Stereoselective Synthesis and Cytoselective Toxicity of Monoterpene-Fused 2-Imino-1,3-thiazines
Abstract
:1. Introduction
2. Results and Discussion
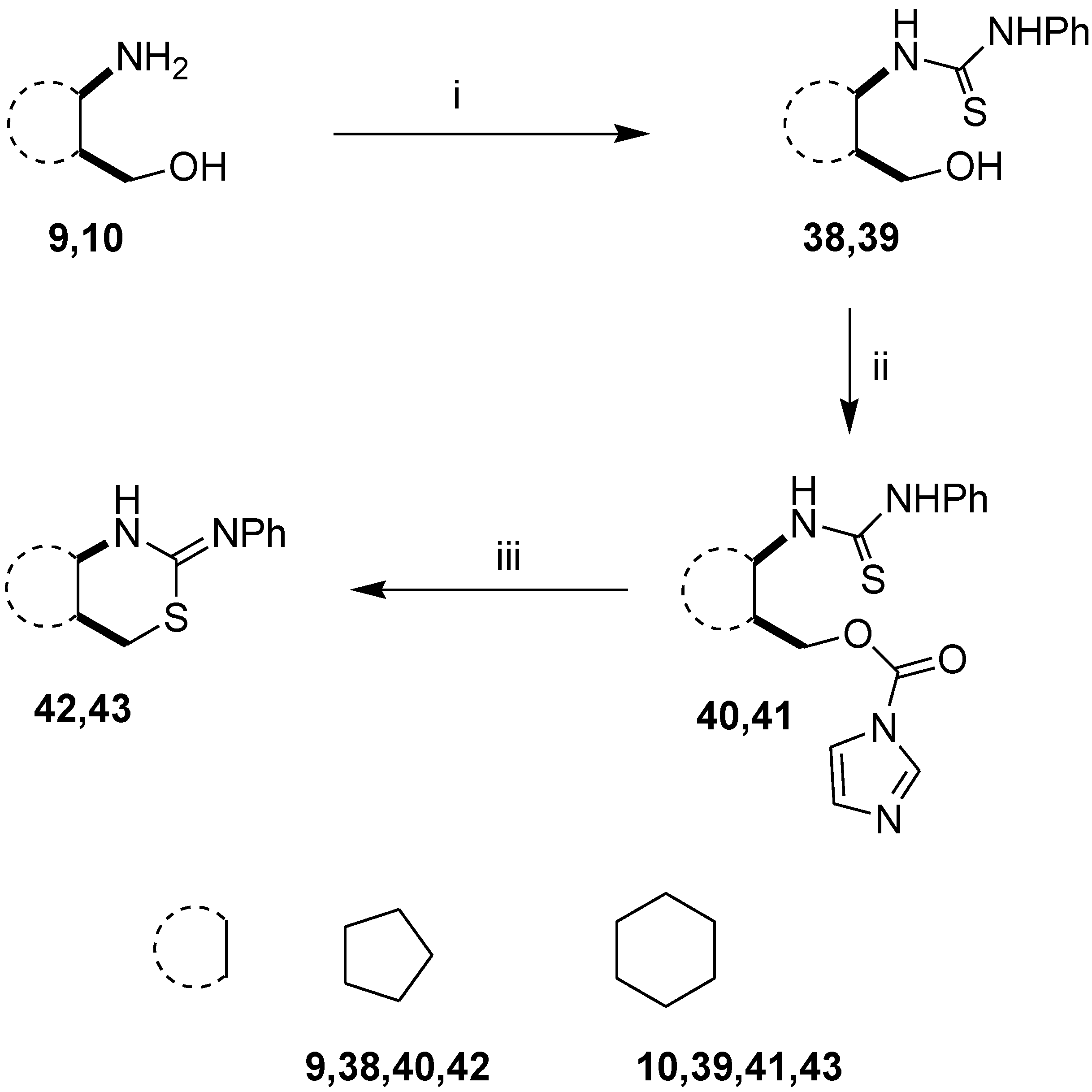
2.1. Syntheses of Alicyclic and Monoterpene-Based 1,3-Amino Alcohols

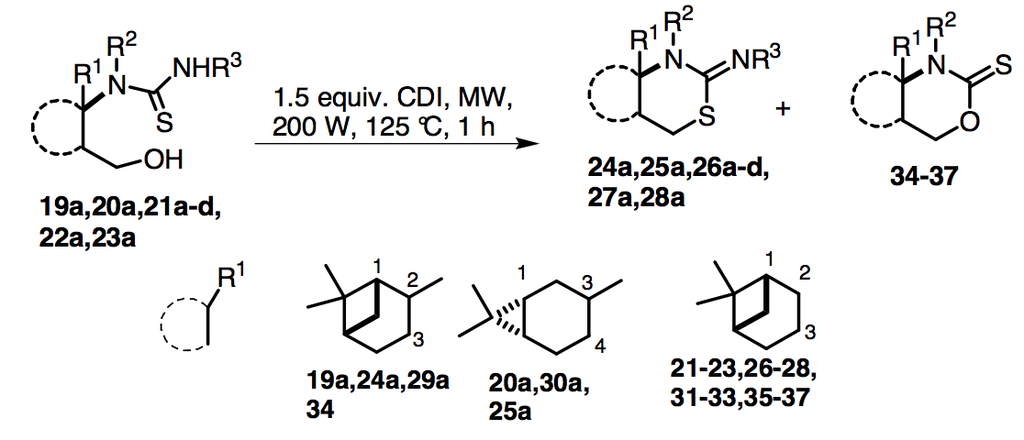
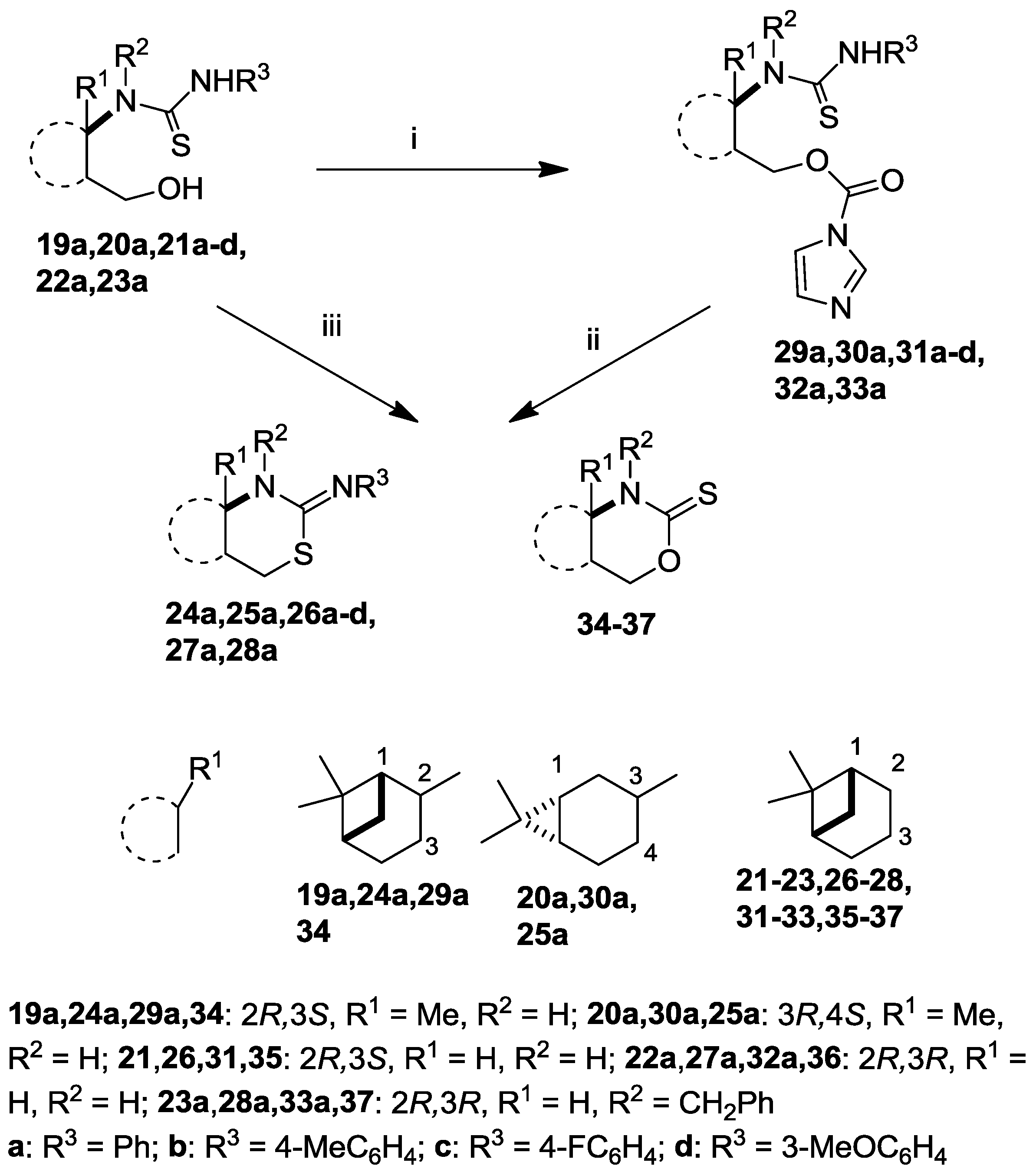
2.2. Syntheses of 2-Imino-1,3-thiazine Derivatives

| General Structure | R1 | R2 | R3 | Compound No. |
|---|---|---|---|---|
 | Me | H | Ph | 19a |
| H | H | Ph | 21a | |
| H | H | 4-MeC6H4 | 21b | |
| H | H | 4-FC6H4 | 21c | |
| H | H | 3-MeOC6H4 | 21d | |
 | H | H | Ph | 22a |
| H | CH2Ph | Ph | 23a | |
 | Me | H | Ph | 20a |
 | Me | H | Ph | 29a |
| H | H | Ph | 31a | |
| H | H | 4-MeC6H4 | 31b | |
| H | H | 4-FC6H4 | 31c | |
| H | H | 3-MeOC6H4 | 31d | |
 | H | H | Ph | 32a |
| H | CH2Ph | Ph | 33a | |
 | H | H | H | 30a |

| General Structure | R1 | R2 | R3 | Compound No. |
|---|---|---|---|---|
 | Me | H | Ph | 24a |
| H | H | Ph | 26a | |
| H | H | 4-MeC6H4 | 26b | |
| H | H | 4-FC6H4 | 26c | |
| H | H | 3-MeOC6H4 | 26d | |
 | H | H | Ph | 27a |
| H | CH2Ph | Ph | 28a | |
 | Me | H | Ph | 25a |
2.3. Antiproliferative Activities
| Compnd. | Conc. | Growth Inhibition, % ± SEM a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HeLa | A2780 | MCF7 | A431 | |||||||
| 24a | 10 µM | - | 67.45 ± 0.83 | 39.40 ± 2.41 | 73.01 ± 1.52 | |||||
| 30 µM | 25.59 ± 1.93 | 86.02 ± 0.27 | 62.08 ± 1.78 | 83.15 ± 1.25 | ||||||
| 25a | 10 µM | - | 65.24 ± 1.65 | 65.91 ± 0.96 | 60.54 ± 1.79 | |||||
| 30 µM | 96.40 ± 0.28 | 96.42 ± 0.15 | 86.09 ± 1.20 | 94.17 ± 0.51 | ||||||
| 26a | 10 µM | - | - | 26.52 ± 2.79 | 67.70 ± 1.49 | |||||
| 30 µM | 22.03 ± 1.57 | 68.93 ± 0.97 | 50.45 + 1.79 | 85.68 ± 0.74 | ||||||
