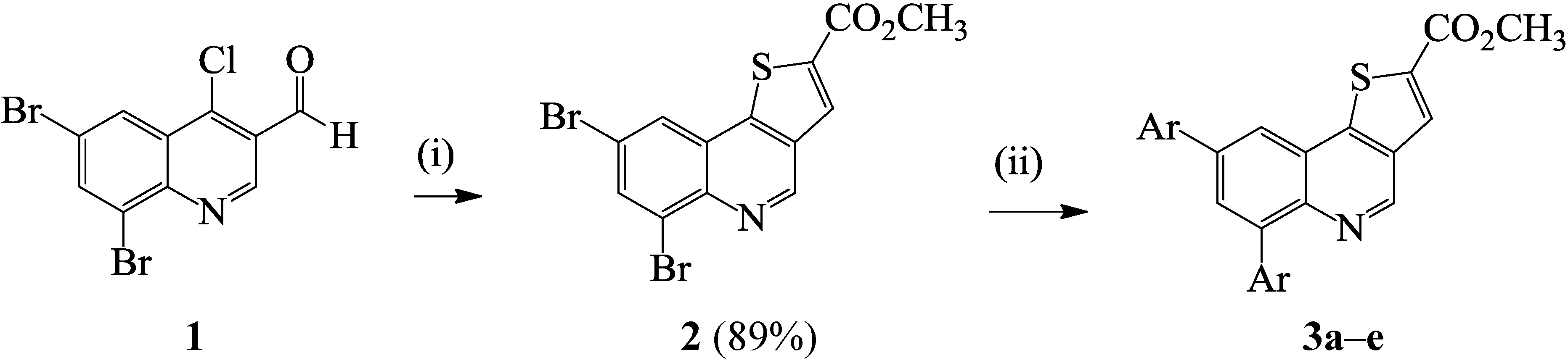
| Compound | Ar | % Yield |
|---|---|---|
| 3a | -C6H5 | 92 |
| 3b | 4-FC6H4- | 65 |
| 3c | 4-MeOC6H4- | 63 |
| 3d | 4-FC6H4CH=CH- | 96 |
| 3e | 4-ClC6H4CH=CH- | 81 |
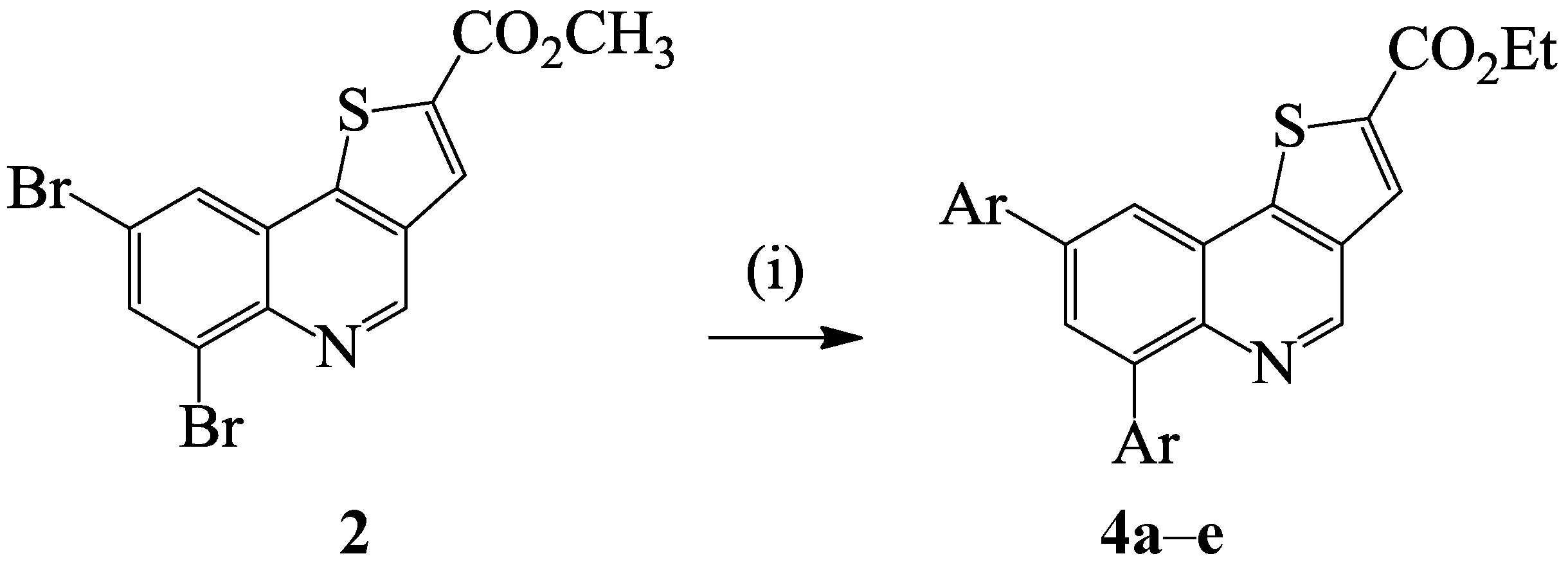
| Compound | Ar | % Yield |
|---|---|---|
| 4a | -C6H5 | 96 |
| 4b | 4-FC6H4- | 70 |
| 4c | 4-ClC6H4- | 71 |
| 4d | 4-MeOC6H4- | 62 |
| 4e | 4-ClC6H4CH=CH- | 67 |

2.2. Cytotoxic Activity against Human Breast Cancer Cell Line MCF-7
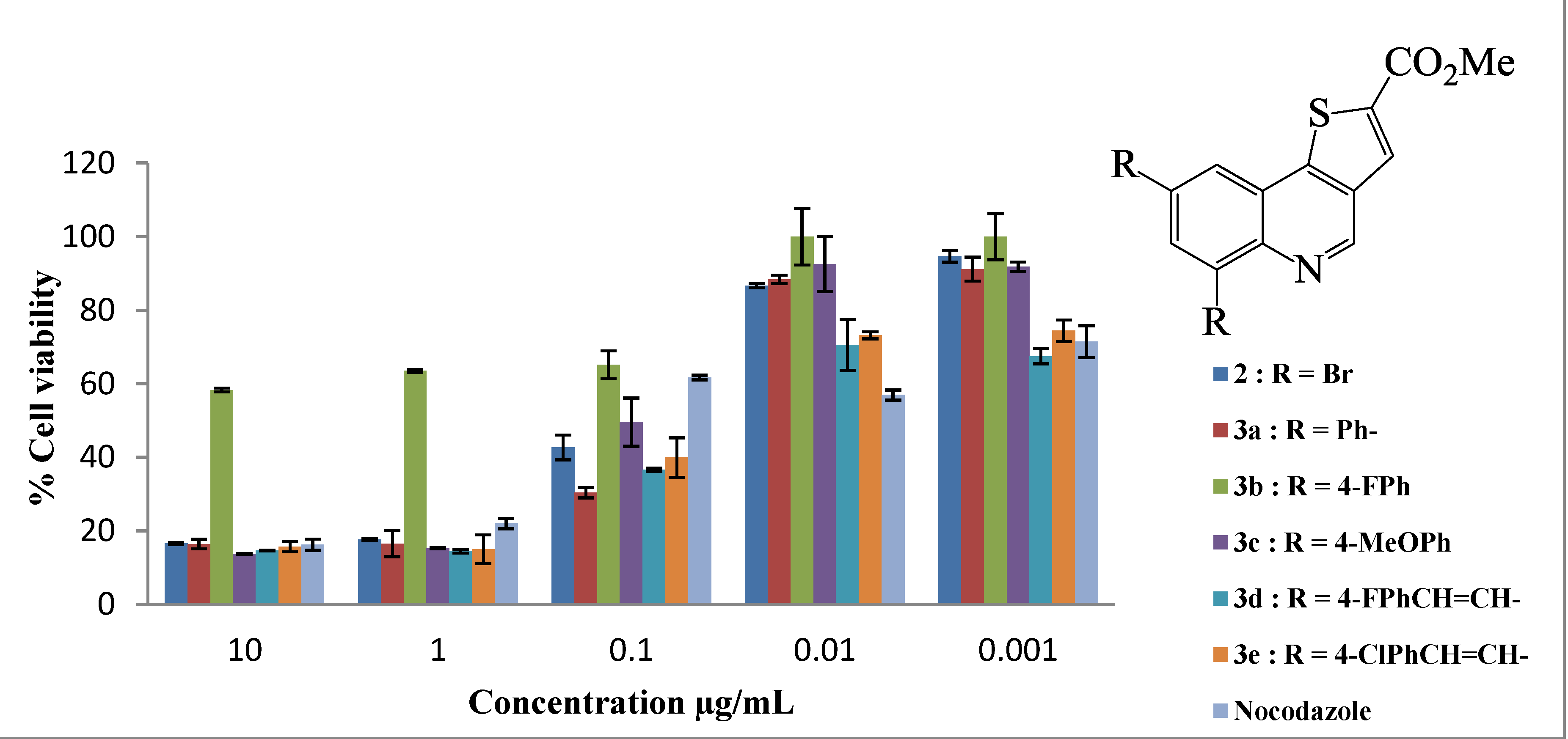
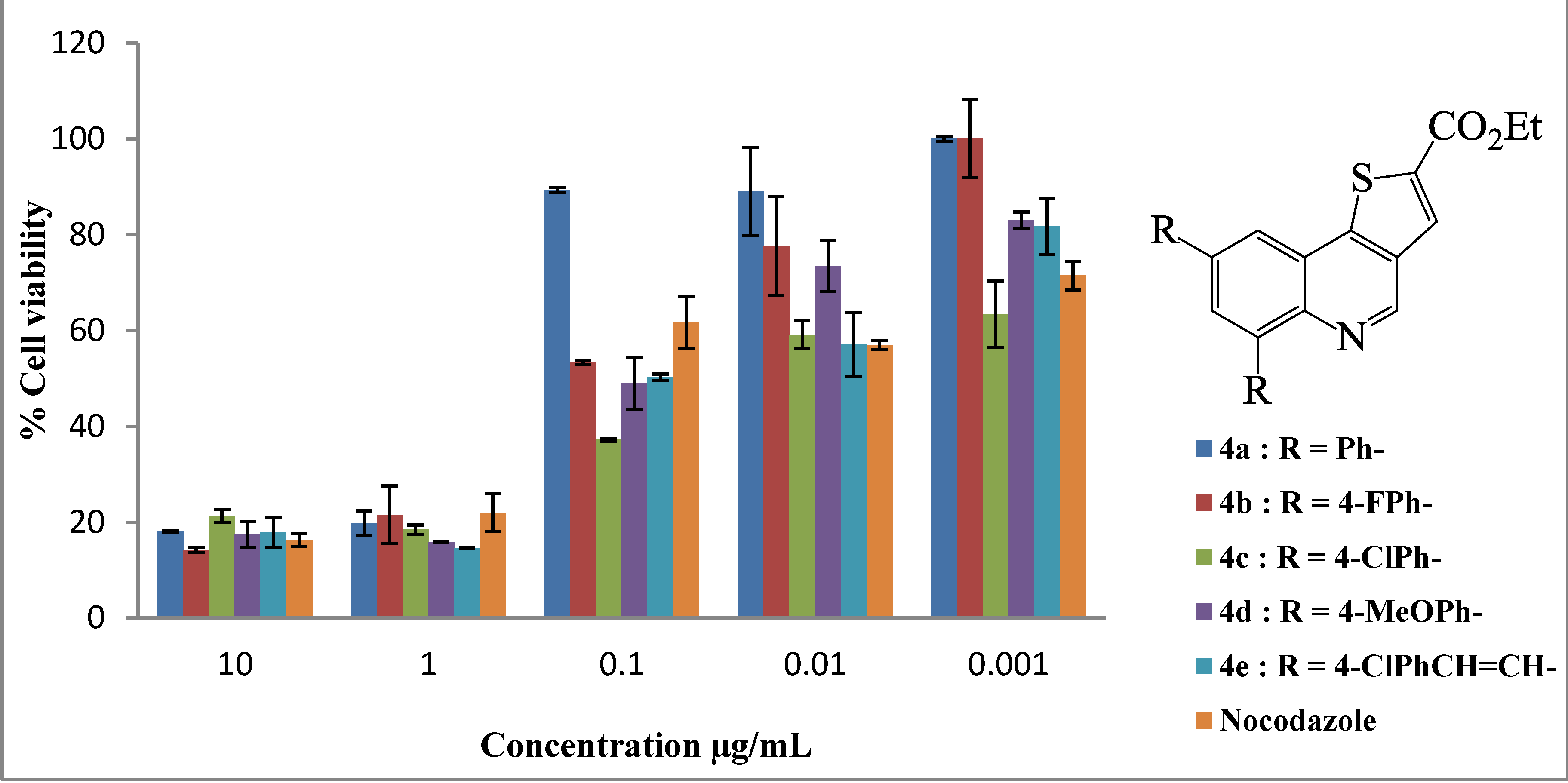
| Compound | LC50 (µg/mL) |
|---|---|
| 2 | 0.11 ± 0.01 |
| 3a | 0.30 ± 0.03 |
| 3b | ˃10.00 ± 0.004 |
| 3c | 0.094 ± 0.03 |
| 3d | 0.014 ± 0.002 |
| 3e | 0.022 ± 0.003 |
| 4a | 1.84 ± 0.02 |
| 4b | 1.84 ± 0.003 |
| 4c | 0.01 ± 0.03 |
| 4d | 0.16 ± 0.04 |
| 4e | 0.04 ± 0.02 |
| Nocodazole | 0.13 ± 0.009 |
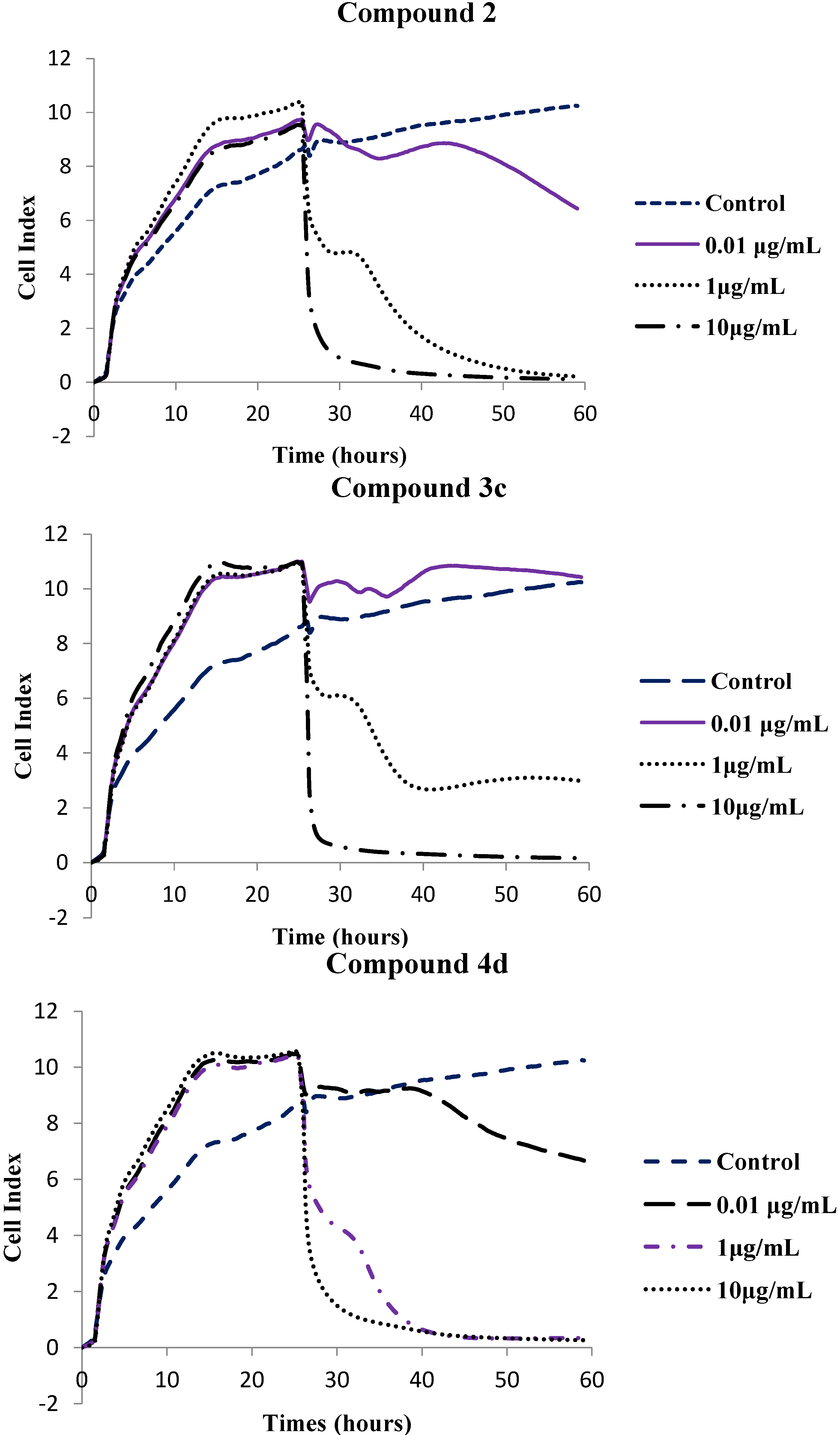
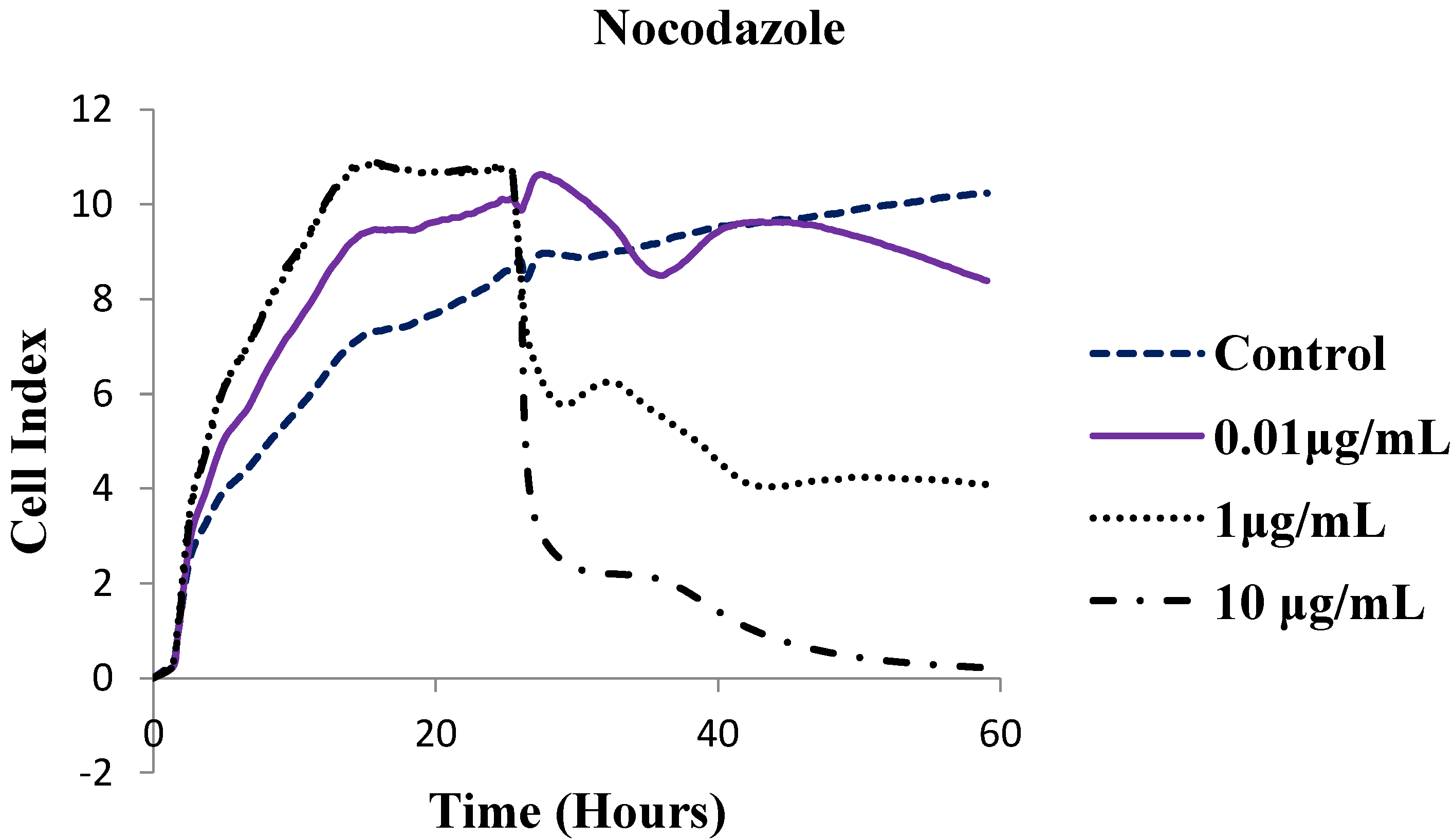
| Compound | IC50 (µg/mL) |
|---|---|
| 2 | 0.167 |
| 3c | 0.094 |
| 4d | 0.169 |
| Nocodazole | 0.171 |
3. Experimental Section
3.1. General Information
