Biological Evaluation of the Activity of Some Benzimidazole-4,7-dione Derivatives
Abstract
:1. Introduction
| Prodrug | Chemical Structure | Chemical Class | Mechanism of Action * | Mechanism of Cytotoxicity | One-Electron Reductases | Two-Electron Reductases | KO2 (µM) |
|---|---|---|---|---|---|---|---|
| TPZ |  | Aromatic N-oxide | 1, 3 [R.] *** | Complex DNA damage | CYPOR ** iNOS ** | NQO1 ** | ±1 |
| SN30000 |  | Aromatic N-oxide | 1, 3 [R.] *** | Complex DNA damage | CYPOR ** | ---- | ±1 |
| AQ4N |  | Aliphatic N-oxide | 2, 5 [Y] *** | Topoisomerase II inhibition | iNOS ** | CYP3A4 ** CYP2S1 ** | ---- |
| EP-0152R plus CB1954 |  | Nitro | 1/2, 4, 5, 6 [Y, Z] *** | DNA interstrand crosslink | CYPOR ** iNOS ** | NQO1 ** NQO2 ** | ---- |
| EO9 |  | Quinone | 1, 4 [X, Y] *** | DNA interstrand crosslink | CYPOR ** | NQO1 ** | ---- |
2. Results and Discussion
2.1. Chemistry
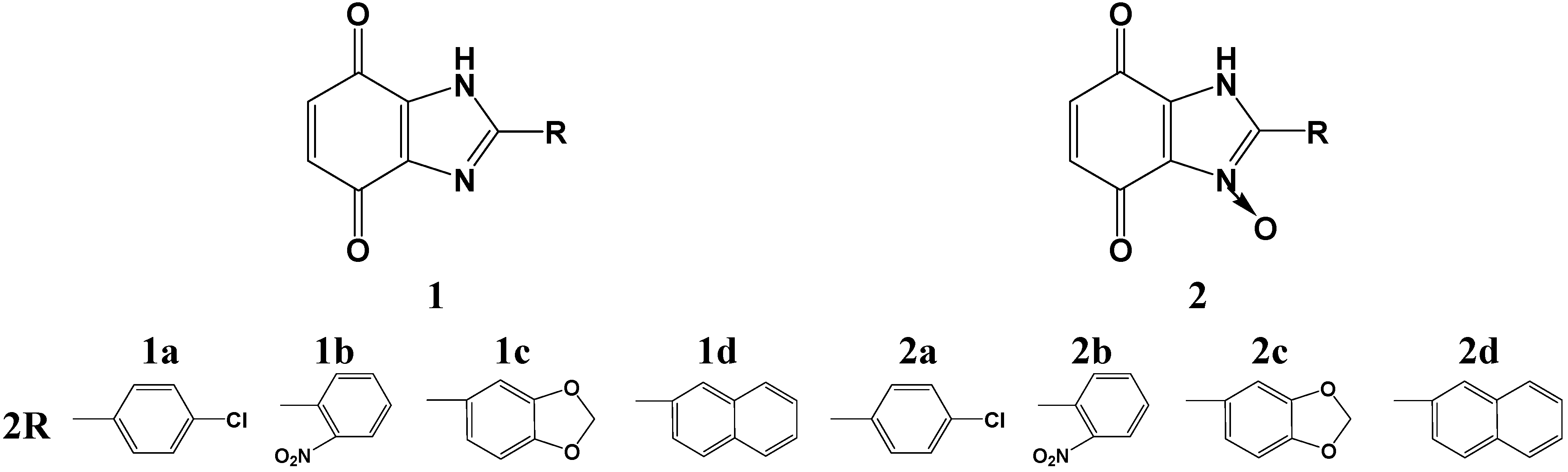
2.2. Biological Activities
2.3. Effect of Benzimidazole-4,7-diones on Cancer Cell Viability in Normoxia
| Compounds | IC50 [μM] | Differential Cytotoxicity O/H | ||
|---|---|---|---|---|
| Normoxia (O) | Hypoxia (H) | |||
| 1a | A549 | 30.2 ± 1.2 | 36.1 ± 1.2 | 0.83 |
