Design, Synthesis and SAR Studies of NAD Analogues as Potent Inhibitors towards CD38 NADase
Abstract
:1. Introduction
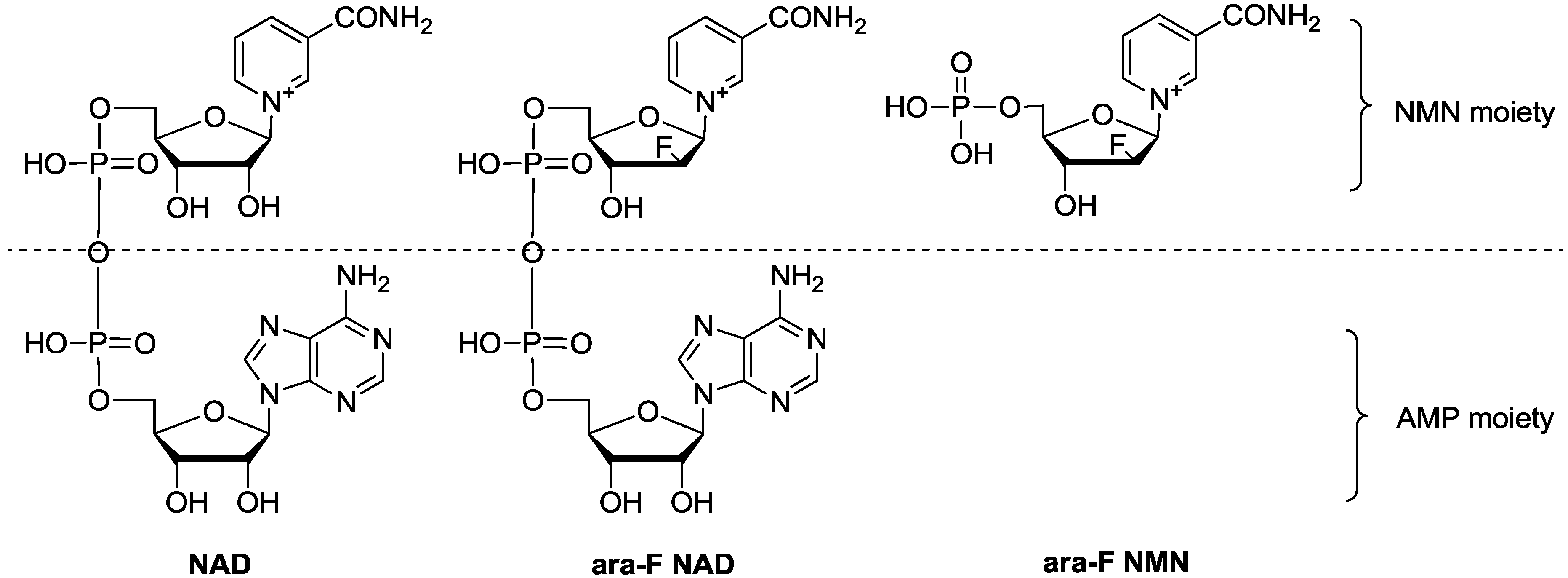
2. Results and Discussion
2.1. Chemistry
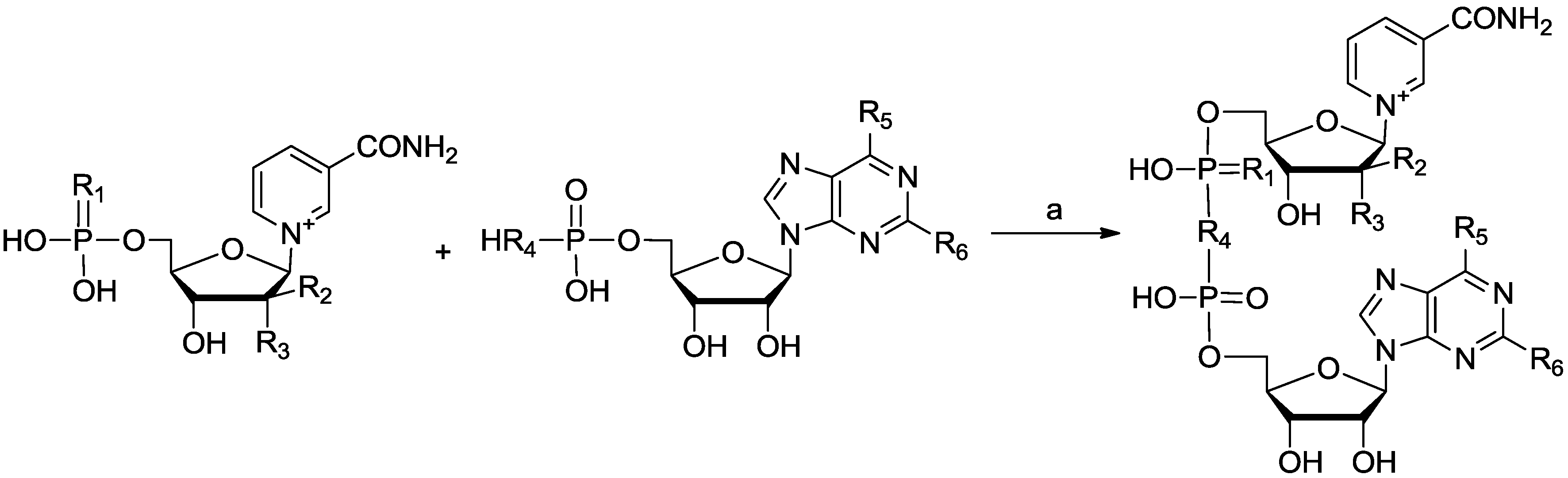
| Entry | R1 | R2 | R3 | R4 | R5 | R6 | Product No. | Yield |
|---|---|---|---|---|---|---|---|---|
| 1 | O | F | H | O | NH2 | H | Ara-F NAD | 40% |
| 2 | O | CH3 | F | O | NH2 | H | 7 | 10% |
| 3 | S | F | H | O | NH2 | H | 10 | 29% |
| 4 | O | F | H | HOPO3 | NH2 | H | 11 | 6% |
| 5 | O | F | H | O | OH | H | 12 | 15% |
| 6 | O | F | H | O | OH | NH2 | 13 | 10% |
| 7 | O | F | H | O | OCH3 | H | 19 | 35% |



2.2. Biological Evaluation
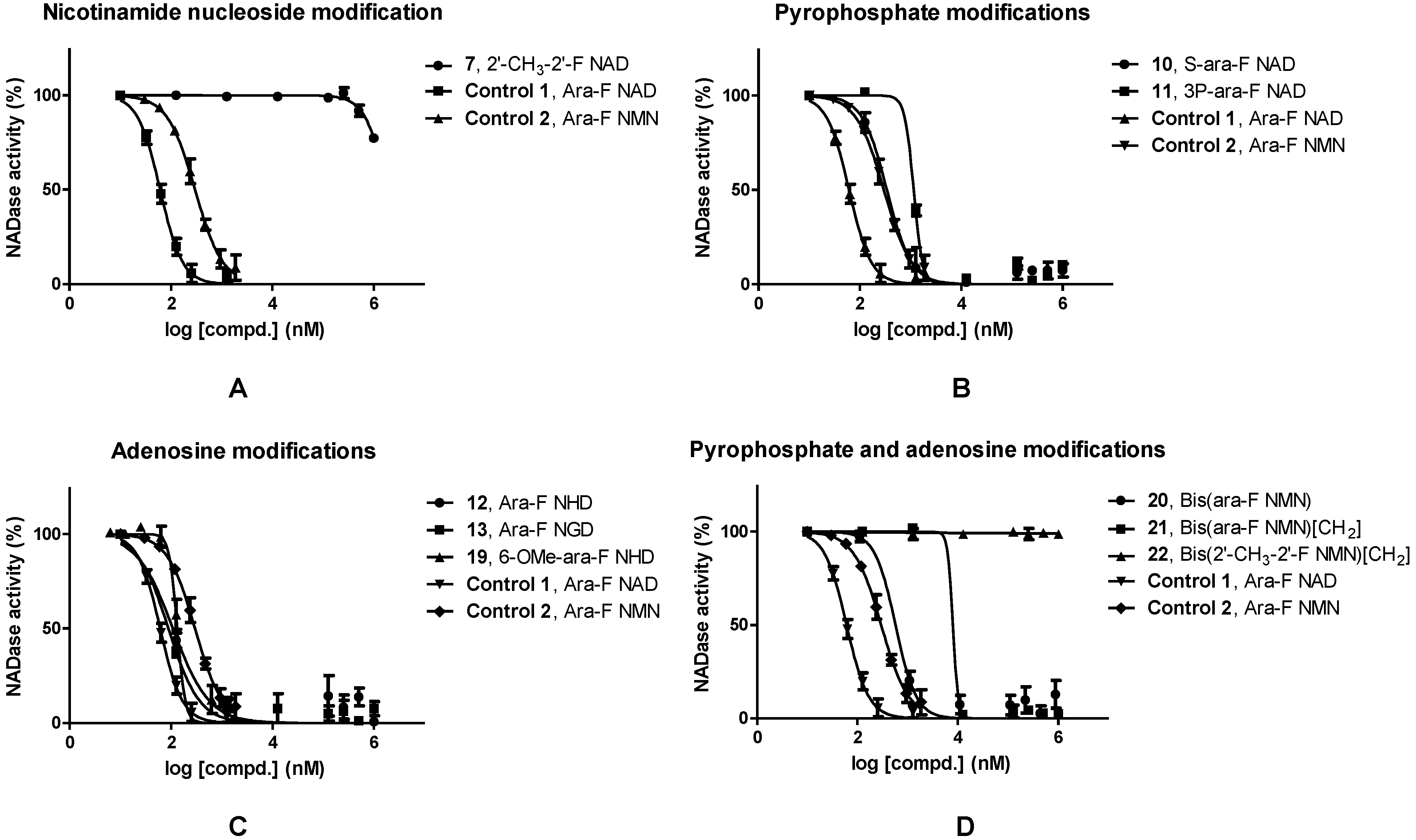
| Modified Approach | Compd. No. | Compd. Name | IC50/nM |
|---|---|---|---|
| Control 1 | 23 | Ara-F NAD | 61.1 |
| Control 2 | 8 | Ara-F NMN | 297 |
| Nicotinamide nucleoside | 7 | 2′-CH3-2′-F NAD | 1.81 × 106 |
| Pyrophosphate | 10 | S-ara-F NAD | 341 |
| 11 | 3P-ara-F NAD | 1.15 × 103 | |
| Adenosine | 12 | Ara-F NHD | 109 |
| 13 | Ara-F NGD | 89.3 | |
| 19 | 6-OMe-ara-F NHD | 133 | |
| Dimer | 20 | Bis(ara-F NMN) | 575 |
| 21 | Bis(ara-F NMN)[CH2] | 7.98 × 103 | |
| 22 | Bis(2′-CH3-2′-F NMN)[CH2] | n.s. |
3. Experimental Section
3.1. General Information
3.2. Chemistry
General Procedure: Coupling Reaction to Synthesize NAD Analogues
3.3. Enzyme Activity Assay
4. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef]
- Lin, H.N. Nicotinamide adenine dinucleotide: Beyond a redox coenzyme. Org. Biomol. Chem. 2007, 5, 2541–2554. [Google Scholar] [CrossRef]