| 26b | 10 µM | - | 45.45 ± 2.01 | - | 65.20 ± 1.47 | |||||
| 30 µM | 37.02 ± 2.20 | 58.67 ± 1.29 | 43.66 ± 2.32 | 74.49 ± 1.02 | ||||||
| 26c | 10 µM | 44.90 ± 0.88 | 40.02 ± 0.88 | 21.55 ± 0.99 | 32.93 ± 1.20 | |||||
| 30 µM | 53.60 ± 1.07 | 56.86 ± 1.17 | 30.93 ± 1.29 | 34.20 ± 1.02 | ||||||
| 26d | 10 µM | 27.40 ± 0.52 | 20.87 ± 1.87 | - | 34.92 ± 2.66 | |||||
| 30 µM | 33.67 ± 2.61 | 92.17 ± 0.51 | 63.84 ± 2.36 | 82.46 ± 1.11 | ||||||
| 27a | 10 µM | - | 37.26 ± 2.35 | 43.83 ± 0.99 | 77.32 ± 0.94 | |||||
| 30 µM | 21.57 ± 0.92 | 42.80 ± 2.78 | 47.15 ± 2.93 | 80.05 ± 1.15 | ||||||
| 28a | 10 µM | 46.83 ± 1.42 | 23.10 ± 1.00 | - | - | |||||
| 30 µM | 42.24 ± 2.48 | 50.23 ± 0.52 | 23.84 ± 1.08 | - | ||||||
| 35 | 10 µM | - | - | - | - | |||||
| 30 µM | - | - | - | - | ||||||
| 42 | 10 µM | - | - | - | - | |||||
| 30 µM | - | 41.23 ± 1.30 | - | - | ||||||
| 43 | 10 µM | - | - | - | - | |||||
| 30 µM | 24.07 ± 2.96 | - | 32.59 ± 1.49 | - | ||||||
| Cisplatin | 10 µM | 42.61 ± 2.33 | 83.57 ± 1.21 | 53.03 ± 2.29 | 88.54 ± 0.50 | |||||
| 30 µM | 99.93 ± 0.26 | 95.02 ± 0.28 | 86.90 ± 1.24 | 90.18 ± 1.78 | ||||||
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Thioureas 21–23
 = +52.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (1H, d, J = 9.9 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.63–1.70 (1H, m), 1.72–1.79 (1H, m), 1.88–1.94 (1H, m), 1.98–2.18 (3H, m), 2.54–2.65 (1H, m), 3.48-3.63 (2H, m), 5.15 (1H, t, J = 8.2 Hz), 7.16 (1H, d, J = 8.5 Hz), 7.19-7.44 (5H, m), 7.68 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 26.8, 30.0, 32.6, 39.3, 40.7, 46.4, 57.0, 65.1, 125.7, 127.6, 130.3, 136.5, 180.2. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.39; H, 8.13; N, 9.01; S, 10.42%.