| WM 115 | 26.4 ± 1.6 | 32.5 ± 1.2 | 0.81 | |
| 1b | A549 | 100.0 ± 1.8 | 47.4 ± 1.1 | 2.13 |
| WM 115 | 101.0 ± 1.2 | 45.8 ± 1.8 | 2.20 | |
| 1c | A549 | 80.9 ± 1.9 | 51.2 ± 1.5 | 1.58 |
| WM 115 | 77.9 ± 1.3 | 48.5 ± 1.3 | 1.60 | |
| 1d | A549 | 479.5 ± 3.6 | 232.4 ± 1.4 | 2.06 |
| WM 115 | 399.5 ± 2.2 | 229.2 ± 0.4 | 1.74 | |
| 2a | A549 | 115.7 ± 1.9 | 35.0 ± 1.6 | 3.28 |
| WM 115 | 105.0 ± 0.7 | 33.0 ± 1.9 | 3.18 | |
| 2b | A549 | 500.6 ± 1.2 | 116.0 ± 0.8 | 4.31 |
| WM 115 | 490.6 ± 1.9 | 115.0 ± 1.2 | 4.26 | |
| 2c | A549 | 79.5 ± 1.9 | 44.0 ± 2.5 | 1.80 |
| WM 115 | 65.5 ± 1.3 | 40.0 ± 2.0 | 1.63 | |
| 2d | A549 | 252 ± 2.5 | 96.8 ± 1.9 | 2.59 |
| WM 115 | 189 ± 1.5 | 95.2 ± 2.1 | 1.98 | |
| T | A549 | 166.2 ± 1.6 | 36.0 ± 1.2 | 4.61 |
| WM 115 | 156.2 ± 1.6 | 34.0 ± 1.2 | 4.59 | |
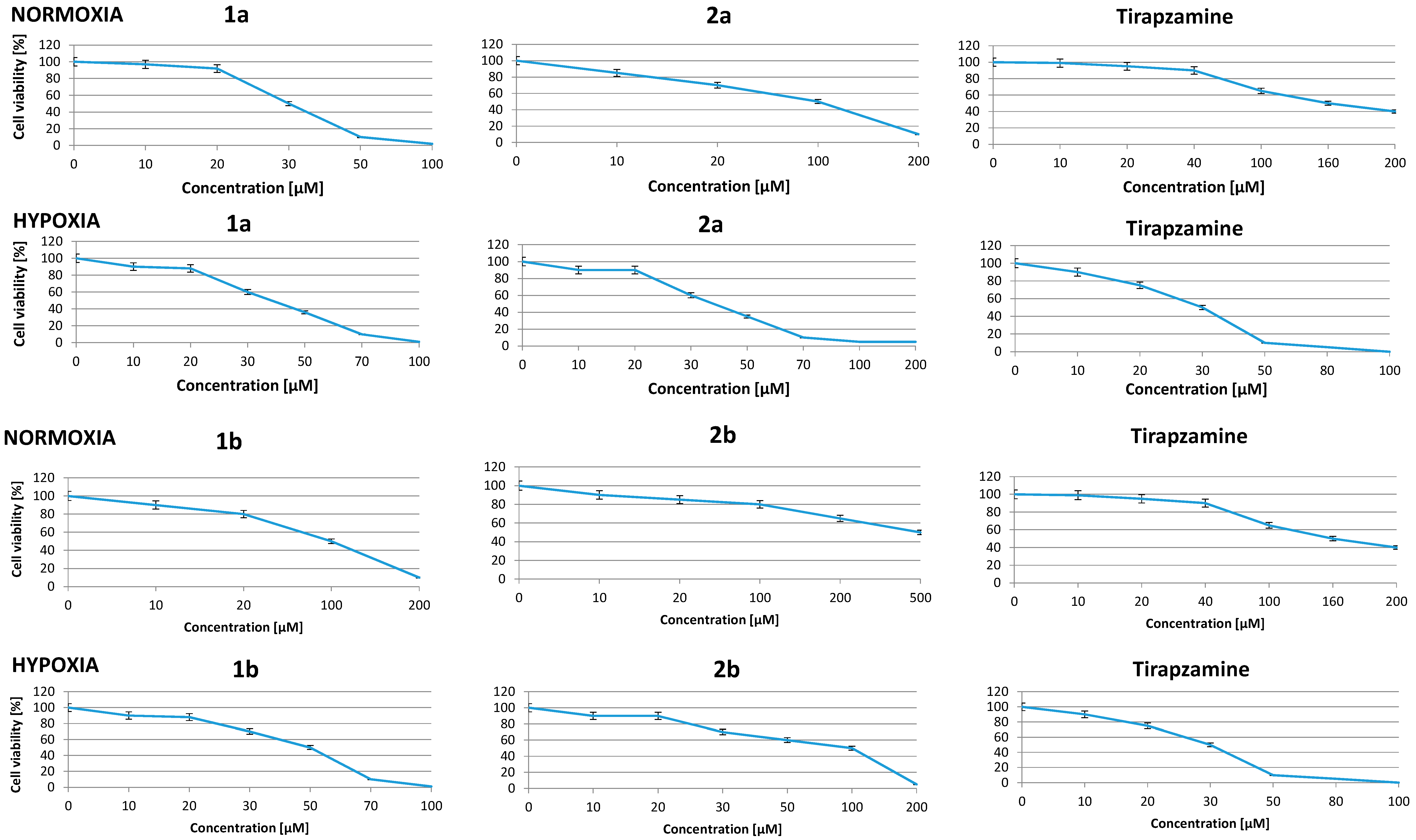
2.4. Effect of New Compounds on Viability of Hypoxic Cells
2.5. Effect of Compounds on Cell Apoptosis
2.6. Effect of Compounds on DNA Damage in Normoxic and Hypoxic Cancer Cells
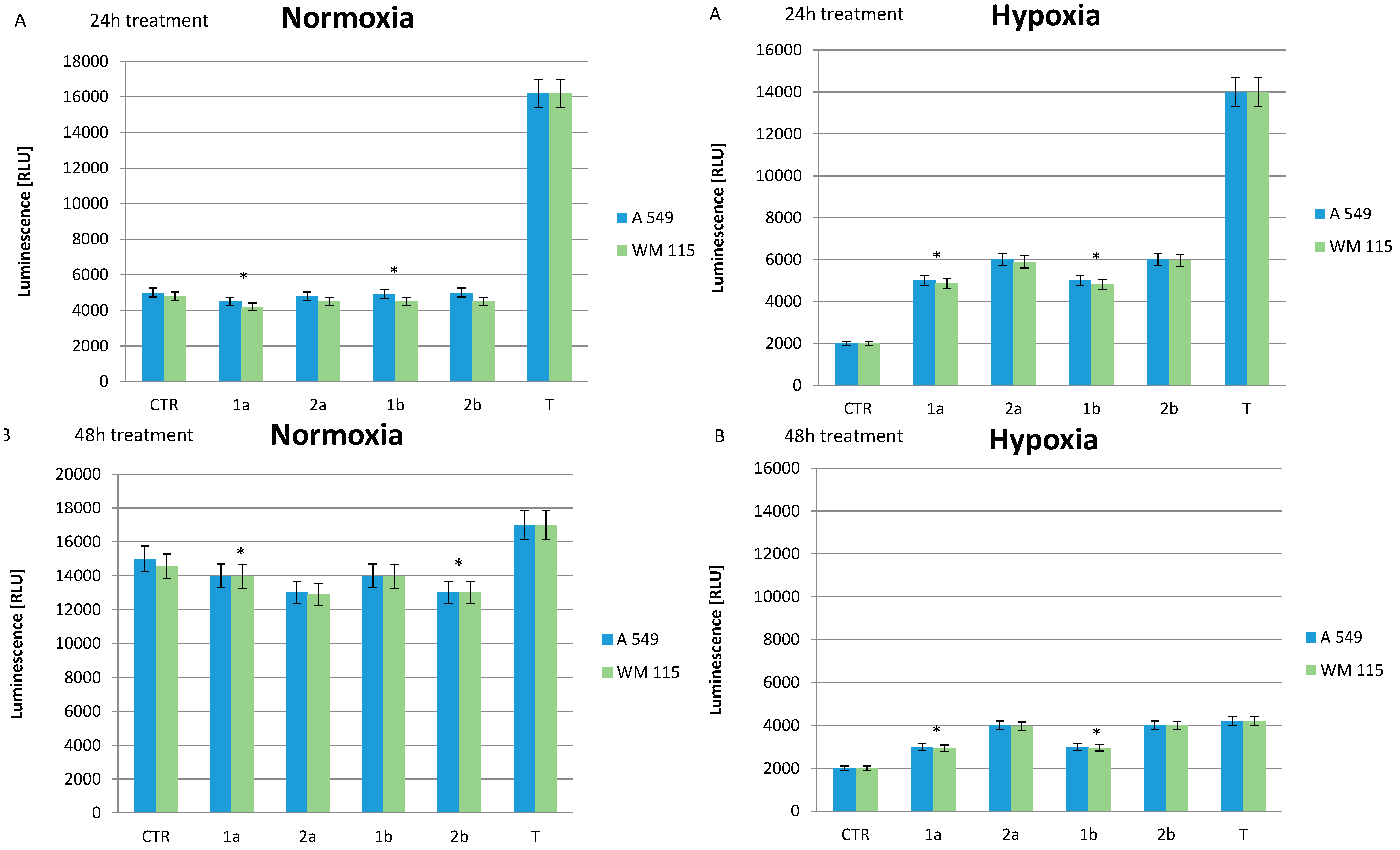
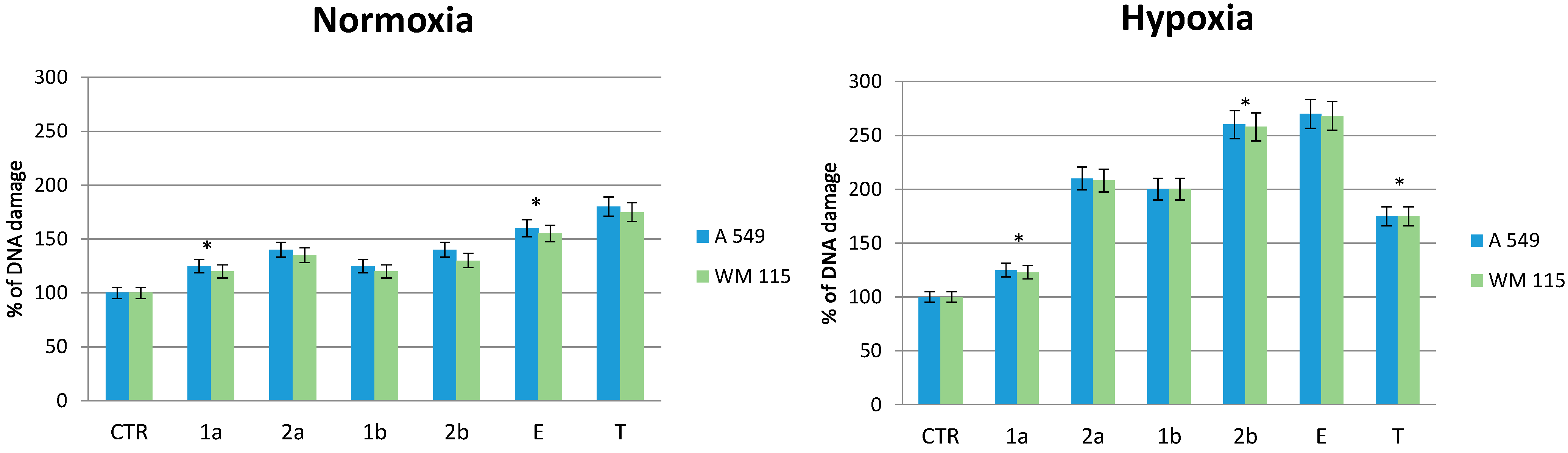
3. Experimental Section
3.1. Biochemistry Experiment Procedures
3.1.1. Cell Culture