- Liu, Q.; Kriksunov, I.A.; Graeff, R.; Lee, H.C.; Hao, Q. Structural basis for formation and hydrolysis of the calcium messenger cyclic ADP-ribose by human CD38. J. Biol. Chem. 2007, 282, 5853–5861. [Google Scholar]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Wilkinson, A.; Day, J.; Bowater, R. Bacterial DNA ligases. Mol. Microbiol. 2001, 40, 1241–1248. [Google Scholar] [CrossRef]
- Reinherz, E.L.; Kung, P.C.; Goldstein, G.; Levey, R.H.; Schlossman, S.F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc. Natl. Acad. Sci. USA 1980, 77, 1588–1592. [Google Scholar] [CrossRef]
- Kato, I.; Yamamoto, Y.; Fujimura, M.; Noguchi, N.; Takasawa, S.; Okamoto, H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i and insulin secretion. J. Biol. Chem. 1999, 274, 1869–1872. [Google Scholar] [CrossRef]
- Partida-Sanchez, S.; Cockayne, D.; Monard, S.; Jacobson, E.L.; Oppenheimer, N.; Garvy, B.; Kusser, K.; Goodricj, S.; Howard, M.; Harmsen, A.; et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001, 7, 1209–1216. [Google Scholar] [CrossRef]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef]
- Galione, A.; Lee, H.C.; Busa, W.B. Ca2+-induced Ca2+ release in sea urchin egg homogenates: Modulation by cyclic ADP-ribose. Science 1991, 253, 1143–1146. [Google Scholar] [CrossRef]
- Churchill, G.C.; Okada, Y.; Thomas, J.M.; Genazzani, A.A.; Patel, S.; Galione, A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 2002, 111, 703–708. [Google Scholar] [CrossRef]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef]
- Sauve, A.A.; Munshi, C.; Lee, H.C.; Schramm, V.L. The reaction mechanism for CD38. A single intermediate is responsible for cyclization, hydrolysis, and base-exchange chemistries. Biochemistry-USA 1998, 37, 13239–13249. [Google Scholar] [CrossRef]