= +52.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (1H, d, J = 9.9 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.63–1.70 (1H, m), 1.72–1.79 (1H, m), 1.88–1.94 (1H, m), 1.98–2.18 (3H, m), 2.54–2.65 (1H, m), 3.48-3.63 (2H, m), 5.15 (1H, t, J = 8.2 Hz), 7.16 (1H, d, J = 8.5 Hz), 7.19-7.44 (5H, m), 7.68 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 26.8, 30.0, 32.6, 39.3, 40.7, 46.4, 57.0, 65.1, 125.7, 127.6, 130.3, 136.5, 180.2. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.39; H, 8.13; N, 9.01; S, 10.42%. = +63.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (1H, d, J = 10.1 Hz), 1.01 (3H, s), 1.22 (3H, s), 1.62–1.68 (1H, m), 1.73–1.86 (1H, m), 1.87–1.93 (1H, m), 1.98–2.16 (3H, m), 2.35 (3H, s), 2.54–2.64 (1H, m), 3.47–3.61 (2H, m), 5.15 (1H, br s), 7.05 (1H, d, J = 8.5 Hz), 7.09 (2H, d, J = 8.2 Hz), 7.20 (2H, d, J = 8.2 Hz). 13C-NMR (CDCl3) δ (ppm) 21.2, 21.5, 26.8, 26.9, 30.0, 32.8, 39.4, 40.8, 46.5, 57.0, 65.3, 126.0, 131.0, 132.8, 137.7, 151.9, 180.1. Anal. Calcd for C18H26N2OS (318.48): C, 67.88; H, 8.23; N, 8.80; S, 10.07%; Found: C, 68.13; H, 8.35; N, 8.69; S, 9.94%.
= +63.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (1H, d, J = 10.1 Hz), 1.01 (3H, s), 1.22 (3H, s), 1.62–1.68 (1H, m), 1.73–1.86 (1H, m), 1.87–1.93 (1H, m), 1.98–2.16 (3H, m), 2.35 (3H, s), 2.54–2.64 (1H, m), 3.47–3.61 (2H, m), 5.15 (1H, br s), 7.05 (1H, d, J = 8.5 Hz), 7.09 (2H, d, J = 8.2 Hz), 7.20 (2H, d, J = 8.2 Hz). 13C-NMR (CDCl3) δ (ppm) 21.2, 21.5, 26.8, 26.9, 30.0, 32.8, 39.4, 40.8, 46.5, 57.0, 65.3, 126.0, 131.0, 132.8, 137.7, 151.9, 180.1. Anal. Calcd for C18H26N2OS (318.48): C, 67.88; H, 8.23; N, 8.80; S, 10.07%; Found: C, 68.13; H, 8.35; N, 8.69; S, 9.94%. = +55.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 9.8 Hz), 1.00 (3H, s), 1.22 (3H, s), 1.62–1.72 (1H, m), 1.87–1.94 (1H, m), 1.98–2.16 (4H, m), 2.51–2.63 (1H, m), 3.51 (1H, dd, J = 4.6, 10.5 Hz), 3.61 (1H, dd, J = 3.2, 10.7 Hz), 5.11 (1H, br s), 7.01–7.26 (4H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.6, 26.7, 29.9, 32.4, 39.3, 40.7, 46.3, 57.0, 65.0, 117.1 (d, J = 23.7 Hz), 128.1 (d, J = 8.5 Hz), 162.6, 180.1. Anal. Calcd for C17H23FN2OS (322.44): C, 63.32; H, 7.19; N, 8.69; S, 9.94%; Found: C, 63.49; H, 7.29; N, 8.48; S, 9.77%.
= +55.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 9.8 Hz), 1.00 (3H, s), 1.22 (3H, s), 1.62–1.72 (1H, m), 1.87–1.94 (1H, m), 1.98–2.16 (4H, m), 2.51–2.63 (1H, m), 3.51 (1H, dd, J = 4.6, 10.5 Hz), 3.61 (1H, dd, J = 3.2, 10.7 Hz), 5.11 (1H, br s), 7.01–7.26 (4H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.6, 26.7, 29.9, 32.4, 39.3, 40.7, 46.3, 57.0, 65.0, 117.1 (d, J = 23.7 Hz), 128.1 (d, J = 8.5 Hz), 162.6, 180.1. Anal. Calcd for C17H23FN2OS (322.44): C, 63.32; H, 7.19; N, 8.69; S, 9.94%; Found: C, 63.49; H, 7.29; N, 8.48; S, 9.77%. = +57.0 (c 0.20, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.99 (1H, d, J = 8.8 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.65–1.74 (1H, m), 1.78–1.92 (2H, m), 1.99–2.19 (3H, m), 2.55–2.66 (1H, m), 3.53 (1H, dd, J = 4.5, 10.7 Hz), 3.61 (1H, br d, J = 10.5 Hz), 3.79 (3H, s), 5.17 (1H, br s), 6.72–6.83 (3H, m), ), 7.23–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.7, 26.8, 29.9, 32.6, 39.3, 40.8, 46.4, 55.8, 57.1, 65.1, 110.9, 113.1, 117.3, 128.8, 131.1, 137.6, 161.1, 179.9. Anal. Calcd for C18H26N2O2S (334.48): C, 64.64; H, 7.84; N, 8.39; S, 9.59%; Found: C, 64.73; H, 7.99; N, 8.11; S, 9.38%.
= +57.0 (c 0.20, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.99 (1H, d, J = 8.8 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.65–1.74 (1H, m), 1.78–1.92 (2H, m), 1.99–2.19 (3H, m), 2.55–2.66 (1H, m), 3.53 (1H, dd, J = 4.5, 10.7 Hz), 3.61 (1H, br d, J = 10.5 Hz), 3.79 (3H, s), 5.17 (1H, br s), 6.72–6.83 (3H, m), ), 7.23–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.7, 26.8, 29.9, 32.6, 39.3, 40.8, 46.4, 55.8, 57.1, 65.1, 110.9, 113.1, 117.3, 128.8, 131.1, 137.6, 161.1, 179.9. Anal. Calcd for C18H26N2O2S (334.48): C, 64.64; H, 7.84; N, 8.39; S, 9.59%; Found: C, 64.73; H, 7.99; N, 8.11; S, 9.38%. = −19.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.2 Hz), 1.57–1.64 (1H, m), 1.71–1.88 (2H, m), 1.92–2.00 (1H, m), 2.06–2.13 (1H, m), 3.42–3.50 (1H, m), 3.66–3.83 (2H, m), 4.91 (1H, t, J = 7.7 Hz), 6.16 (1H, d, J = 9.4 Hz), 7.18 (2H, d, J = 7.7 Hz), 7.29 (1H, t, J = 7.0 Hz), 7.43 (2H, t, J = 7.5 Hz), 7.82 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.8, 23.8, 27.1, 27.3, 39.3, 40.4, 41.1, 46.6, 56.6, 64.4, 125.3, 127.7, 130.7, 135.8, 159.6, 179.5. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.31; H, 7.80; N, 9.30; S, 10.41%.
= −19.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.2 Hz), 1.57–1.64 (1H, m), 1.71–1.88 (2H, m), 1.92–2.00 (1H, m), 2.06–2.13 (1H, m), 3.42–3.50 (1H, m), 3.66–3.83 (2H, m), 4.91 (1H, t, J = 7.7 Hz), 6.16 (1H, d, J = 9.4 Hz), 7.18 (2H, d, J = 7.7 Hz), 7.29 (1H, t, J = 7.0 Hz), 7.43 (2H, t, J = 7.5 Hz), 7.82 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.8, 23.8, 27.1, 27.3, 39.3, 40.4, 41.1, 46.6, 56.6, 64.4, 125.3, 127.7, 130.7, 135.8, 159.6, 179.5. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.31; H, 7.80; N, 9.30; S, 10.41%. = +7.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.07 (3H, s), 1.26 (3H, s), 1.65 (1H, d, J = 10.2 Hz), 1.75–1.86 (1H, m), 1.89–2.01 (3H, m), 2.09–2.15 (1H, m), 2.23–2.31 (1H, m), 3.55–3.63 (1H, m), 3.92–3.99 (1H, m), 4.73–4.90 (2H, m), 6.01 (1H, br s), 7.03–7.45 (10H, m). 13C-NMR (CDCl3) δ (ppm) 19.8, 25.6, 27.3, 27.5, 35.6, 40.0, 42.8, 45.8, 49.0, 59.6, 62.1, 126.4, 126.5, 126.6, 128.5, 128.9, 129.7, 139.3, 139.8, 183.1. Anal. Calcd for C24H30N2OS (394.57): C, 73.06; H, 7.66; N, 7.10; S, 8.13%; Found: C, 73.40; H, 7.86; N, 7.05; S, 8.38%.