3.1.2. DNA Damage Assay
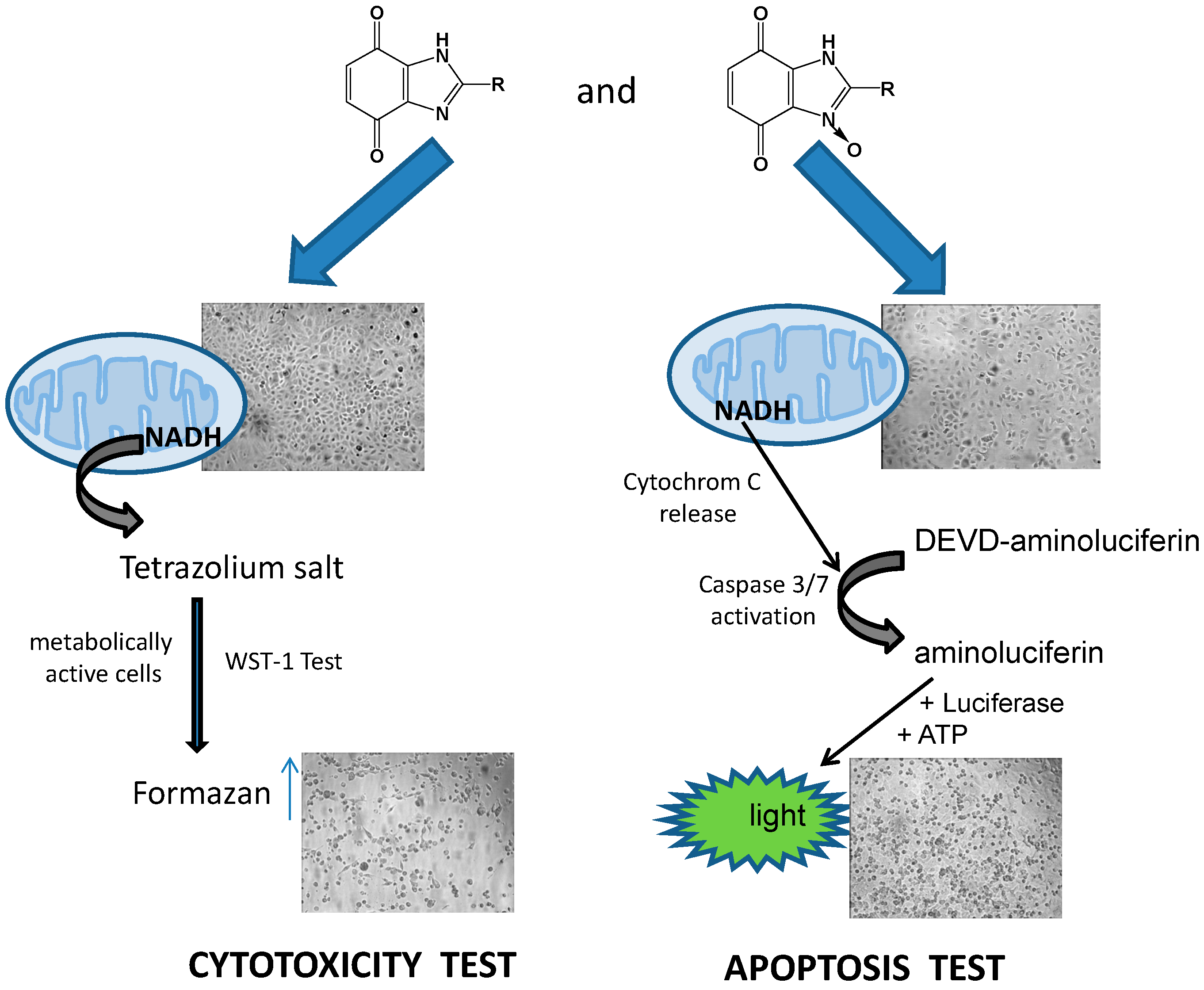
3.1.3. Cell Viability/Cytotoxicity Assay
3.1.4. Apoptosis Assay
3.1.5. Cell Morphology
3.1.6. Statistical Analysis of the Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, J.M.; Wilson, R.W. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar]
- Chung-Faye, G.; Palmer, D.; Anderson, D.; Clark, J.; Downes, M.; Baddeley, J.; Hussain, S.; Murray, P.I.; Searle, P.; Seymour, L.; et al. Virus-directed, enzyme prodrug therapy with nitroimidazole reductase: A phase I and phamacokinetic study of its prodrug, CB1954. Clin. Cancer Res. 2001, 7, 2662–2668. [Google Scholar]
- Tomasz, M. Mitomycin C: Small, fast and deadly (but very selective). Chem. Biol. 1995, 2, 575–579. [Google Scholar]
- Denny, W.A. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 2000, 1, 25–29. [Google Scholar]
- Brown, J.M. The hypoxic cell: A target for selective cancer therapy—eighteenth bruce F. cain memorial award lecture. Cancer Res. 1999, 59, 5863–5870. [Google Scholar]
- Koch, C.J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993, 53, 3992–3997. [Google Scholar]