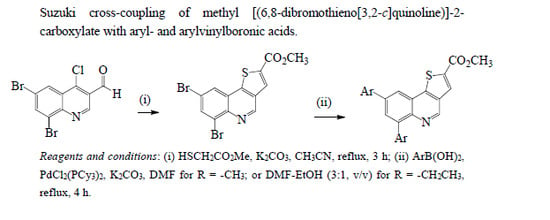
3.1.1. Synthesis of Methyl [(6,8-Dibromothieno[3,2-c]quinoline)]-2-carboxylate (2)
3.1.2. Typical Procedure for the Suzuki-Miyaura Cross-Coupling of 2 with Arylboronic Acids
3.1.3. Typical Procedure for the Preparation of Compounds 4a–e
3.2. Cell Culture and Conditions
3.2.1. Tetrazolium-Based Cytotoxicity Analysis
3.2.2. xCelligency Real Time Cell Analysis Proliferation Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castrillo, A.; Pennington, D.J.; Otto, F.; Parker, P.J.; Owen, M.J.; Bosca, L. Protein kinase Cε is required for macrophage activation and defense against bacterial infection. J. Exp. Med. 2001, 194, 1231–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarak, I.; Kralj, M.; Suman, L.; Pavlovic, G.; Dogan, J.; Piantaninda, I.; Zinic, M.; Pavelic, K.; Karminski-Zamola, G. Novel cyano- and N-isopropylamidino-substituted derivatives of benzo[b]thiophene-2-carboxanilides and benzo[b]thieno[2,3-c]quinolones: Synthesis, photo-chemical synthesis, crystal structure determination, and antitumor evaluation. J. Med. Chem. 2005, 48, 2346–2360. [Google Scholar] [CrossRef] [PubMed]
- Cesare, P.; Dekker, L.V.; Sardini, A.; Parker, P.J.; McNaughton, P.A. Specific involvement of PKC-ε in sensitization of the neuronal response to painful heat. Neuron 1999, 23, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, K.; Doucet, H. One-pot synthesis of furo- or thienoquinolines through sequential imination and intramolecular palladium-catalyzed direct arylation. Eur. J. Org. Chem. 2012, 2012, 6745–6751, and references cited therein. [Google Scholar] [CrossRef]
- Pierre, F.; Chua, P.C.; O’Brien, S.E.; Siddiqui-Jain, A.; Bourbon, P.; Haddach, M.; Michaux, J.; Nagasawa, J.; Schwaebe, M.K.; Stefan, E.; et al. Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J. Med. Chem. 2011, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, A.A.; Aleksanyan, I.L.; Sargsyan, K.S. Synthesis of substituted 2,4-dimethylthieno[3,2-c]quinolines. Russ. J. Org. Chem. 2007, 43, 422–425. [Google Scholar] [CrossRef]
- Gupta, M.C.L.N.; Darbarwar, M. A facile synthesis of 3-hydroxythieno[3,2-c]quinolin-4(5H)-ones. Indian J. Chem. 1995, 34, 432–435. [Google Scholar]
- Majumdar, K.C.; Ghosh, M. Tandem cyclization: One pot regioselective synthesis of thieno[3,2-c]quinolin-4(5H)-one derivatives. Tetrahedron 2002, 58, 10047–10052. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Saha, D.; Ghosh, M. Studies in thio-Claisen rearrangement. Synthesis of thieno-[3,2-c]-quinolone derivatives from 4-allylthioquinolin-2(1H)-ones. Synth. Commun. 2005, 35, 947–9547. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Sadek, K.U.; El-Nabi, H.A.A.; Mohamed, A.A.E.; Ebraheem, E.A.; Smith, M.B. Fused quinoline heterocycles. VI: Synthesis of 5H-1-thia-3,5,6-triazaaceanthrylenes and 5H-1-thia-3,4,5,6-tetraazaaceanthrylenes. J. Heterocycl. Chem. 2005, 42, 567–574. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Ahmed, E.A.; Sadek, K.U. Recent developments in the chemistry of pyrazolo[4,3-c]quinolines. Tetrahedron 2012, 68, 1637–1667. [Google Scholar] [CrossRef]
- Maguire, M.P.; Sheets, K.R.; McVety, K.; Spada, A.P.; Zilberstein, A. A New series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J. Med. Chem. 1994, 37, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Zouhiri, F.; Danet, M.; Bénard, C.; Normand-Bayle, M.; Mouscadet, J.-F.; Leh, H.; Thomas, C.M.; Mbemba, G.; d’Angelo, J.; Desmaële, D. HIV-1 replication inhibitors of the styrylquinoline class: Introduction of an additional carboxyl group at the C-5 position of the quinoline. Tetrahedron Lett. 2005, 46, 2201–2205. [Google Scholar] [CrossRef]
- Zamboni, R.; Belley, M.; Champion, E.; Charette, L.; DeHaven, R.; Frenette, R.; Gauthier, J.Y.; Jones, T.R.; Leger, S.; Masson, P.; et al. Development of a novel series of styrylquinoline compounds as high-affinity leukotriene D4 receptor antagonists: Synthetic and structure-activity studies leading to the discovery of (±)-3-[[[3-[2-(7-chloro-2-quinolinyl)-(E)-ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]propionic acid. J. Med. Chem. 1992, 35, 3832–3844. [Google Scholar] [CrossRef]
- Maluleka, M.M.; Mphahlele, M.J. 6,8-Dibromo-4-chloroquinoline-3-carbaldehyde as a synthon in the development of novel 1,6,8-triaryl-1H-pyrazolo[4,3-c]quinolines. Tetrahedron 2013, 69, 699–704. [Google Scholar] [CrossRef]
- Vasumathi, N.; Ramana, D.V.; Ramadas, S.R. Studies of organosulfur compounds. Part VI. A simple method of synthesis of thieno[3,2-c]thiopyran involving an unusual oxidation under mild conditions. Synth. Commun. 1990, 20, 2749–2757. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar] [CrossRef]
- Haman, B.C.; Hartwig, J.F. Sterically hindered chelating alkyl phosphines provide large rate accelerations in palladium-catalyzed amination of aryl iodides, bromides, and chlorides, and the first amination of aryl tosylates. J. Am. Chem. Soc. 1998, 120, 7369–7370. [Google Scholar] [CrossRef]
- Garcia, Y.; Schoenebeck, F.; Legault, C.Y.; Merlic, C.A.; Houk, K.N. Theoretical bond dissociation energies of halo-heterocycles: Trends and relationships to regioselectivity in palladium-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 2009, 131, 6632–6639. [Google Scholar] [CrossRef] [PubMed]
- Khoza, T.A.; Maluleka, M.M.; Mama, N.; Mphahlele, M.J. Synthesis and photophysical properties of 2-aryl-6,8-bis(arylethenyl)-4-methoxyquinolines. Molecules 2012, 17, 14186–14204. [Google Scholar] [CrossRef] [PubMed]