= +7.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.07 (3H, s), 1.26 (3H, s), 1.65 (1H, d, J = 10.2 Hz), 1.75–1.86 (1H, m), 1.89–2.01 (3H, m), 2.09–2.15 (1H, m), 2.23–2.31 (1H, m), 3.55–3.63 (1H, m), 3.92–3.99 (1H, m), 4.73–4.90 (2H, m), 6.01 (1H, br s), 7.03–7.45 (10H, m). 13C-NMR (CDCl3) δ (ppm) 19.8, 25.6, 27.3, 27.5, 35.6, 40.0, 42.8, 45.8, 49.0, 59.6, 62.1, 126.4, 126.5, 126.6, 128.5, 128.9, 129.7, 139.3, 139.8, 183.1. Anal. Calcd for C24H30N2OS (394.57): C, 73.06; H, 7.66; N, 7.10; S, 8.13%; Found: C, 73.40; H, 7.86; N, 7.05; S, 8.38%.3.2.2. General Procedure for the Reactions of Thioureas 19–23 with CDI to Yield Intermediates 29–33
 = +32.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (3H, s), 1.07 (1H, d, J = 10.1 Hz), 1.22 (3H, s), 1.42 (3H, s), 1.60–1.76 (1H, m), 1.92–2.20 (3H, m), 2.90–3.00 (1H, m), 4.35 (2H, d, J = 5.2 Hz), 5.26 (1H, t, J = 9.4 Hz), 6.25 (1H, d, J = 9.0 Hz), 7.05–7.39 (7H, m), 7.80 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.3, 22.5, 26.7, 26.6, 29.6, 30.1, 39.5, 40.8, 46.5, 55.6, 71.5, 117.5, 125.3, 128.5, 130.0, 130.4, 131.2, 135.5, 148.7, 181.5. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 63.89; H, 6.91; N, 13.73; S, 7.65%.
= +32.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (3H, s), 1.07 (1H, d, J = 10.1 Hz), 1.22 (3H, s), 1.42 (3H, s), 1.60–1.76 (1H, m), 1.92–2.20 (3H, m), 2.90–3.00 (1H, m), 4.35 (2H, d, J = 5.2 Hz), 5.26 (1H, t, J = 9.4 Hz), 6.25 (1H, d, J = 9.0 Hz), 7.05–7.39 (7H, m), 7.80 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.3, 22.5, 26.7, 26.6, 29.6, 30.1, 39.5, 40.8, 46.5, 55.6, 71.5, 117.5, 125.3, 128.5, 130.0, 130.4, 131.2, 135.5, 148.7, 181.5. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 63.89; H, 6.91; N, 13.73; S, 7.65%. = –140.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.58 (1H, t, J = 8.5 Hz), 0.76–0.85 (2H, m), 0.93 (3H, s), 1.05 (3H, s), 1.28–1.55 (3H, m), 1.67 (3H, s), 1.72–1.82 (1H, m), 3.36 (1H, dd, J = 2.7, 11.1 Hz), 3.75 (1H,dd, J = 7.1, 14.0 Hz), 3.84 (1H, dd, J = 11.3, 16.7 Hz), 3.95 (1H, dd, J = 2.7, 11.2 Hz), 7.19 (1H, br s), 7.22–7.46 (7H, m). 13LC-NMR (CDCl3) δ (ppm) 15.6, 19.1, 19.3, 24.0, 29.0, 30.1, 31.1, 45.4, 63.0, 69.1, 122.5, 126.6, 126.9, 127.6, 128.8, 130.1, 137.0, 143.0, 180.0. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.21; H, 6.97; N, 13.23; S, 7.71%.
= –140.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.58 (1H, t, J = 8.5 Hz), 0.76–0.85 (2H, m), 0.93 (3H, s), 1.05 (3H, s), 1.28–1.55 (3H, m), 1.67 (3H, s), 1.72–1.82 (1H, m), 3.36 (1H, dd, J = 2.7, 11.1 Hz), 3.75 (1H,dd, J = 7.1, 14.0 Hz), 3.84 (1H, dd, J = 11.3, 16.7 Hz), 3.95 (1H, dd, J = 2.7, 11.2 Hz), 7.19 (1H, br s), 7.22–7.46 (7H, m). 13LC-NMR (CDCl3) δ (ppm) 15.6, 19.1, 19.3, 24.0, 29.0, 30.1, 31.1, 45.4, 63.0, 69.1, 122.5, 126.6, 126.9, 127.6, 128.8, 130.1, 137.0, 143.0, 180.0. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.21; H, 6.97; N, 13.23; S, 7.71%. = +39.0 (c 0.32, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.86 (1H, d, J = 10.3 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.65–1.72 (1H, m), 1.95–2.26 (4H, m), 2.90–3.00 (1H, m), 4.39 (2H, d, J = 5.4 Hz), 5.29 (1H, t, J = 9.2 Hz), 6.26 (1H, d, J = 9.0 Hz), 7.00–7.30 (7H, m), 7.85 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 29.6, 30.1, 39.5, 40.7, 46.5, 55.8, 71.5, 117.3, 125.5, 128.1, 130.1, 130.5, 131.0, 135.9, 148.9, 181.0. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.54; H, 6.60; N, 13.84; S, 7.91%.
= +39.0 (c 0.32, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.86 (1H, d, J = 10.3 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.65–1.72 (1H, m), 1.95–2.26 (4H, m), 2.90–3.00 (1H, m), 4.39 (2H, d, J = 5.4 Hz), 5.29 (1H, t, J = 9.2 Hz), 6.26 (1H, d, J = 9.0 Hz), 7.00–7.30 (7H, m), 7.85 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 29.6, 30.1, 39.5, 40.7, 46.5, 55.8, 71.5, 117.3, 125.5, 128.1, 130.1, 130.5, 131.0, 135.9, 148.9, 181.0. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.54; H, 6.60; N, 13.84; S, 7.91%. = +81.0 (c 0.265, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (3H, s), 1.19 (1H, d, J = 10.0 Hz), 1.24 (3H, s), 1.66–1.75 (1H, m), 1.91–1.99 (2H, m), 2.06–2.16 (1H, m), 2.19–2.30 (1H, m), 2.24 (3H, s), 2.79–2.91 (1H, m), 4.34–4.44 (2H, m), 5.14 (1H, t, J = 8.8 Hz), 7.01–7.09 (3H, m), 7.25 (1H, d, J = 8.2 Hz), 7.57 (1H, s), 7.72 (1H, d, J = 8.9 Hz), 8.23 (1H, s), 9.28 (1H, s). 13C-NMR (DMSO–d6) δ (ppm) 21.3, 21.5, 27.1, 27.2, 29.9, 30.4, 39.3, 47.0, 53.7, 59.0, 71.8, 118.4, 124.1, 129.7, 131.0, 134.2, 137.2, 137.7, 149.3, 181.6. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.39; H, 6.93; N, 13.27; S, 7.56%.