- Siim, B.G.; Pruijn, F.B.; Sturman, J.R.; Hogg, A.; Hay, M.P.; Brown, J.M.; Wilson, W.R. Selective potentiation of the hypoxic cytotoxicity of tirapazamine by its 1-N-oxide metabolite SR 4317. Cancer Res. 2004, 64, 736–742. [Google Scholar]
- Siemann, D.W.; Hinchman, C.A. Potentiation of cisplatin activity by the bioreductive agent tirapazamine. Radiother. Oncol. 1998, 47, 215–220. [Google Scholar]
- Papadopoulou, M.V.; Bloomer, W.D. NLCQ-1 (NSC 709257): Exploiting hypoxia with a weak DNA-intercalating bioreductive drug. Clin. Cancer Res. 2003, 9, 5714–5720. [Google Scholar]
- Patterson, H.; McKeown, S.R. AQ4N: A new approach to hypoxia-activated cancer chemotherapy. Brit. J. Cancer 2000, 83, 1589–1593. [Google Scholar]
- Błaszczak-Świątkiewicz, K.; Mirowski, M.; Kaplińska, K.; Kruszyński, R.; Trzęsowska-Kruszyńska, A.; Mikiciuk-Olasik, E. New benzimidazole derivatives with potential cytotoxic activity-study of their stability by RP-HPLC. Acta Biochim. Pol. 2012, 59, 279–288. [Google Scholar]
- Jin, S.; Kim, J.S.; Sim, S.P.; Liu, A.; Pilch, D.S.; Liu, L.F.; LaVoie, E.J. Heterocyclic bibenzimidazole derivatives as topoisomerase I inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 719–723. [Google Scholar]