- Liu, Q.; Kriksunov, I.A.; Graeff, R.; Munshi, C.; Lee, H.C.; Hao, Q. Structural basis for the mechanistic understanding of human CD38-controlled multiple catalysis. J. Biol. Chem. 2006, 281, 32861–32869. [Google Scholar] [CrossRef]
- Liu, Q.; Kriksunov, I.A.; Jiang, H.; Graeff, R.; Lin, H.; Lee, H.C.; Hao, Q. Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38. Chem. Biol. 2008, 15, 1068–1078. [Google Scholar]
- Kwong, A.K.Y.; Chen, Z.; Zhang, H.M.; Leung, F.P.; Lam, C.M.C.; Ting, K.Y.; Zhang, L.R.; Hao, Q.; Zhang, L.H.; Lee, H.C. Catalysis-based inhibitors of the calcium signaling function of CD38. Biochemistry 2012, 51, 555–564. [Google Scholar] [CrossRef]
- Zhou, Y.; Ting, K.Y.; Lam, C.M.C.; Kwong, A.K.Y.; Xia, J.; Jin, H.W.; Liu, Z.M.; Zhang, L.R.; Lee, H.C.; Zhang, L.H. Design, synthesis and biological evaluation of noncovalent inhibitors of human CD38 NADase. ChemMedChem 2012, 7, 223–228. [Google Scholar] [CrossRef]
- Wu, D.Y.; Ting, K.Y.; Duan, Y.K.; Li, N.; Li, J.G.; Zhang, L.R.; Lee, H.C.; Zhang, L.H. Synthesis and activity of novel indole derivatives as inhibitors of CD38. Acta Pharm. Sin. B 2013, 3, 245–253. [Google Scholar] [CrossRef]
- Berthelier, V.; Tixier, J.M.; Muller-Steffner, H.; Schuber, F.; Deterre, P. Human CD38 is an authentic NAD(P)+ glycohydrolase. Biochem. J. 1998, 330, 1383–1390. [Google Scholar]
- Sauve, A.A.; Deng, H.T.; Angeletti, R.H.; Schramm, V.L. A covalent intermediate in CD38 is responsible for ADP-ribosylation and cyclization reactions. J. Am. Chem. Soc. 2000, 122, 7855–7859. [Google Scholar] [CrossRef]