= +81.0 (c 0.265, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (3H, s), 1.19 (1H, d, J = 10.0 Hz), 1.24 (3H, s), 1.66–1.75 (1H, m), 1.91–1.99 (2H, m), 2.06–2.16 (1H, m), 2.19–2.30 (1H, m), 2.24 (3H, s), 2.79–2.91 (1H, m), 4.34–4.44 (2H, m), 5.14 (1H, t, J = 8.8 Hz), 7.01–7.09 (3H, m), 7.25 (1H, d, J = 8.2 Hz), 7.57 (1H, s), 7.72 (1H, d, J = 8.9 Hz), 8.23 (1H, s), 9.28 (1H, s). 13C-NMR (DMSO–d6) δ (ppm) 21.3, 21.5, 27.1, 27.2, 29.9, 30.4, 39.3, 47.0, 53.7, 59.0, 71.8, 118.4, 124.1, 129.7, 131.0, 134.2, 137.2, 137.7, 149.3, 181.6. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.39; H, 6.93; N, 13.27; S, 7.56%. = +44.0 (c 0.35, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.87 (1H, d, J = 10.5 Hz), 1.03 (3H, s), 1.24 (3H, s), 1.65–1.72 (1H, m), 1.96–2.26 (4H, m), 2.91–3.01 (1H, m), 4.37–4.46 (2H, m), 5.26 (1H, t, J = 9.9 Hz), 6.20 (1H, br s), 6.94–7.15 (5H, m), 7.30 (1H, s), 7.78 (1H, br s), 8.14 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 21.0, 26.5, 26.6, 29.7, 30.1, 39.4, 40.6, 46.6, 55.4, 71.6, 117.2, 117.3, 117.4, 127.7, 127.8, 148.8, 160.4, 162.9, 181.3. Anal. Calcd for C21H25FN4O2S (416.51): C, 60.56; H, 6.05; N, 13.45; S, 7.70%; Found: C, 60.79; H, 6.41; N, 13.08; S, 7.53%.
= +44.0 (c 0.35, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.87 (1H, d, J = 10.5 Hz), 1.03 (3H, s), 1.24 (3H, s), 1.65–1.72 (1H, m), 1.96–2.26 (4H, m), 2.91–3.01 (1H, m), 4.37–4.46 (2H, m), 5.26 (1H, t, J = 9.9 Hz), 6.20 (1H, br s), 6.94–7.15 (5H, m), 7.30 (1H, s), 7.78 (1H, br s), 8.14 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 21.0, 26.5, 26.6, 29.7, 30.1, 39.4, 40.6, 46.6, 55.4, 71.6, 117.2, 117.3, 117.4, 127.7, 127.8, 148.8, 160.4, 162.9, 181.3. Anal. Calcd for C21H25FN4O2S (416.51): C, 60.56; H, 6.05; N, 13.45; S, 7.70%; Found: C, 60.79; H, 6.41; N, 13.08; S, 7.53%. = +9.0 (c 0.26, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (1H, d, J = 10.6 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.66–1.72 (1H, m), 1.97–2.02 (1H, m), 2.06–2.28 (3H, m), 2.90–3.00 (1H, m), 3.73 (3H, s), 4.34–4.44 (2H, m), 5.30 (1H, t, J = 9.8 Hz), 6.41 (1H, d, J = 9.7 Hz), 6.60–6.69 (3H, m), 7.02 (1H, s), 7.17 (1H, t, J = 8.1 Hz), 7.22 (1H, s), 7.79 (1H, s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.2, 26.6, 26.7, 29.6, 30.2, 39.6, 40.7, 46.6, 55.8, 55.9, 71.5, 110.6, 111.2, 113.4, 117.1, 117.3, 131.0, 131.3, 137.0, 148.9, 161.4, 180.8. Anal. Calcd for C22H28N4O3S (428.55): C, 61.66; H, 6.59; N, 13.07; S, 7.48%; Found: C, 61.83; H, 6.84; N, 12.89; S, 7.53%.
= +9.0 (c 0.26, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (1H, d, J = 10.6 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.66–1.72 (1H, m), 1.97–2.02 (1H, m), 2.06–2.28 (3H, m), 2.90–3.00 (1H, m), 3.73 (3H, s), 4.34–4.44 (2H, m), 5.30 (1H, t, J = 9.8 Hz), 6.41 (1H, d, J = 9.7 Hz), 6.60–6.69 (3H, m), 7.02 (1H, s), 7.17 (1H, t, J = 8.1 Hz), 7.22 (1H, s), 7.79 (1H, s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.2, 26.6, 26.7, 29.6, 30.2, 39.6, 40.7, 46.6, 55.8, 55.9, 71.5, 110.6, 111.2, 113.4, 117.1, 117.3, 131.0, 131.3, 137.0, 148.9, 161.4, 180.8. Anal. Calcd for C22H28N4O3S (428.55): C, 61.66; H, 6.59; N, 13.07; S, 7.48%; Found: C, 61.83; H, 6.84; N, 12.89; S, 7.53%. = −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.26 (3H, s), 1.31 (1H, d, J = 9.5 Hz), 1.61–1.72 (1H, m), 1.94–2.19 (5H, m), 4.54 (1H, dd, J = 6.6, 10.8 Hz), 4.65 (1H, dd, J = 6.6, 10.7 Hz), 5.04 (1H, t, J = 8.7 Hz), 6.16 (1H, d, J = 9.2 Hz), 7.06 (1H, s), 7.13 (2H, d, J = 7.8 Hz), 7.24–7.29 (1H, m), 7.40 (2H, t, J = 7.9 Hz), 7.46 (1H, br s), 8.13 (1H, br s), 8.16 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.9, 23.6, 26.7, 27.5, 35.6, 40.2, 40.646.2, 55.7, 70.4, 117.6, 125.2, 127.5, 130.6, 131.0, 136.5, 149.2, 180.3. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.43; H, 6.75; N, 13.85; S, 8.16%.
= −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.26 (3H, s), 1.31 (1H, d, J = 9.5 Hz), 1.61–1.72 (1H, m), 1.94–2.19 (5H, m), 4.54 (1H, dd, J = 6.6, 10.8 Hz), 4.65 (1H, dd, J = 6.6, 10.7 Hz), 5.04 (1H, t, J = 8.7 Hz), 6.16 (1H, d, J = 9.2 Hz), 7.06 (1H, s), 7.13 (2H, d, J = 7.8 Hz), 7.24–7.29 (1H, m), 7.40 (2H, t, J = 7.9 Hz), 7.46 (1H, br s), 8.13 (1H, br s), 8.16 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.9, 23.6, 26.7, 27.5, 35.6, 40.2, 40.646.2, 55.7, 70.4, 117.6, 125.2, 127.5, 130.6, 131.0, 136.5, 149.2, 180.3. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.43; H, 6.75; N, 13.85; S, 8.16%. = −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.09 (3H, s), 1.32 (3H, s), 1.68 (1H, d, J = 10.1 Hz), 1.80–1.89 (1H, m), 1.95–2.11 (2H, m), 2.20 (1H, t, J = 5.4 Hz), 2.33–2.50 (2H, m), 4.65 (1H, dd, J = 7.3, 11.6 Hz), 4.80–4.89 (3H, m), 6.50 (1H, br d, J = 10.6 Hz), 7.03–7.55 (12H, m), 8.20 (1H, s). 13C-NMR (CDCl3) δ (ppm) 20.1, 25.7, 27.1, 28.1, 33.3, 39.9, 42.5, 45.8, 49.1, 58.9, 70.3, 117.7, 126.3, 126.5, 128.7, 128.9, 129.2, 130.9, 131.8, 136.1, 137.1, 140.0, 149.2, 184.2. Anal. Calcd for C28H32N4O2S (488.22): C, 68.82; H, 6.60; N, 11.47; S, 6.56%; Found: C, 68.63; H, 6.49; N, 11.72; S, 6.83%.