- Alpan, A.S.; Gunes, H.S.; Topcu, Z. 1H-Benzimidazole derivatives as mammalian DNA topoisomerase I inhibitors. Acta Biochim. Pol. 2007, 54, 561–565. [Google Scholar]
- Garuti, L.; Roberti, M.; Malagoli, M.; Rossi, T.; Castelli, M. Synthesis and antiproliferative activity of some benzimidazole-4,7-dione derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 2193–2195. [Google Scholar]
- Antonini, I.; Claudi, F.; Cristalli, G.; Franchetti, P.; Grifantini, M.; Martelli, S. Heterocyclic quinones with potential antitumor activity. Synthesis and antitumor activuty of some benzimidazole-4,7-dione derivatives. J. Med. Chem. 1988, 31, 260–264. [Google Scholar]
- Garuti, L.; Roberti, M.; Pizzirani, D.; Pession, A.; Leoncini, E.; Cenci, V.; Hrelia, S. Differential antiproliferative activity of new benzimidazole-4,7-diones. Il Farmaco 2004, 59, 663–668. [Google Scholar]
- Boufatah, N.; Gellis, A.; Maldonado, J.; Vanelle, P. Efficient microwave-assisted synthesis of new sulfonylbenzimidazole-4,7-diones: Heterocyclic quinones with potential antitumor activity. Tetrahedron 2004, 60, 9131–9137. [Google Scholar]
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998, 58, 1408–1416. [Google Scholar]
- Błaszczak-Swiątkiewicz, K.; Almeida, D.C.; Perry, M.D.J.; Mikiciuk-Olasik, E. Synthesis, anticancer activity and UPLC analysis of the stability of some new benzimidazole-4,7-dione derivatives. Molecules 2014, 19, 400–413. [Google Scholar]
- Sample Availability: Samples of the compounds 1–2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczak-Świątkiewicz, K.; Mikiciuk-Olasik, E. Biological Evaluation of the Activity of Some Benzimidazole-4,7-dione Derivatives. Molecules 2014, 19, 15361-15373. https://doi.org/10.3390/molecules191015361
Błaszczak-Świątkiewicz K, Mikiciuk-Olasik E. Biological Evaluation of the Activity of Some Benzimidazole-4,7-dione Derivatives. Molecules. 2014; 19(10):15361-15373. https://doi.org/10.3390/molecules191015361
Chicago/Turabian StyleBłaszczak-Świątkiewicz, Katarzyna, and Elżbieta Mikiciuk-Olasik. 2014. "Biological Evaluation of the Activity of Some Benzimidazole-4,7-dione Derivatives" Molecules 19, no. 10: 15361-15373. https://doi.org/10.3390/molecules191015361
APA StyleBłaszczak-Świątkiewicz, K., & Mikiciuk-Olasik, E. (2014). Biological Evaluation of the Activity of Some Benzimidazole-4,7-dione Derivatives. Molecules, 19(10), 15361-15373. https://doi.org/10.3390/molecules191015361