- Shrimp, J.H.; Hu, J.; Dong, M.; Wang, B.S.; MacDonald, R.J.; Jiang, H.; Hao, Q.; Yen, A.; Lin, H. Revealing CD38 cellular localization using a cell permeable, mechanism-based fluorescent small molecule probe. J. Am. Chem. Soc. 2014, 136, 5656–5663. [Google Scholar] [CrossRef]
- Gebeyehu, G.; Marquez, V.E.; Cott, A.V.; Cooney, D.A.; Kelley, J.A.; Jayaram, H.N.; Ahluwalia, G.S.; Dion, R.L.; Wilson, Y.A.; Johns, D.G. Ribavirin, tiazofurin, and selenazofurin: mononucleotides and nicotinamide adenine dinucleotide analogues. Synthesis, structure, and interactions with IMP dehydrogenase. J. Med. Chem. 1985, 28, 99–105. [Google Scholar] [CrossRef]
- Chen, Z.; Kwong, A.K.Y.; Yang, Z.J.; Zhang, L.R.; Lee, H.C.; Zhang, L.H. Studies on the synthesis of nicotinamide nucleoside and nucleotide analogues and their inhibitions towards CD38 NADase. Heterocycles 2011, 83, 2837–2850. [Google Scholar] [CrossRef]
- Kristinsson, H.; Nebel, K.; O’Sullivan, A.C.; Struber, F.; Winkler, T.; Yamaguchi, Y. A novel synthesis of sulfamoyl nucleosides. Tetrahedron 1994, 50, 6825–6838. [Google Scholar] [CrossRef]
- Zhang, F.; Yamada, S.; Gu, Q.M.; Jing, P.; Sih, C.J. Synthesis and characterization of cyclic ATP-ribose: A potent mediator of calcium release. Bioorg. Med. Chem. Lett. 1996, 6, 1203–1208. [Google Scholar] [CrossRef]
- Reddy, P.G.; Chun, B.K.; Zhang, H.R.; Rachakonda, S.; Ross, B.S.; Sofia, M.J. Stereoselective synthesis of PSI-352938: A β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyl-3′,5′-cyclic phosphate nucleotide prodrug for the treatment of HCV. J. Org. Chem. 2011, 76, 3782–3790. [Google Scholar] [CrossRef]