= −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.09 (3H, s), 1.32 (3H, s), 1.68 (1H, d, J = 10.1 Hz), 1.80–1.89 (1H, m), 1.95–2.11 (2H, m), 2.20 (1H, t, J = 5.4 Hz), 2.33–2.50 (2H, m), 4.65 (1H, dd, J = 7.3, 11.6 Hz), 4.80–4.89 (3H, m), 6.50 (1H, br d, J = 10.6 Hz), 7.03–7.55 (12H, m), 8.20 (1H, s). 13C-NMR (CDCl3) δ (ppm) 20.1, 25.7, 27.1, 28.1, 33.3, 39.9, 42.5, 45.8, 49.1, 58.9, 70.3, 117.7, 126.3, 126.5, 128.7, 128.9, 129.2, 130.9, 131.8, 136.1, 137.1, 140.0, 149.2, 184.2. Anal. Calcd for C28H32N4O2S (488.22): C, 68.82; H, 6.60; N, 11.47; S, 6.56%; Found: C, 68.63; H, 6.49; N, 11.72; S, 6.83%.3.2.3. General Procedure for the Synthesis of 2-Imino-1,3-thiazines 24–28 and 2-Thioxo-1,3-oxazines 34–37
 = +142.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.24 (3H, s), 1.30 (1H, d, J = 10.5 Hz), 1.50–1.58 (1H, m), 1.92–1.99 (2H, m), 2.13–2.30 (2H, m), 2.61–2.73 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.92 (1H, t, J = 8.9 Hz), 6.88–7.30 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 33.1, 34.1, 35.7, 39.0, 41.1, 47.7, 54.9, 122.9, 123.7, 129.3, 137.7, 160.4. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.39; H, 8.00; N, 9.21; S, 11.11%.
= +142.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.24 (3H, s), 1.30 (1H, d, J = 10.5 Hz), 1.50–1.58 (1H, m), 1.92–1.99 (2H, m), 2.13–2.30 (2H, m), 2.61–2.73 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.92 (1H, t, J = 8.9 Hz), 6.88–7.30 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 33.1, 34.1, 35.7, 39.0, 41.1, 47.7, 54.9, 122.9, 123.7, 129.3, 137.7, 160.4. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.39; H, 8.00; N, 9.21; S, 11.11%. = +112.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (3H, s), 1.26 (3H, s), 1.28 (1H, d, J = 11.8 Hz), 1.45–1.52 (1H, m), 1.97–2.24 (4H, m), 2.64–2.75 (1H, m), 3.85–3.88 (1H, m), 3.91 (1H, dd, J = 10.2, 21.1 Hz), 4.24 (1H, dd, J = 6.1, 10.8 Hz), 7.53 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.4, 25.3, 26.6, 26.7, 28.0, 39.6, 40.5, 46.1, 54.2, 72.6, 192.5. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.77; H, 8.36; N, 6.41; S, 15.00%.
= +112.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (3H, s), 1.26 (3H, s), 1.28 (1H, d, J = 11.8 Hz), 1.45–1.52 (1H, m), 1.97–2.24 (4H, m), 2.64–2.75 (1H, m), 3.85–3.88 (1H, m), 3.91 (1H, dd, J = 10.2, 21.1 Hz), 4.24 (1H, dd, J = 6.1, 10.8 Hz), 7.53 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.4, 25.3, 26.6, 26.7, 28.0, 39.6, 40.5, 46.1, 54.2, 72.6, 192.5. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.77; H, 8.36; N, 6.41; S, 15.00%. = +170.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.57 (1H, m), 1.88–1.99 (2H, m), 2.11–2.29 (2H, m), 2.31 (3H, s), 2.59–2.71 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.91 (1H, d, J = 8.3 Hz), 5.01 (1H, br s), 6.80 (1H, d, J = 8.1 Hz), 7.08 (1H, d, J = 8.1 Hz). 13C-NMR (CDCl3) δ (ppm) 20.9, 21.3, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.8, 122.5, 129.9, 133.0, 146.2, 159.7. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 71.99; H, 8.21; N, 9.10; S, 10.70%.
= +170.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.57 (1H, m), 1.88–1.99 (2H, m), 2.11–2.29 (2H, m), 2.31 (3H, s), 2.59–2.71 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.91 (1H, d, J = 8.3 Hz), 5.01 (1H, br s), 6.80 (1H, d, J = 8.1 Hz), 7.08 (1H, d, J = 8.1 Hz). 13C-NMR (CDCl3) δ (ppm) 20.9, 21.3, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.8, 122.5, 129.9, 133.0, 146.2, 159.7. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 71.99; H, 8.21; N, 9.10; S, 10.70%. = +234.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.8, 129.9, 150.6, 160.6. Anal. Calcd for C18H24N2OS (316.46): C, 68.32; H, 7.64; N, 8.85; S,10.13%; Found: C, 68.45; H, 7.93; N, 8.50; S,9.89%.
= +234.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.8, 129.9, 150.6, 160.6. Anal. Calcd for C18H24N2OS (316.46): C, 68.32; H, 7.64; N, 8.85; S,10.13%; Found: C, 68.45; H, 7.93; N, 8.50; S,9.89%. = +272.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.9, 150.6, 160.6. Anal. Calcd for C17H21FN2S (304.43): C, 67.07; H, 6.95; N, 9.20; S, 10.53%; Found: C, 67.41; H, 7.13; N, 9.01; S 10.27%.
= +272.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.9, 150.6, 160.6. Anal. Calcd for C17H21FN2S (304.43): C, 67.07; H, 6.95; N, 9.20; S, 10.53%; Found: C, 67.41; H, 7.13; N, 9.01; S 10.27%. = +165.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.82 (3H, s), 1.25 (3H, s), 1.43–1.53 (1H, m), 1.67 (1H, d, J = 10.6 Hz), 1.91–2.16 (5H, m), 2.91–3.03 (2H, m), 3.44 (1H, d, J = 8.1 Hz), 6.95–7.28 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 23.8, 27.3, 29.9, 33.8, 41.1, 41.5, 46.6, 58.1, 121.5, 123.0, 129.2, 145.2, 160.8. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.35; H, 7.83; N, 9.65; S, 11.17%.
= +165.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.82 (3H, s), 1.25 (3H, s), 1.43–1.53 (1H, m), 1.67 (1H, d, J = 10.6 Hz), 1.91–2.16 (5H, m), 2.91–3.03 (2H, m), 3.44 (1H, d, J = 8.1 Hz), 6.95–7.28 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 23.8, 27.3, 29.9, 33.8, 41.1, 41.5, 46.6, 58.1, 121.5, 123.0, 129.2, 145.2, 160.8. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.35; H, 7.83; N, 9.65; S, 11.17%. = +331.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.83 (3H, s), 1.33 (3H, s), 1.54 (1H, t, J = 12.5 Hz), 1.78 (1H, d, J = 10.7 Hz), 1.87–1.95 (1H, m), 2.03–2.12 (1H, m), 2.23–2.31 (1H, m), 2.40–2.53 (1H, m), 3.62 (1H, d, J = 10.2 Hz), 4.31 (1H, dd, J = 10.1, 12.5 Hz), 4.52 (1H, dd, J = 4.7, 10.0 Hz), 8.29 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 19.6, 23.2, 23.5, 27.5,32.6, 41.6, 42.4, 43.1, 54.9, 75.6, 188.2. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.73; H, 8.46; N, 6.52; S, 15.01%.
