Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and Its Application in Removal of 2,4-Dichlorophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of HRP-immobilized Magnetic Particles
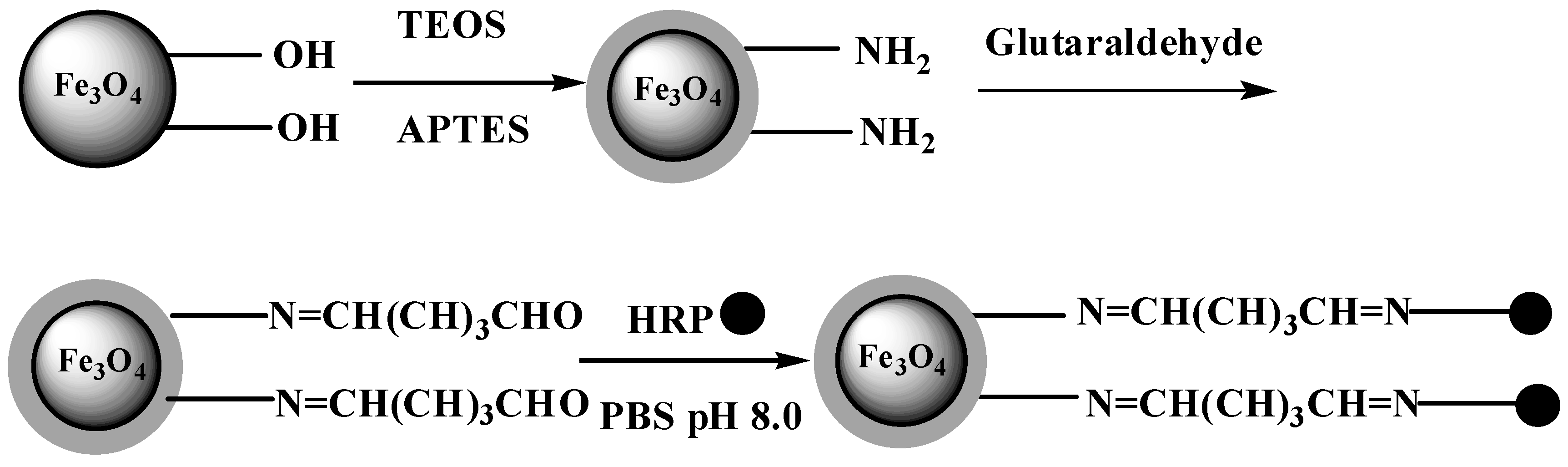
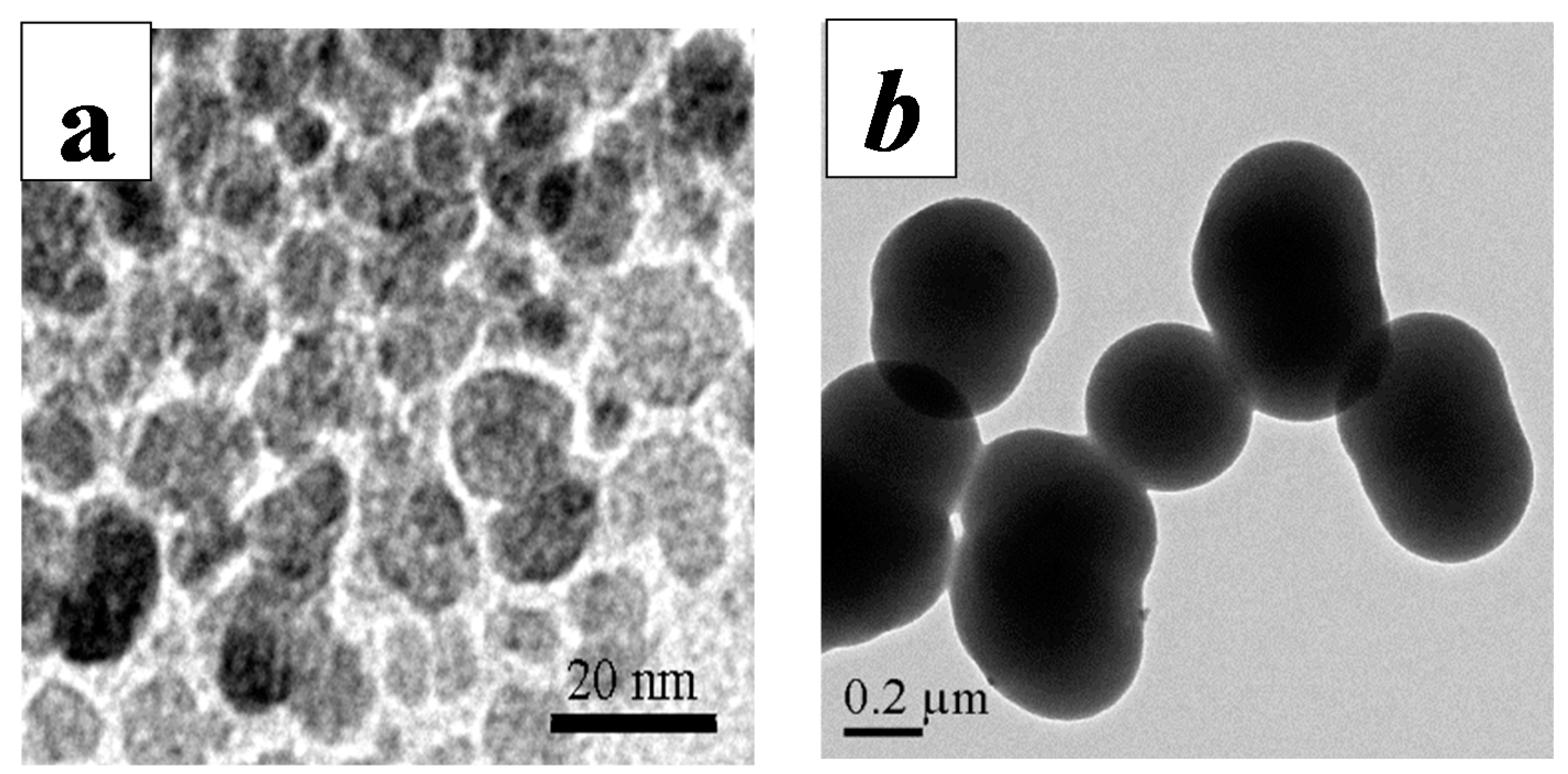
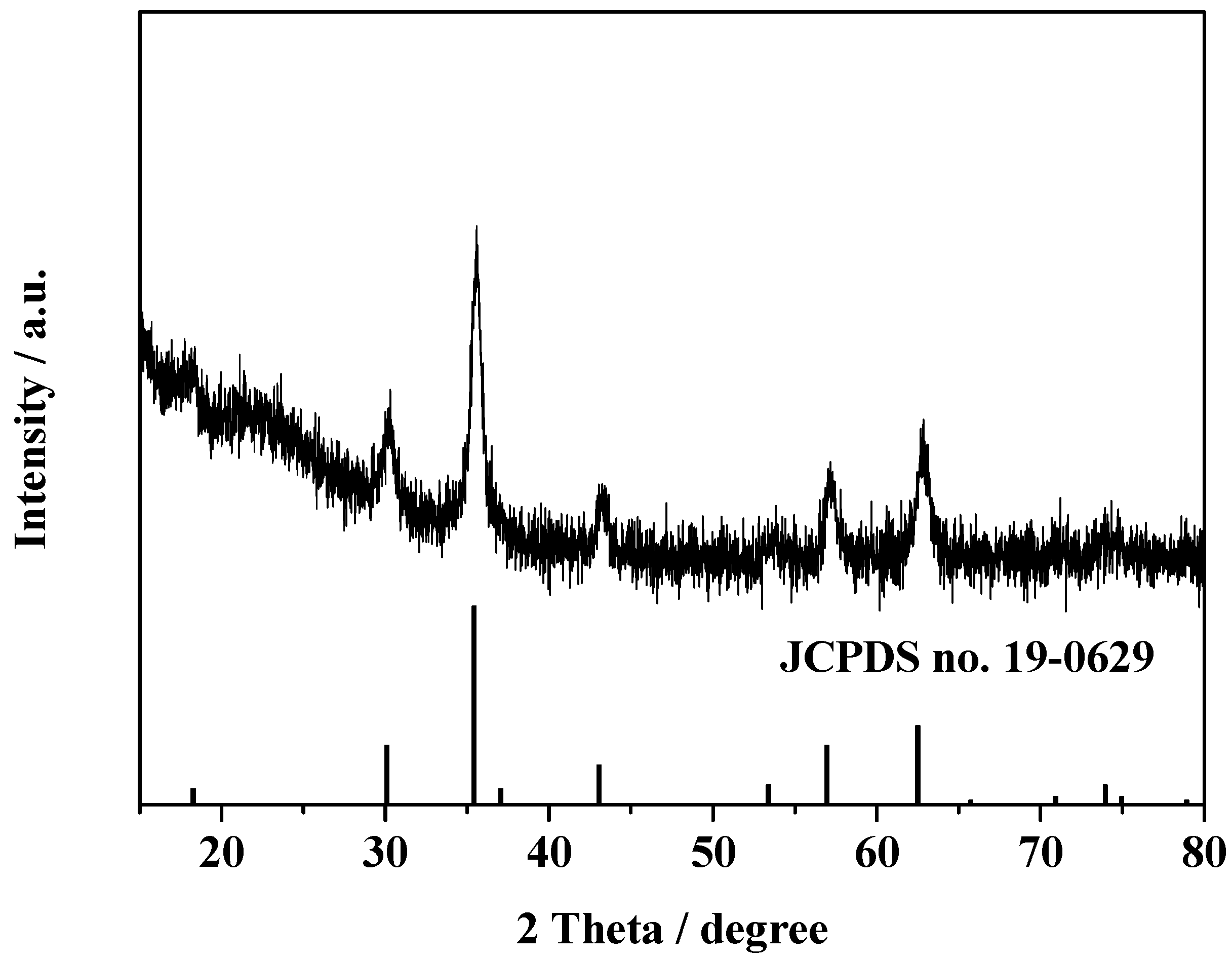
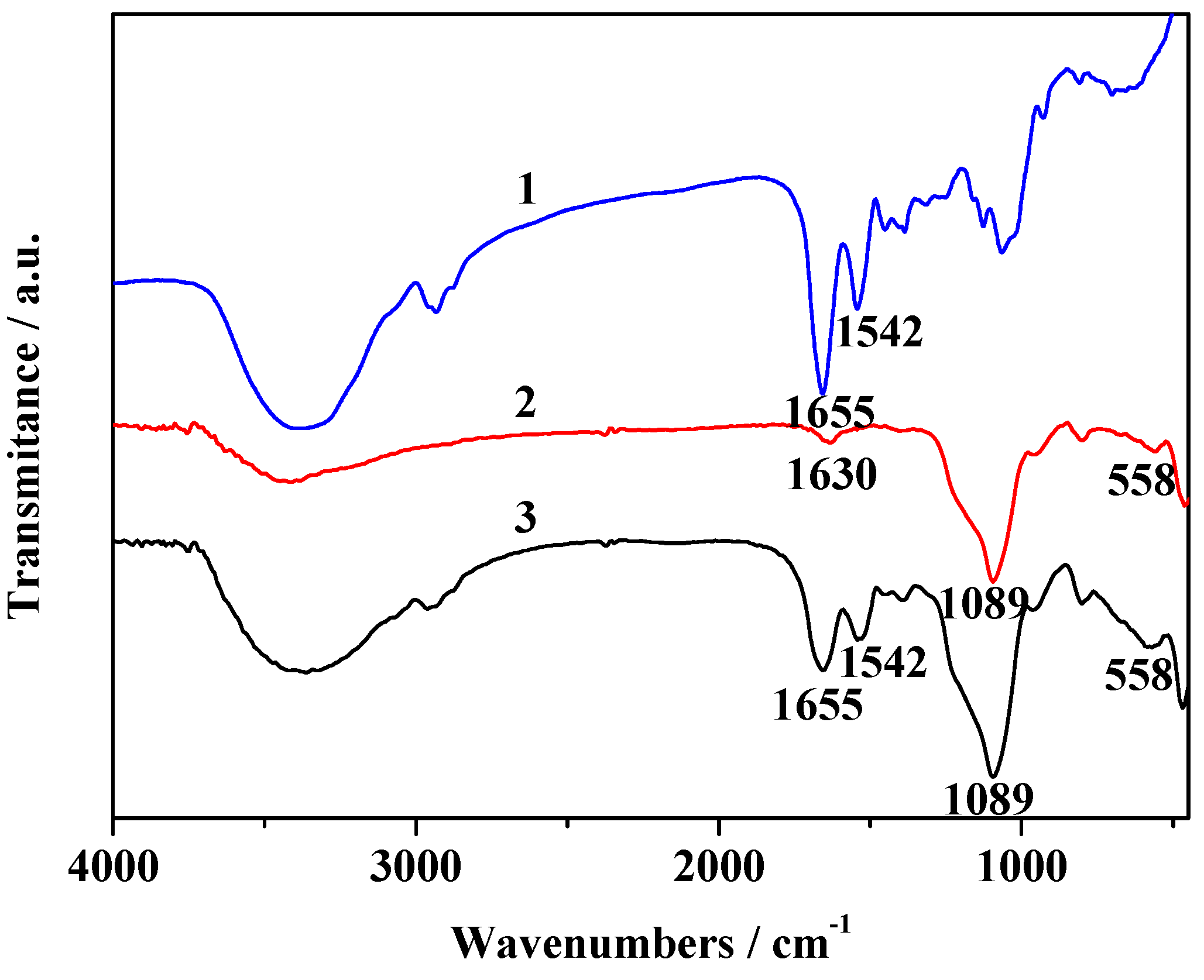
2.2. Characterization
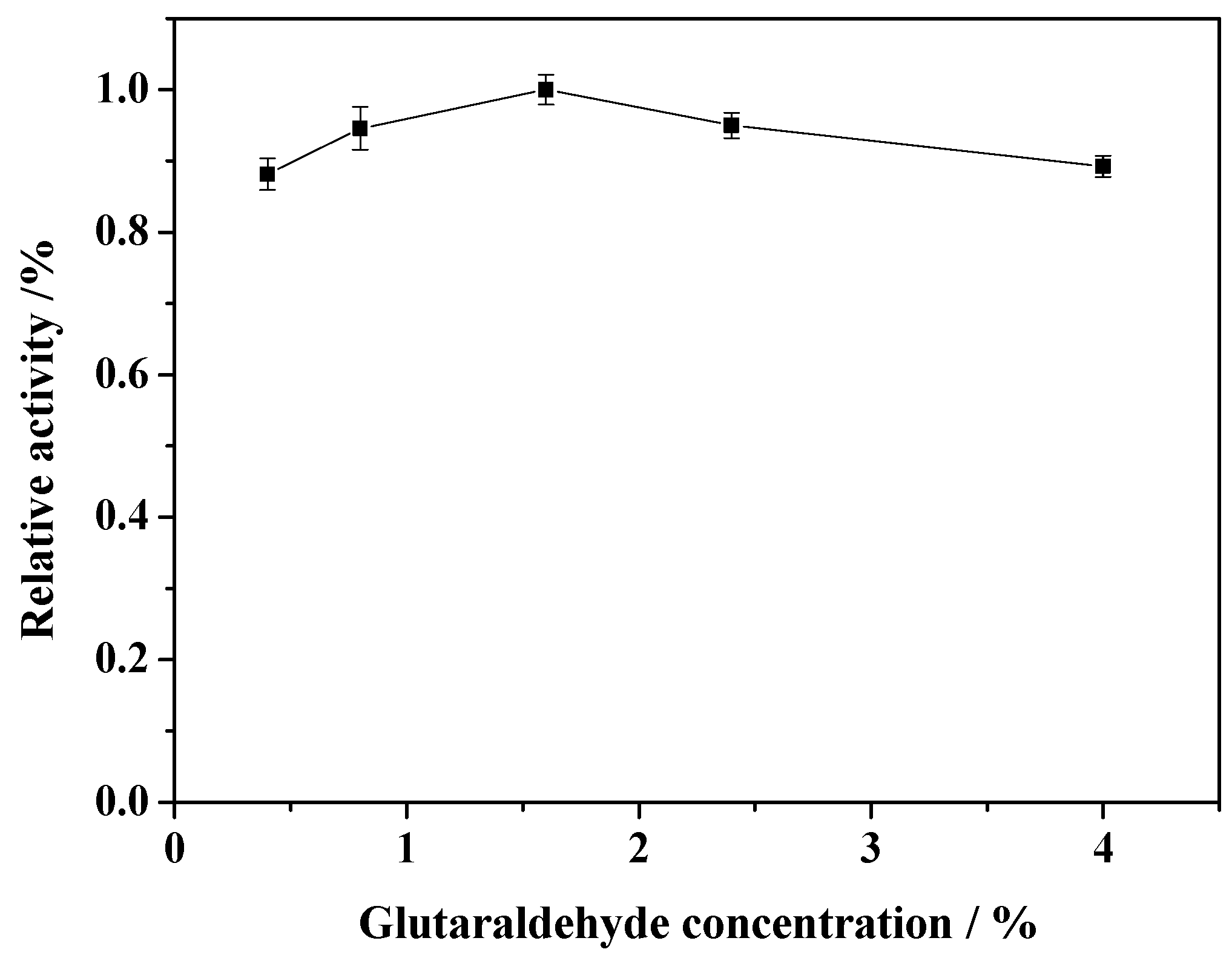
2.3. Effect of Concentration of GA
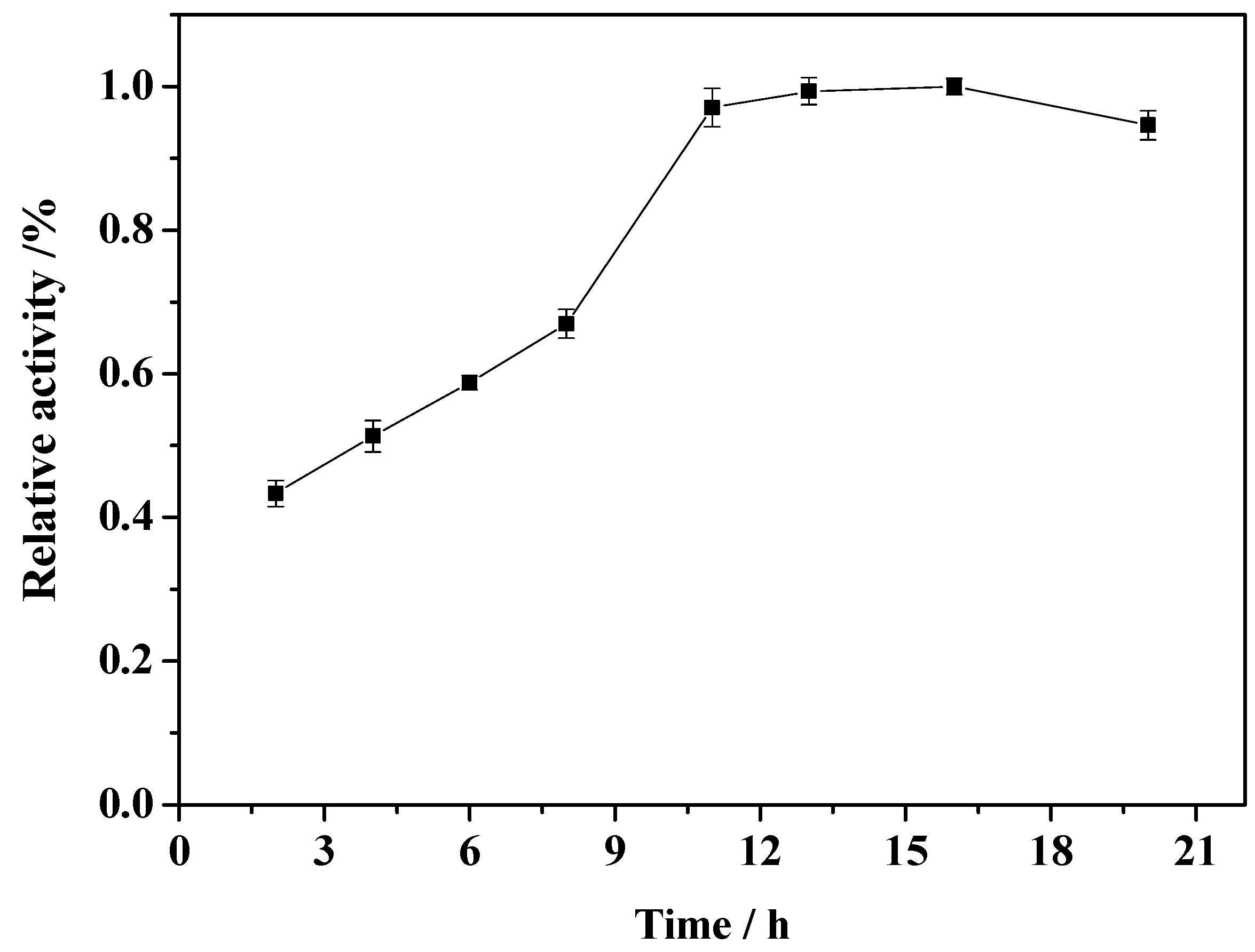
2.4. Effect of Immobilization Time
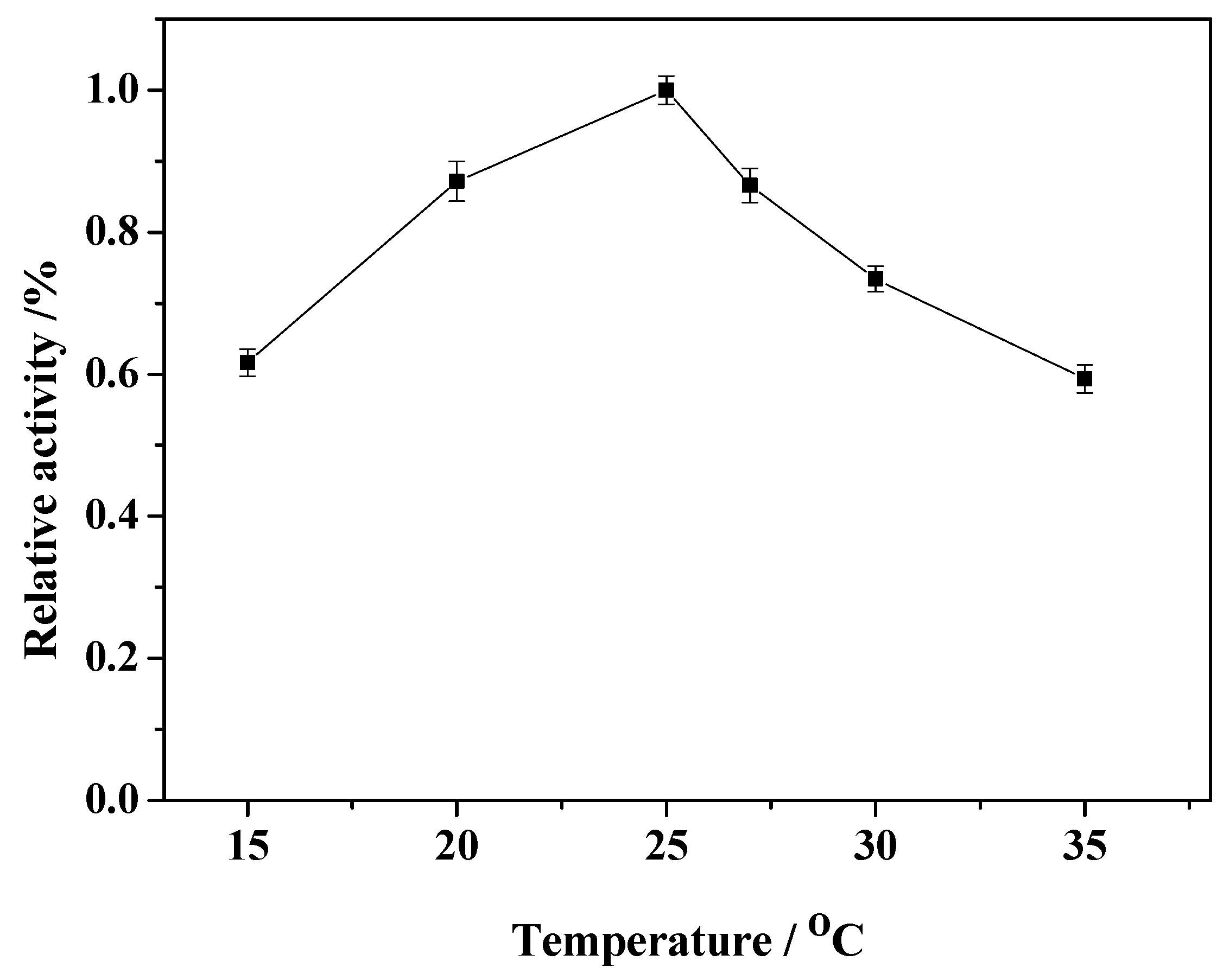
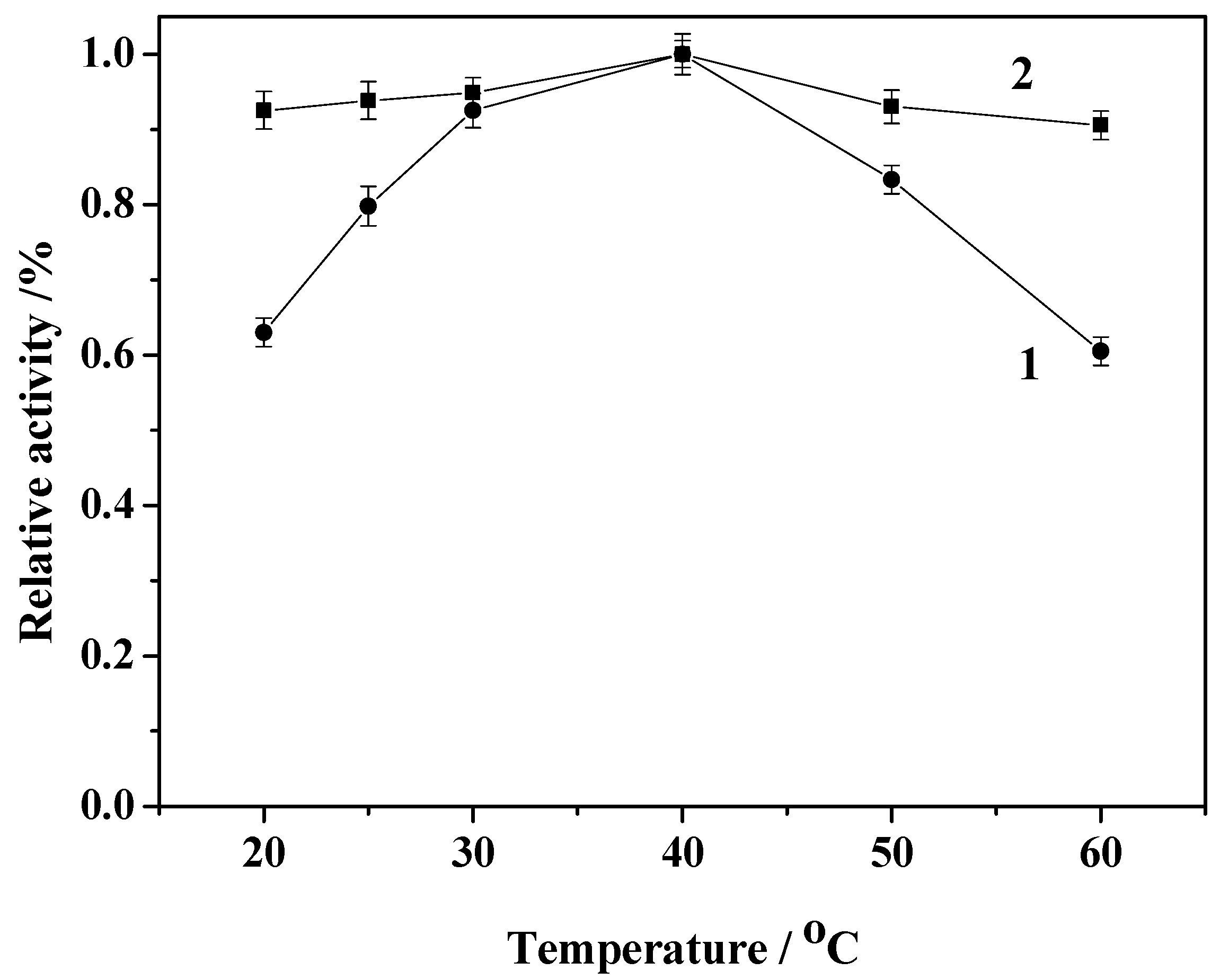
2.5. Effect of Reaction Temperature
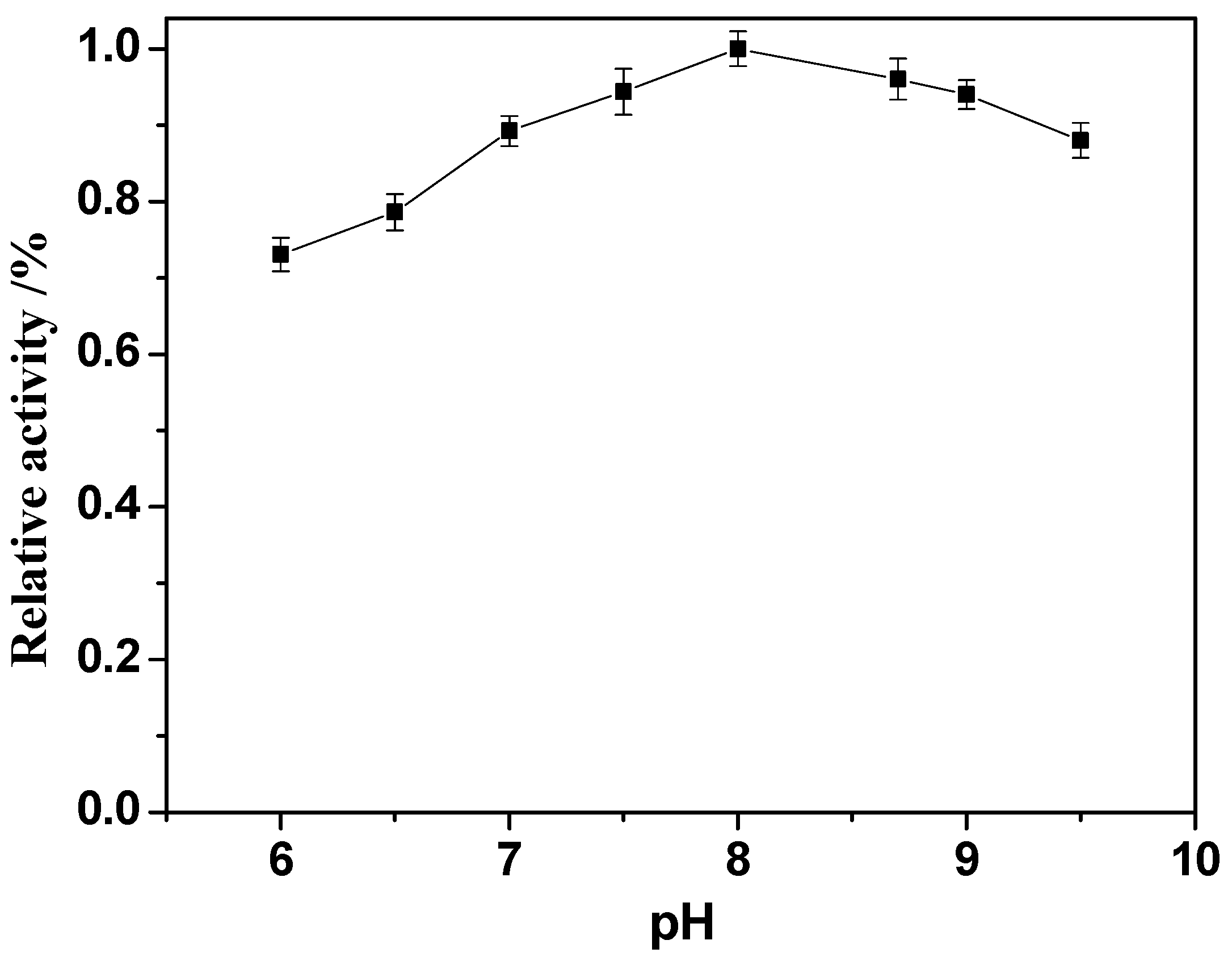
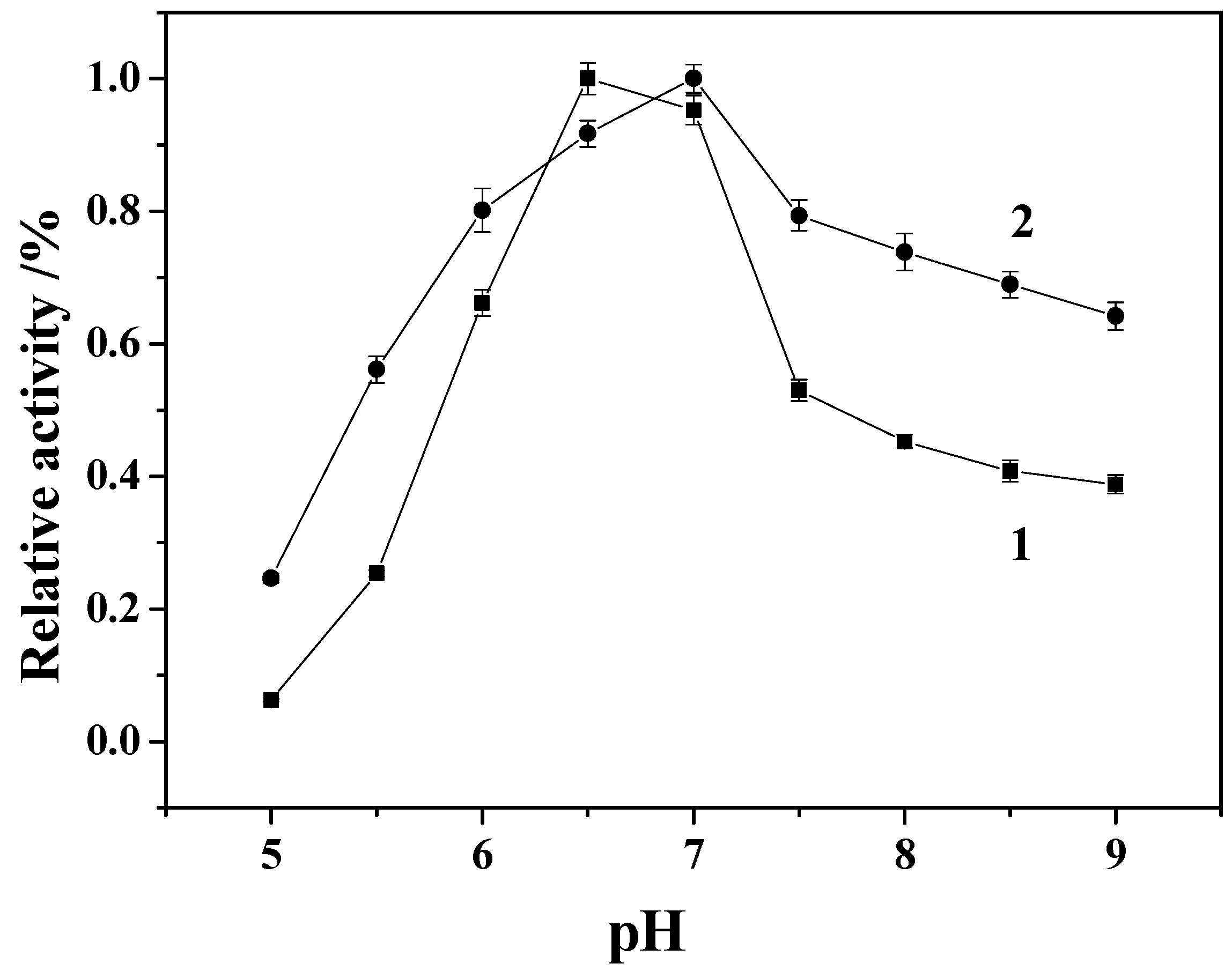
2.6. Effect of pH
2.7. Stability of the Immobilized Enzyme
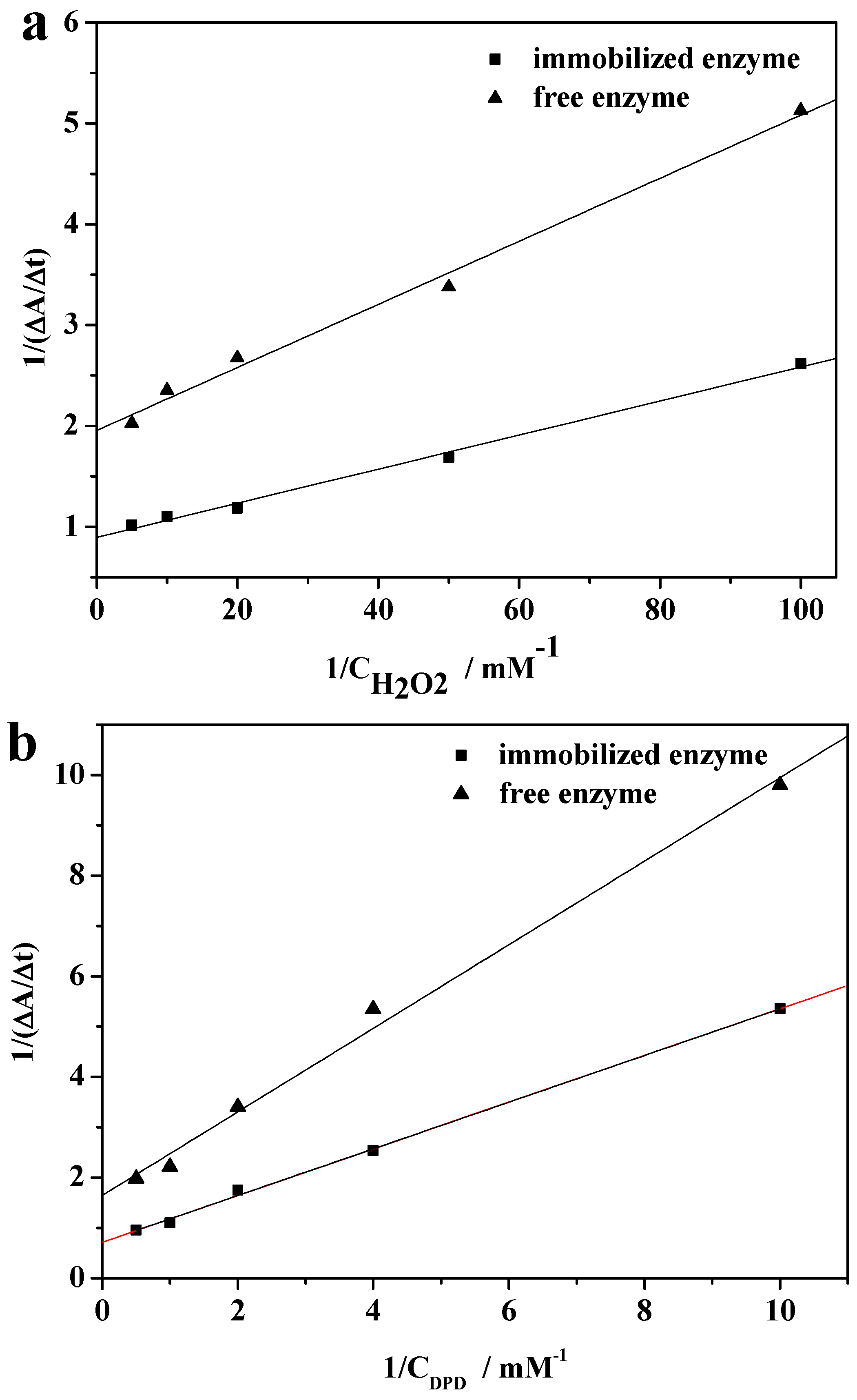
2.8. Kinetic Parameters
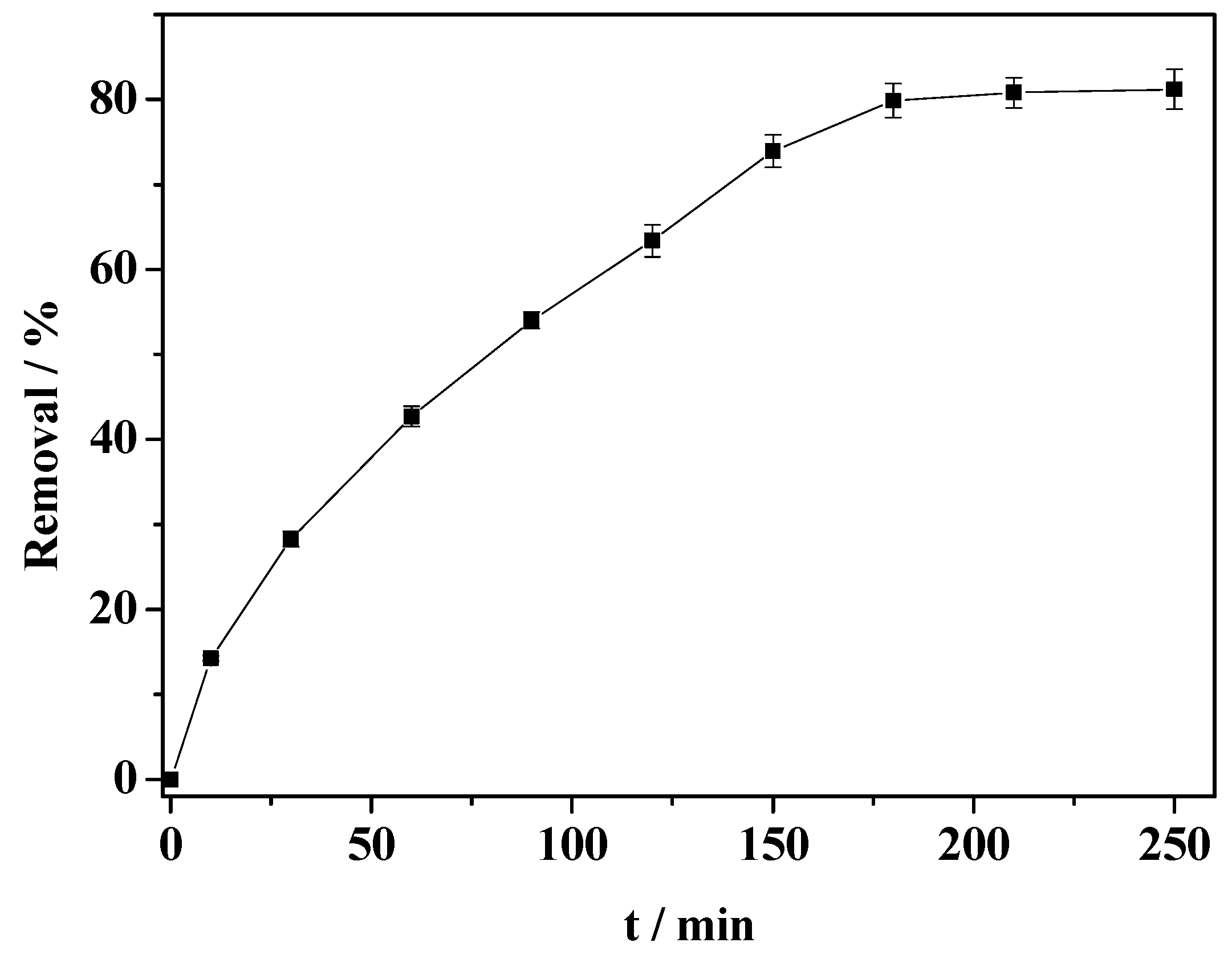
2.9. Catalytic Degradation of 2,4-Dichlorophenol