- Moreau, C.; Kirchberger, T.; Swarbrick, J.M.; Bartlett, S.J.; Fliegert, R.; Yorgan, T.; Bauche, A.; Harneit, A.; Guse, A.H.; Potter, B.V.L. Structure-activity relationship of adenosine 5′-diphosphoribose at the transient receptor potential melastatin 2 (TRPM2) channel: Rational design of antagonists. J. Med. Chem. 2013, 56, 10079–10102. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (compounds 7, 8, 10–13, 19–23) are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhu, W.; Wang, X.; Li, J.; Zhang, K.; Zhang, L.; Zhao, Y.-J.; Lee, H.C.; Zhang, L. Design, Synthesis and SAR Studies of NAD Analogues as Potent Inhibitors towards CD38 NADase. Molecules 2014, 19, 15754-15767. https://doi.org/10.3390/molecules191015754
Wang S, Zhu W, Wang X, Li J, Zhang K, Zhang L, Zhao Y-J, Lee HC, Zhang L. Design, Synthesis and SAR Studies of NAD Analogues as Potent Inhibitors towards CD38 NADase. Molecules. 2014; 19(10):15754-15767. https://doi.org/10.3390/molecules191015754
Chicago/Turabian StyleWang, Shengjun, Wenjie Zhu, Xuan Wang, Jianguo Li, Kehui Zhang, Liangren Zhang, Yong-Juan Zhao, Hon Cheung Lee, and Lihe Zhang. 2014. "Design, Synthesis and SAR Studies of NAD Analogues as Potent Inhibitors towards CD38 NADase" Molecules 19, no. 10: 15754-15767. https://doi.org/10.3390/molecules191015754
APA StyleWang, S., Zhu, W., Wang, X., Li, J., Zhang, K., Zhang, L., Zhao, Y.-J., Lee, H. C., & Zhang, L. (2014). Design, Synthesis and SAR Studies of NAD Analogues as Potent Inhibitors towards CD38 NADase. Molecules, 19(10), 15754-15767. https://doi.org/10.3390/molecules191015754