= +331.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.83 (3H, s), 1.33 (3H, s), 1.54 (1H, t, J = 12.5 Hz), 1.78 (1H, d, J = 10.7 Hz), 1.87–1.95 (1H, m), 2.03–2.12 (1H, m), 2.23–2.31 (1H, m), 2.40–2.53 (1H, m), 3.62 (1H, d, J = 10.2 Hz), 4.31 (1H, dd, J = 10.1, 12.5 Hz), 4.52 (1H, dd, J = 4.7, 10.0 Hz), 8.29 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 19.6, 23.2, 23.5, 27.5,32.6, 41.6, 42.4, 43.1, 54.9, 75.6, 188.2. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.73; H, 8.46; N, 6.52; S, 15.01%. = +567.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.77 (3H, s), 1.20 (3H, s), 1.47 (1H, dt, J = 5.1, 13.6 Hz), 1.52 (1H, d, J = 10.8 Hz), 1.91–1.97 (1H, m), 2.04–2.14 (2H, m), 2.25–2.42 (2H, m), 2.88–2.99 (2H, m), 3.81 (1H, d, J = 8.1 Hz), 4.51 (1H, d, J = 16.4 Hz), 5.31 (1H, d, J = 16.6 Hz), 6.78–6.83 (2H, m), 6.94–6.99 (1H, m), 7.15–7.32 (7H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 24.2, 27.5, 31.1, 34.0, 35.8, 40.3, 40.4, 42.5, 49.0, 60.2, 122.7, 122.9, 126.7, 12.8, 128.7, 129.1, 140.2, 150.5, 152.6. Anal. Calcd for C24H28N2S (376.56): C, 76.55; H, 7.49; N, 7.44; S, 8.52%; Found: C, 76.86; H, 7.63; N, 7.21; S, 8.30%.
= +567.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.77 (3H, s), 1.20 (3H, s), 1.47 (1H, dt, J = 5.1, 13.6 Hz), 1.52 (1H, d, J = 10.8 Hz), 1.91–1.97 (1H, m), 2.04–2.14 (2H, m), 2.25–2.42 (2H, m), 2.88–2.99 (2H, m), 3.81 (1H, d, J = 8.1 Hz), 4.51 (1H, d, J = 16.4 Hz), 5.31 (1H, d, J = 16.6 Hz), 6.78–6.83 (2H, m), 6.94–6.99 (1H, m), 7.15–7.32 (7H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 24.2, 27.5, 31.1, 34.0, 35.8, 40.3, 40.4, 42.5, 49.0, 60.2, 122.7, 122.9, 126.7, 12.8, 128.7, 129.1, 140.2, 150.5, 152.6. Anal. Calcd for C24H28N2S (376.56): C, 76.55; H, 7.49; N, 7.44; S, 8.52%; Found: C, 76.86; H, 7.63; N, 7.21; S, 8.30%. = +132.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71 (3H, s), 1.23 (3H, s), 1.42–1.57 (2H, m), 1.83–1.91 (1H, m), 1.95–2.06 (2H, m), 2.20 (1H, t, J = 5.14), 2.43–2.51 (1H, m), 3.64 (1H, d, J = 9.6 Hz), 4.17 (1H, dd, J = 9.6, 12.2 Hz), 4.36 (1H, dd, J = 4.9, 9.6 Hz), 4.49 (1H, d, J = 16.2 Hz), 4.68 (1H, d, J = 16.2 Hz), 7.19–7.32 (5H, m). 13C-NMR (CDCl3) δ (ppm) 19.5, 23.5, 24.0, 27.8, 34.1, 40.6, 41.5, 41.6, 48.1, 57.1, 72.2, 127.3, 127.4, 128.9, 138.7, 155.8. Anal. Calcd for C18H23NOS (301.45): C, 71.72; H, 7.69; N, 4.65; s, 10.64%; Found: C, 71.98; H, 7.83; N, 4.39; S, 10.39%.
= +132.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71 (3H, s), 1.23 (3H, s), 1.42–1.57 (2H, m), 1.83–1.91 (1H, m), 1.95–2.06 (2H, m), 2.20 (1H, t, J = 5.14), 2.43–2.51 (1H, m), 3.64 (1H, d, J = 9.6 Hz), 4.17 (1H, dd, J = 9.6, 12.2 Hz), 4.36 (1H, dd, J = 4.9, 9.6 Hz), 4.49 (1H, d, J = 16.2 Hz), 4.68 (1H, d, J = 16.2 Hz), 7.19–7.32 (5H, m). 13C-NMR (CDCl3) δ (ppm) 19.5, 23.5, 24.0, 27.8, 34.1, 40.6, 41.5, 41.6, 48.1, 57.1, 72.2, 127.3, 127.4, 128.9, 138.7, 155.8. Anal. Calcd for C18H23NOS (301.45): C, 71.72; H, 7.69; N, 4.65; s, 10.64%; Found: C, 71.98; H, 7.83; N, 4.39; S, 10.39%. = –123.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.05 (3H, s), 1.28 (3H, s), 1.38 (1H, d, J = 12.5 Hz), 1.41 (3H,s), 1.69–1.98 (2H, m), 2.10–2.23 (2H, m), 2.42–2.53 (1H, m), 2.63 (1H, dd, J = 8.0, 12.8 Hz), 2.97 (1H, dd, J = 3.9, 12.9 Hz), 5.04 (1H, br s), 6.87–7.06 (3H, m), 7.22–7.30 (2H, m). 13C-NMR (CDCl3) δ (ppm) 24.1, 27.8, 28.2, 31.7, 33.4, 33.9, 40.3, 40.6, 55.3, 60.9, 122.7, 123.4, 129.2, 148.6, 158.4. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 72.19; H, 8.33; N, 9.11; S, 10.60%.
= –123.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.05 (3H, s), 1.28 (3H, s), 1.38 (1H, d, J = 12.5 Hz), 1.41 (3H,s), 1.69–1.98 (2H, m), 2.10–2.23 (2H, m), 2.42–2.53 (1H, m), 2.63 (1H, dd, J = 8.0, 12.8 Hz), 2.97 (1H, dd, J = 3.9, 12.9 Hz), 5.04 (1H, br s), 6.87–7.06 (3H, m), 7.22–7.30 (2H, m). 13C-NMR (CDCl3) δ (ppm) 24.1, 27.8, 28.2, 31.7, 33.4, 33.9, 40.3, 40.6, 55.3, 60.9, 122.7, 123.4, 129.2, 148.6, 158.4. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 72.19; H, 8.33; N, 9.11; S, 10.60%. = +36.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 11.1 Hz), 1.06 (3H, s), 1.31 (3H, s), ), 1.35 (3H, s), 1.79–1.87 (1H, m), 1.95–2.03 (2H, m), 2.14–2.42 (3H, m), 4.08 (1H, d, J = 11.0 Hz), 4.18 (1H, d, J = 10.7 Hz), 7.43 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.7, 27.2, 33.1, 34.1, 35.5, 39.3, 40.2, 41.2, 47.4, 55.1, 158.2. Anal. Calcd for C12H19NOS (225.35): C, 63.96; H, 8.50; N, 6.22; S, 14.23%; Found: C, 64.25; H, 8.79; N, 6.01; S, 13.88%.