2.10. Reusability
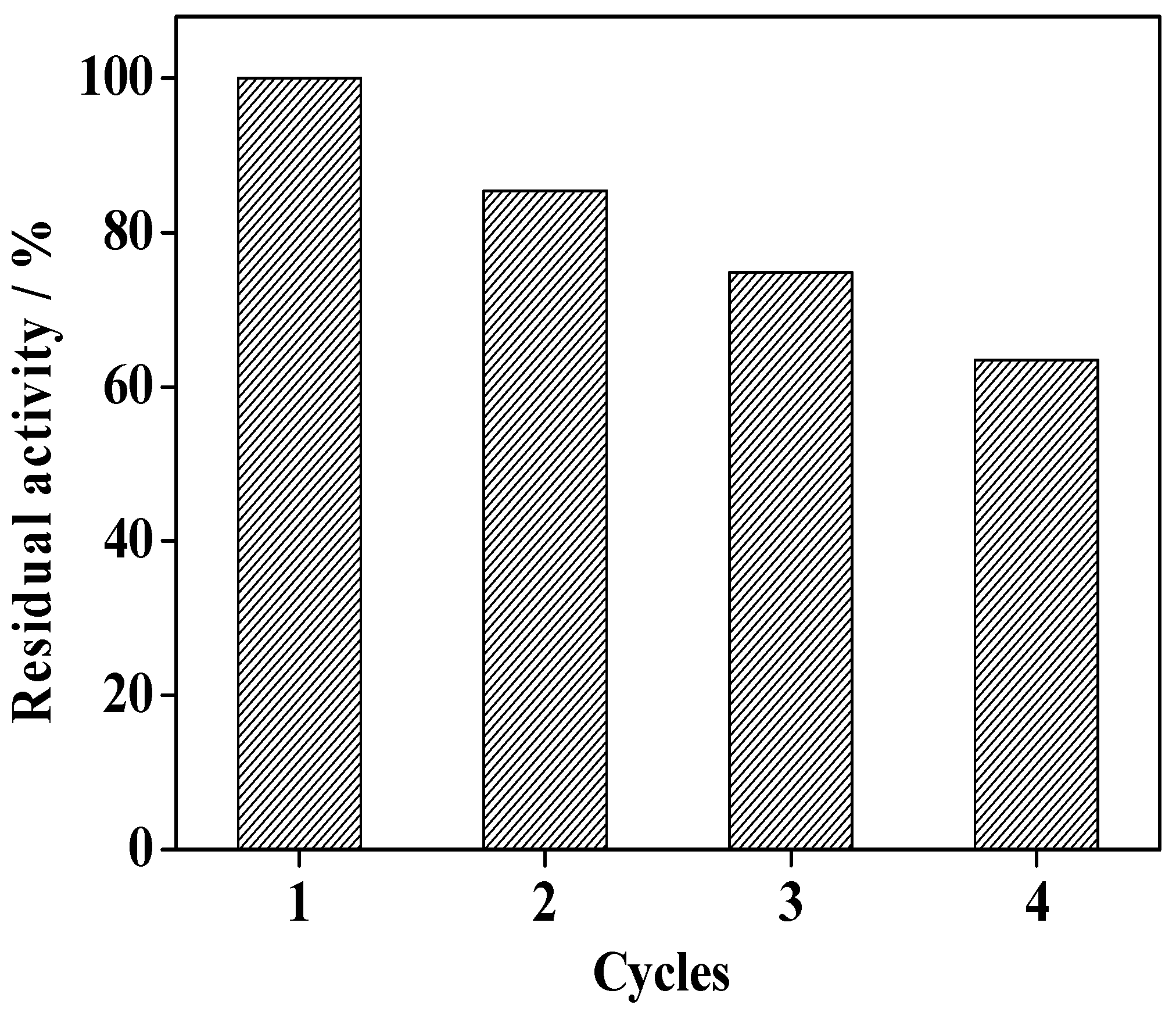
3. Experimental Section
3.1. Reagents
3.2. Preparation of NH2-Modified Fe3O4/SiO2 Particles
3.3. HRP Immobilization
3.4. Catalytic Experiments
3.5. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bayramoğlu, G.; Arıca, M.Y. Enzymatic removal of phenol and p-Chlorophenol in enzyme reactor: Horseradish peroxidase immobilized on magnetic beads. J. Hazard. Mater. 2008, 156, 148–155. [Google Scholar] [CrossRef]
- Magario, I.; García Einschlag, F.S.; Rueda, E.H.; Zygadlo, J.; Ferreira, M.L. Mechanisms of radical generation in the removal of phenol derivatives and pigments using different Fe-based catalytic systems. J. Mol. Catal. A Chem. 2012, 352, 1–20. [Google Scholar] [CrossRef]
- Laurenti, E.; Ghibaudi, E.; Todaro, G.; Ferrari, R.P. Enzymatic degradation of 2,6-Dichlorophenol by horseradish peroxidase: UV-visible and mass spectrophotometric characterization of the reaction products. J. Inorg. Biochem. 2002, 92, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, S.; Wang, P.; Wang, H. Degradation of high concentration 2,4-Dichlorophenol by simultaneous photocatalytic-enzymatic process using TiO2/UV and laccase. J. Hazard. Mater. 2012, 205, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhu, L.H.; Jiang, G.D.; Tang, H.Q. Sensitive fluorescent probes for determination of hydrogen peroxide and glucose based on enzyme-immobilized magnetite/silica nanoparticles. Anal. Bioanal. Chem. 2009, 395, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Aybastıer, Ö; Demir, C. Optimization of immobilization conditions of Thermomyces lanuginosus lipase onstyrene-divinylbenzene copolymer using response surface methodology. J. Mol. Catal. B Enzym. 2010, 63, 170–178. [Google Scholar]
- Won, K.; Kim, S.; Kim, K.J.; Park, H.W.; Moon, S.J. Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 90–802. [Google Scholar]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.G.C.M.; Toma, H.E.; Andrade, L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 2013, 85–86, 71–92. [Google Scholar] [CrossRef]
- Chang, Q.; Zhu, L.H.; Luo, Z.H.; Lei, M.; Zhang, S.C.; Tang, H.Q. Sono-assisted preparation of magnetic magnesium-aluminum layered double hydroxides and their application for removing fluoride. Ultrason. Sonochem. 2011, 18, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Majewski, A.P.; Stahlschmidt, U.; Jérôme, V.; Freitag, R.; Müller, A.H.E.; Schmalz, H. PDMAEMA-grafted core-shell-corona particles for nonviral gene delivery and magnetic cell separation. Biomacromolecules 2013, 14, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, A.; Guardia, P.; Curcio, A.; Garcia, M.A.; Cingolani, R.; Manna, L.; Pellegrino, T. Subnanometer local temperature probing and remotely controlled drug release based on azo-functionalized iron oxide nanoparticles. Nano Lett. 2013, 13, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Tang, H.Q. Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Microchim. Acta 2014, 181, 527–534. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Guan, J.; Normandin, F.; Dénommée, S.; Enright, G.; Veres, T.; Simard, B. Multifunctional nano-architecture for biomedical applications. Chem. Mater. 2006, 18, 1920–1927. [Google Scholar] [CrossRef]
- Aissaoui, N.; Bergaoui, L.; Boujday, S.; Lambert, J.-F.; Méthivier, C.; Landoulsi, J. Enzyme immobilization on silane-modified surface through short linkers: Fate of interfacial phases and impact on catalytic activity. Langmuir 2014, 30, 4066–4077. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, L.; Wang, D.; Wang, M.; Lin, Z.; Tang, H. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason. Sonochem. 2010, 17, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Salgueiriño-Maceira, V.; Correa-Duarte, M.A.; Spasova, M.; Liz-Marzán, L.M.; Farle, M. Composite silica spheres with magnetic and luminescent functionalities. Adv. Funct. Mater. 2006, 16, 509–514. [Google Scholar]
- Chui, W.K.; Wan, L.S. Prolonged retention of cross-linked trypsin in calcium alginate microspheres. J. Microencapsul. 1997, 14, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, S.; Gao, G. Immobilization of β-Galactosidase onto magnetic beads. Appl. Biochem. Biotechnol. 2010, 160, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Ranjbakhsh, E.; Bordbar, A.K.; Abbasi, M.; Khosropour, A.R.; Shams, E. Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem. Eng. J. 2012, 179, 272–276. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.-X.; Wang, Z.-J.; Park, D.; Chung, H.Y.; Wang, S.-F.; Yan, L.; Yang, J.-M.; Qian, G.-Y.; Yin, S.-J.; Park, Y.-D. Effect of hesperetin on tyrosinase: Inhibition kinetics integrated computational simulation study. Int. J. Biol. Macromol. 2012, 50, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Deng, K.J.; Zhu, L.H.; Jiang, G.D.; Yu, C.; Tang, H.Q. Determination of hydrogen peroxide with the aid of peroxidase-like Fe3O4 magnetic nanoparticles as the catalyst. Microchim. Acta 2009, 165, 299–305. [Google Scholar]
- Cheng, J.; Yu, S.M.; Zuo, P. Horseradish peroxidase immobilized on aluminum-pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res. 2006, 40, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nicell, J.A.; Wright, H. A model of peroxidase activity with inhibition by hydrogen peroxide. Enzym. Microb. Technol. 1997, 21, 302–310. [Google Scholar] [CrossRef]
- Wu, Y.M.; Taylor, K.E.; Biswas, N.; Bewtra, J.K. A model for the protective effect of additives on the activity of horseradish peroxidase in the removal of phenol. Enzym. Microb. Technol. 1998, 22, 315–322. [Google Scholar]
- Sample Availability: Samples of the compounds Fe3O4 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Tang, H. Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and Its Application in Removal of 2,4-Dichlorophenol. Molecules 2014, 19, 15768-15782. https://doi.org/10.3390/molecules191015768
Chang Q, Tang H. Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and Its Application in Removal of 2,4-Dichlorophenol. Molecules. 2014; 19(10):15768-15782. https://doi.org/10.3390/molecules191015768
Chicago/Turabian StyleChang, Qing, and Heqing Tang. 2014. "Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and Its Application in Removal of 2,4-Dichlorophenol" Molecules 19, no. 10: 15768-15782. https://doi.org/10.3390/molecules191015768
APA StyleChang, Q., & Tang, H. (2014). Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and Its Application in Removal of 2,4-Dichlorophenol. Molecules, 19(10), 15768-15782. https://doi.org/10.3390/molecules191015768