- Piala, A.; Mayi, D.; Handy, S.T. Studies of one-pot double couplings on dibromoquinolines. Tetrahedron 2011, 67, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Kappaun, S.; Sovic, T.; Stelzer, F.; Pogantsch, A.; Zojer, E.; Slugovc, C. Molecular fluorescent pH-probes based on 8-hydroxyquinoline. Org. Biomol. Chem. 2006, 4, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Akrawi, O.A.; Mohammed, H.H.; Langer, P. Synthesis and Suzuki-Miyaura reactions of 3,6,8-tribromoquinoline: A structural revision. Synlett 2013, 1121–1124. [Google Scholar]
- Zhang, Y.; Gao, J.; Li, W.; Lee, H.; Lu, B.Z.; Senanayake, C.H. Synthesis of 8-arylquinolines via one-pot Pd-catalyzed borylation of quinoline-8-yl halides and subsequent Suzuki-Miyaura coupling. J. Org. Chem. 2011, 76, 6394–6400. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.Y.; Evdokimov, A.N.; Kurzin, A.V.; Maiyorova, H.D. Solubility of potassium carbonate in methanol. J. Chem. Eng. Data 2002, 47, 1175–1176, and references cited therein. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Jacques Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ken, N.; Wang, X.; Xu, X.; Abassi, Y. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [PubMed]
- Xing, J.Z.; Zhu, L.; Gabos, S.; Xie, L. Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol. In Vitro 2006, 20, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphahlele, M.J.; Maluleka, M.M.; Makhafola, T.J.; Mabeta, P. Novel Polycarbo-Substituted Alkyl (Thieno[3,2-c]quinoline)-2-Carboxylates: Synthesis and Cytotoxicity Studies. Molecules 2014, 19, 18527-18542. https://doi.org/10.3390/molecules191118527
Mphahlele MJ, Maluleka MM, Makhafola TJ, Mabeta P. Novel Polycarbo-Substituted Alkyl (Thieno[3,2-c]quinoline)-2-Carboxylates: Synthesis and Cytotoxicity Studies. Molecules. 2014; 19(11):18527-18542. https://doi.org/10.3390/molecules191118527
Chicago/Turabian StyleMphahlele, Malose Jack, Marole Maria Maluleka, Tshepiso Jan Makhafola, and Peace Mabeta. 2014. "Novel Polycarbo-Substituted Alkyl (Thieno[3,2-c]quinoline)-2-Carboxylates: Synthesis and Cytotoxicity Studies" Molecules 19, no. 11: 18527-18542. https://doi.org/10.3390/molecules191118527