= +36.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 11.1 Hz), 1.06 (3H, s), 1.31 (3H, s), ), 1.35 (3H, s), 1.79–1.87 (1H, m), 1.95–2.03 (2H, m), 2.14–2.42 (3H, m), 4.08 (1H, d, J = 11.0 Hz), 4.18 (1H, d, J = 10.7 Hz), 7.43 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.7, 27.2, 33.1, 34.1, 35.5, 39.3, 40.2, 41.2, 47.4, 55.1, 158.2. Anal. Calcd for C12H19NOS (225.35): C, 63.96; H, 8.50; N, 6.22; S, 14.23%; Found: C, 64.25; H, 8.79; N, 6.01; S, 13.88%. = −18.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71–0.83 (2H, m), 0.98 (3H, s), 1.07 (3H, s), 1.22 (1H, dd, J = 4.5, 15.8 Hz), 1.28 (3H, s), 1.53–1.71 (2H, m), 1.89–2.01 (2H, m), 2.65 (1H, d, J = 3.4, 12.4 Hz), 3.29 (1H, d, J = 3.5, 11.7 Hz), 7.02–7.12 (3H, m), 7.28–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 15.6, 17.6, 18.2, 19.6, 21.8, 28.8, 28.9, 31.6, 35.4, 52.1, 123.2, 123.4, 123.9, 129.3, 155.0. Anal. Calcd for C18H24N2OS (300.46): C, 71.95; H, 8.05; N, 9.32; S,10.67%; Found: C, 72.20; H, 8.19; N, 9.02; S, 10.54%.
= −18.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71–0.83 (2H, m), 0.98 (3H, s), 1.07 (3H, s), 1.22 (1H, dd, J = 4.5, 15.8 Hz), 1.28 (3H, s), 1.53–1.71 (2H, m), 1.89–2.01 (2H, m), 2.65 (1H, d, J = 3.4, 12.4 Hz), 3.29 (1H, d, J = 3.5, 11.7 Hz), 7.02–7.12 (3H, m), 7.28–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 15.6, 17.6, 18.2, 19.6, 21.8, 28.8, 28.9, 31.6, 35.4, 52.1, 123.2, 123.4, 123.9, 129.3, 155.0. Anal. Calcd for C18H24N2OS (300.46): C, 71.95; H, 8.05; N, 9.32; S,10.67%; Found: C, 72.20; H, 8.19; N, 9.02; S, 10.54%.3.2.4. Alternative Procedure for the Synthesis of 2-Thioxo-1,3-oxazines 35 and 36
3.2.5. General Procedure for the Synthesis of 2-Imino-1,3-thiazines 42 and 43
3.2.6. Determination of Antiproliferative Activities
3.2.7. X-ray Crystallographic Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lait, S.M.; Rankic, D.A.; Keay, B.A. 1,3-Amino alcohols and their derivatives in asymmetric organic synthesis. Chem. Rev. 2007, 107, 767–796. [Google Scholar]
- Lázár, L.; Fülöp, F. 1,3-Oxazines and their benzo derivatives. In Comprehensive Heterocyclic Chemistry. III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; pp. 373–459. [Google Scholar]
- Szakonyi, Z.; Fülöp, F. Monoterpene-based chiral β-amino acid derivatives prepared from natural sources: Syntheses and applications. Amino Acids 2011, 41, 597–608. [Google Scholar]
- Andrés, C.; Gonzáles, I.; Nieto, J.; Rosón, C.D. Lewis acid mediated diastereoselective keto-ene cyclization on chiral perhydro-1,3-benzoxazines: Synthesis of enantiopure cis-3,4-disubstituted 3-hydroxypyrrolidines. Tetrahedron 2009, 65, 9728–9736. [Google Scholar]
- Andrés, C.; Infante, R.; Nieto, J. Perhydro-1,3-benzoxazines derived from (−)-8-aminomenthol as ligands for the catalytic enantioselective addition of diethylzinc to aldehydes. Tetrahedron: Asymmetry 2010, 21, 2230–2237. [Google Scholar]
- Jaworska, M.; Blocka, E.; Kozakiewicz, A.; Welniak, M. α-Pinene-type chiral schiff bases as tridentate ligands in asymmetric addition reactions. Tetrahedron: Asymmetry 2011, 22, 648–657. [Google Scholar]
- Evans, P.A.; Brandt, T.A. Enantioselective allylic substitution using a novel (phosphino-1,3-oxazine)palladium catalyst. Tetrahedron Lett. 1996, 37, 9143–9146. [Google Scholar]
- Evans, P.A.; Brandt, T.A. Enantioselective palladium-catalyzed allylic alkylation using E- and Z-vinylogous sulfonates. Org. Lett. 1999, 1563–1565. [Google Scholar]
- Szakonyi, Z.; Balázs, Á.; Martinek, T.A.; Fülöp, F. Enantioselective addition of diethylzinc to aldehydes catalyzed by γ-amino alcohols derived from (+)- and (−)-α-pinene. Tetrahedron: Asymmetry 2006, 17, 199–204. [Google Scholar]
- Li, X.; Lou, R.; Yeung, C.-H.; Chan, A.S.C.; Wong, W.K. Asymmetric hydrogenation of dehydroamino acid derivatives catalyzed by a new aminophosphine phosphinite ligand derived from ketopinic acid. Tetrahedron: Asymmetry 2000, 11, 2077–2082. [Google Scholar]
- De las Casas Engel, T.; Maroto, B.L.; García Martínez, A.; de la Moya Cerero, S. N/N/O versus N/O/O and N/O amino isoborneols in the enantioselective ethylation of benzaldehyde. Tetrahedron: Asymmetry 2008, 19, 269–272. [Google Scholar]
- Sánches-Carnerero, E.M.; de las Casas Engel, T.; Maroto, B.L.; de la Moya Cerero, S. Polyoxygenated ketopinic-acid-derived γ-amino alcohols in the enantioselective diethylzinc addition to benzaldehyde. Tetrahedron: Asymmetry 2009, 20, 2655–2657. [Google Scholar]
- Szakonyi, Z.; Martinek, T.A.; Hetényi, A.; Fülöp, F. Synthesis and transformations of enantiomeric 1,2-disubstituted monoterpene derivatives. Tetrahedron: Asymmetry 2000, 11, 4571–4579. [Google Scholar]
- Koneva, E.A.; Volcho, K.P.; Korchagina, D.V.; Komarova, N.I.; Kochnev, A.I.; Salakhutdinov, N.F.; Tolstikov, A.G. New chiral Schiff bases derived from (+)- and (−)-α-pinenes in the metal complex catalyzed asymmetric oxidation of sulfides. Russ. Chem. Bull. 2008, 57, 108–117. [Google Scholar]
- Koneva, E.A.; Volcho, K.P.; Korchagina, D.V.; Salakhutdinov, N.F.; Tolstikov, G.A. Synthesis of new chiral schiff bases from (+)-3-carene and their use in asymmetric oxidation of sulfides catalyzed by metal complexes. Russ. J. Org. Chem. 2009, 815–824. [Google Scholar]
- Koneva, E.A.; Suslov, E.V.; Korchagina, D.V.; Genaev, A.M.; Volcho, K.P.; Salakhutdinov, N.F. Catalytic asymmetric addition of diethylzinc to benzaldehyde using α-pinene-derived ligands. Open Catal. J. 2011, 4, 107–112. [Google Scholar]
- Koneva, E.A.; Khomenko, T.M.; Kurbakova, S.Y.; Komarova, N.I.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F.; Tolstikov, A.G.; Tolstikov, G.A. Synthesis of optically active omeprazole by catalysis with vanadyl complexes with chiral Schiff bases. Russ. Chem. Bull. Int. Ed. 2008, 57, 1680–1685. [Google Scholar]
- Koneva, E.A.; Korchagina, D.V.; Gatilov, Y.V.; Genaev, A.M.; Krysin, A.P.; Volcho, K.P.; Tolstikov, A.G.; Salakhutdinov, N.F. New chiral ligands based on (+)-α-pinene. Russ. J. Org. Chem. 2010, 46, 1109–1115. [Google Scholar]
- Fülöp, F.; Bernáth, G.; Pihlaja, K. Synthesis, stereochemistry and transformations of cyclopentane-, cyclohexane-, cycloheptane-, and cyclooctane-fused 1,3-oxazines, 1,3-thiazines, and pyrimidines. Adv. Heterocycl. