Pharmacokinetic Comparisons of Benzoylmesaconine in Rats Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry after Administration of Pure Benzoylmesaconine and Wutou Decoction
Abstract
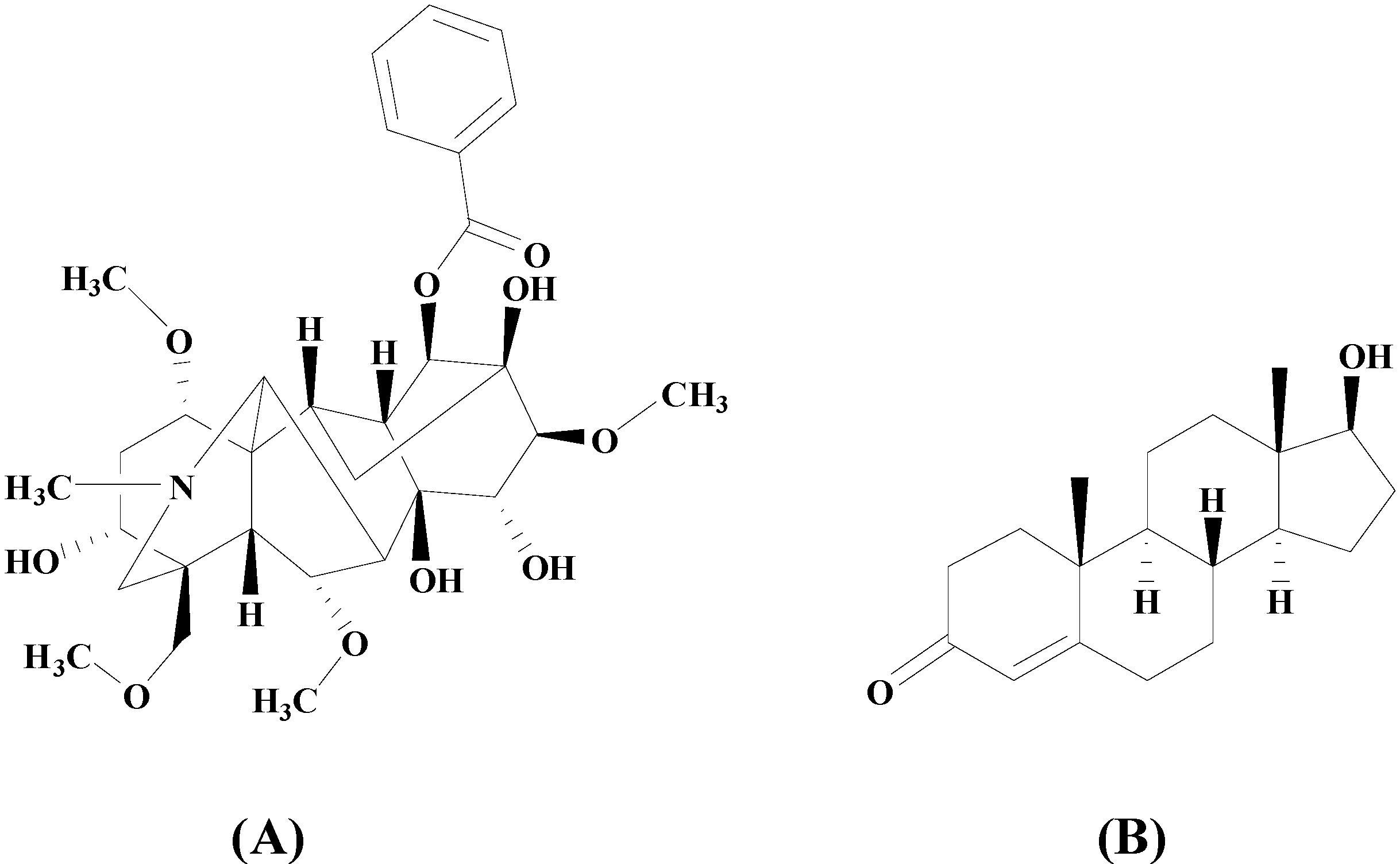
:1. Introduction
2. Results and Discussion
2.1. Validation of UPLC-MS/MS Methods

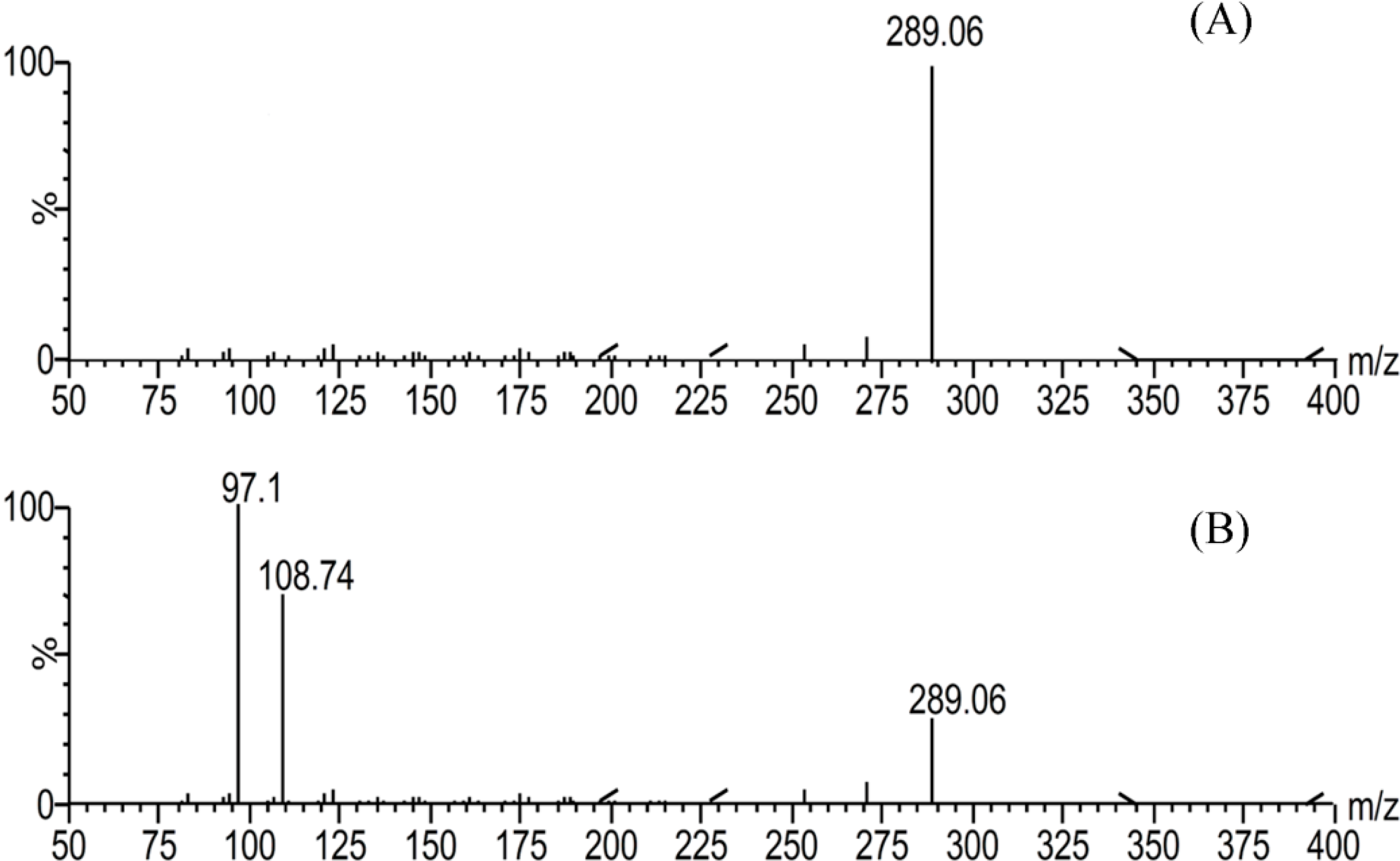

| Concentrations (ng/mL) | Accuracy (RE%) | Precision (RSD%) | |
|---|---|---|---|
| Intra-day | Inter-day | ||
| 1.2 | 17.6 | 6.1 | 13.9 |
| 6 | 12.7 | 6.1 | 7.9 |
| 30 | 10.3 | 3.7 | 4.7 |
| Concentrations (ng/mL) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | |
| 1.2 | 92.0 | 5.4 | 126.1 | 5.9 |
| 6 | 92.9 | 9.3 | 139.0 | 2.3 |
| 30 | 85.2 | 6.4 | 134.1 | 6.5 |
| Concentrations (ng/mL) | Short-Term Storage | Long-Term Storage | Three Freeze-Thaw Cycles | Auto-Sampler Stability | ||||
|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | RSD% | Accuracy (%) | RSD% | Accuracy (%) | RSD% | Accuracy (%) | RSD% | |
| 1.2 | 94.7 | 13.3 | 114.6 | 2.4 | 90.2 | 10.2 | 100.0 | 3.9 |
| 6 | 109.8 | 5.1 | 110.3 | 3.3 | 100.8 | 4.0 | 106.4 | 8.3 |
| 30 | 109.4 | 3.3 | 99.2 | 8.2 | 101.7 | 5.0 | 92.6 | 4.8 |
2.2. Content Determination of BMA in the Lyophilized Powder
2.3. Pharmacokinetic Study
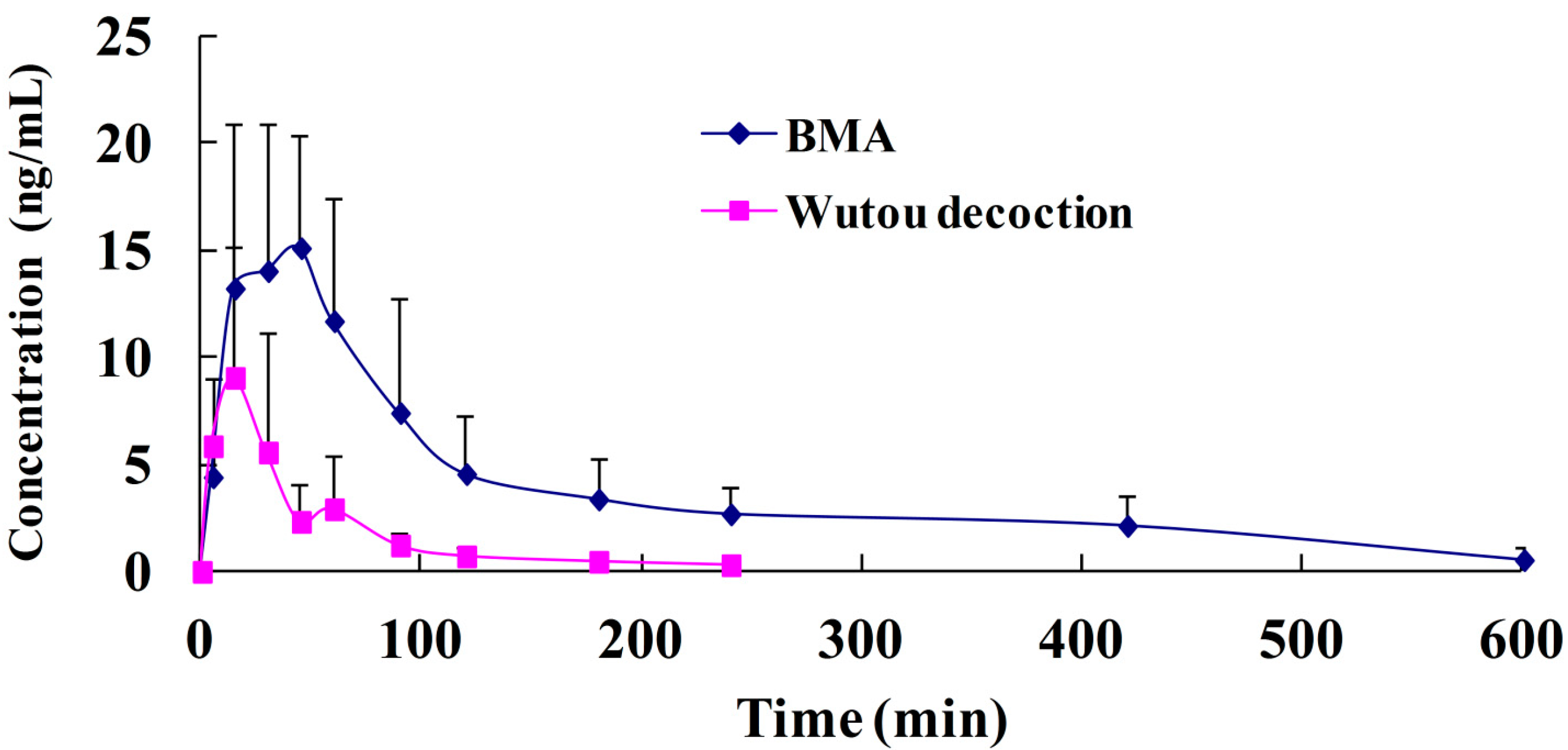
| Parameters | Unit | BMA | Wutou Decoction |
|---|---|---|---|
| AUC(0-t) | ng·min/mL | 2,247.4 ± 1,171.9 | 447.8 ± 292.2 * |
| T1/2 | min | 228.3 ± 117.0 | 61.8 ± 35.1 |
| Cmax | ng/mL | 16.2 ± 6.7 | 10.0 ± 5.8 |
| Tmax | min | 35.0 ± 11.2 | 13.0 ± 4.5 * |
| MRT | min | 155.0 ± 33.2 | 55.8 ± 16.4 * |
| RF | % | - | 19.9 |
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Animals
3.3. Instruments and Conditions
3.4. Preparation of Wutou Decoction Lyophilized Powder
3.5. Preparation of Stock Solutions
3.6. Biosample Collection
3.7. Biosample Preparation
3.8. Method Validation
3.9. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fraenkel, L.; Cunningham, M. High disease activity may not be sufficient to escalate care. Arthrit. Care Res. 2014, 66, 197–203. [Google Scholar] [CrossRef]
- Wisniacki, N.; Amaravadi, L.; Galluppi, G.R.; Zheng, T.S.; Zhang, R.; Kong, J.; Burkly, L.C. Safety, tolerability, pharmacokinetics, and pharmacodynamics of anti-TWEAK monoclonal antibody in patients with rheumatoid arthritis. Clin. Ther. 2013, 35, 1137–1149. [Google Scholar] [CrossRef]
- Van Landeghem, A.A.; de Letter, E.A.; Lambert, W.E.; van Peteghem, C.H.; Piette, M.H. Aconitine involvement in an unusual homicide case. Int. J. Legal Med. 2007, 121, 214–219. [Google Scholar] [CrossRef]
- Singh, S.; Fadnis, P.P.; Sharma, B.K. Aconite poisoning. J. Assoc. Phys. India 1986, 34, 825–826. [Google Scholar]
- Bisset, N.G. Arrow poisons in China. Part II. Aconitum—Botany, chemistry, and pharmacology. J. Ethnopharmacol. 1981, 4, 247–336. [Google Scholar] [CrossRef]
- Ye, L.; Yang, X.; Yang, Z.; Gao, S.; Yin, T.; Liu, W.; Wang, F.; Hu, M.; Liu, Z. The role of efflux transporters on the transport of highly toxic aconitine, mesaconitine, hypaconitine, and their hydrolysates, as determined in cultured Caco-2 and transfected MDCKII cells. Toxicol. Lett. 2013, 216, 86–99. [Google Scholar] [CrossRef]
- Singhuber, J.; Zhu, M.; Prinz, S.; Kopp, B. Aconitum in traditional Chinese medicine: A valuable drug or an unpredictable risk? J. Ethnopharmacol. 2009, 126, 18–30. [Google Scholar] [CrossRef]
- Elliott, S.P. A case of fatal poisoning with the aconite plant: Quantitative analysis in biological fluid. Sci. Justice 2002, 42, 111–115. [Google Scholar] [CrossRef]
- Pullela, R.; Young, L.; Gallagher, B.; Avis, S.P.; Randell, E.W. A case of fatal aconitine poisoning by Monkshood ingestion. J. Forensic Sci. 2008, 53, 491–494. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Xie, Y.; Zhou, H.; Wang, J.R.; Liu, Z.Q.; Wong, Y.F.; Cai, X.; Xu, H.X.; Liu, L. Quantification of aconitum alkaloids in aconite roots by a modified RP-HPLC method. Phytochem. Anal. 2005, 16, 415–421. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hayakawa, Y.; Oyama, T.; Isono, T.; Ohmiya, Y.; Ikeda, Y.; Asami, A.; Noguchi, M. Analgesic effect of benzoylmesaconine. Nihon Yakurigaku Zasshi 1993, 102, 399–404. [Google Scholar] [CrossRef]
- Suzuki, Y.; Oyama, T.; Ishige, A.; Isono, T.; Asami, A.; Ikeda, Y.; Noguchi, M.; Omiya, Y. Antinociceptive mechanism of the aconitine alkaloids mesaconitine and benzoylmesaconine. Planta Med. 1994, 60, 391–394. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mori, K.; Kobayashi, H.; Pollard, R.B.; Suzuki, F. The regulation of burn-associated infections with herpes simplex virus type 1 or Candida albicans by a non-toxic aconitine-hydrolysate, benzoylmesaconine. Part 1: Antiviral and anti-fungal activities in thermally injured mice. Immunol. Cell Biol. 1998, 76, 202–208. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takahashi, H.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. Therapeutic effects of IL-12 combined with benzoylmesaconine, a non-toxic aconitine-hydrolysate, against herpes simplex virus type 1 infection in mice following thermal injury. Burns 2003, 29, 37–42. [Google Scholar] [CrossRef]
- Ye, L.; Yang, X.S.; Lu, L.L.; Chen, W.Y.; Zeng, S.; Yan, T.M.; Dong, L.N.; Peng, X.J.; Shi, J.; Liu, Z.Q. Monoester-diterpene aconitum alkaloid metabolism in human liver microsomes: Predominant role of CYP3A4 and CYP3A5. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Ye, L.; Gao, S.; Feng, Q.; Liu, W.; Yang, Z.; Hu, M.; Liu, Z. Development and validation of a highly sensitive UPLC-MS/MS method for simultaneous determination of aconitine, mesaconitine, hypaconitine, and five of their metabolites in rat blood and its application to a pharmacokinetics study of aconitine, mesaconitine, and hypaconitine. Xenobiotica 2012, 42, 518–525. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, M.H.; Chen, L.J.; Li, R.; Wang, X.H.; Duan, J.G.; Zhao, X.; Wei, Y.Q. Simultaneous quantitation of aconitine, mesaconitine, hypaconitine, benzoylaconine, benzoylmesaconine and benzoylhypaconine in human plasma by liquid chromatography-tandem mass spectrometry and pharmacokinetics evaluation of “SHEN-FU” injectable powder. J. Chromatogr. B 2008, 873, 173–179. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Song, X.; Qin, K.; Guo, H.; Wu, L.; Cai, H.; Cai, B. Comparative pharmacokinetics studies of benzoylhypaconine, benzoylmesaconine, benzoylaconine and hypaconitine in rats by LC-MS method after administration of Radix Aconiti Lateralis Praeparata extract and Dahuang Fuzi Decoction. Biomed. Chromatogr. 2014, 28, 966–973. [Google Scholar] [CrossRef]
- Peng, W.W.; Li, W.; Li, J.S.; Cui, X.B.; Zhang, Y.X.; Yang, G.M.; Wen, H.M.; Cai, B.C. The effects of Rhizoma Zingiberis on pharmacokinetics of six Aconitum alkaloids in herb couple of Radix Aconiti Lateralis-Rhizoma Zingiberis. J. Ethnopharmacol. 2013, 148, 579–586. [Google Scholar] [CrossRef]
- Usui, K.; Hayashizaki, Y.; Hashiyada, M.; Nakano, A.; Funayama, M. Simultaneous determination of 11 aconitum alkaloids in human serum and urine using liquid chromatography-tandem mass spectrometry. Legal Med. 2012, 14, 126–133. [Google Scholar] [CrossRef]
- Tang, L.; Gong, Y.; Lv, C.; Ye, L.; Liu, L.; Liu, Z. Pharmacokinetics of aconitine as the targeted marker of Fuzi (Aconitum carmichaeli) following single and multiple oral administrations of Fuzi extracts in rat by UPLC/MS/MS. J. Ethnopharmacol. 2012, 141, 736–741. [Google Scholar] [CrossRef]
- Song, J.Z.; Han, Q.B.; Qiao, C.F.; But, P.P.; Xu, H.X. Development and validation of a rapid capillary zone electrophoresis method for the determination of aconite alkaloids in aconite roots. Phytochem. Anal. 2010, 21, 137–143. [Google Scholar]
- Izzo, A.A.; Ernst, E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 2009, 69, 1777–1798. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, K.P.; Roy, S.; Khan, F.; Sharma, A. Pharmacovigilance: Effects of herbal components on human drugs interactions involving cytochrome P450. Bioinformation 2008, 3, 198–204. [Google Scholar] [CrossRef]
- Peter, K.; Schinnerl, J.; Felsinger, S.; Brecker, L.; Bauer, R.; Breiteneder, H.; Xu, R.; Ma, Y. A novel concept for detoxification: Complexation between aconitine and liquiritin in a Chinese herbal formula (‘Sini Tang’). J. Ethnopharmacol. 2013, 149, 562–569. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Davey, A.K.; Chen, Y.X.; Wang, J.P.; Liu, X.Q. Effects of diammonium glycyrrhizinate on the pharmacokinetics of aconitine in rats and the potential mechanism. Xenobiotica 2009, 39, 955–963. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound BMA is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.-M.; Wang, Y.; Ye, L.; Zeng, S.; Zheng, Z.-J.; Li, Q.; Lu, L.-L.; Liu, Z.-Q. Pharmacokinetic Comparisons of Benzoylmesaconine in Rats Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry after Administration of Pure Benzoylmesaconine and Wutou Decoction. Molecules 2014, 19, 16757-16769. https://doi.org/10.3390/molecules191016757
Dai P-M, Wang Y, Ye L, Zeng S, Zheng Z-J, Li Q, Lu L-L, Liu Z-Q. Pharmacokinetic Comparisons of Benzoylmesaconine in Rats Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry after Administration of Pure Benzoylmesaconine and Wutou Decoction. Molecules. 2014; 19(10):16757-16769. https://doi.org/10.3390/molecules191016757
Chicago/Turabian StyleDai, Pei-Min, Ying Wang, Ling Ye, Shan Zeng, Zhi-Jie Zheng, Qiang Li, Lin-Liu Lu, and Zhong-Qiu Liu. 2014. "Pharmacokinetic Comparisons of Benzoylmesaconine in Rats Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry after Administration of Pure Benzoylmesaconine and Wutou Decoction" Molecules 19, no. 10: 16757-16769. https://doi.org/10.3390/molecules191016757




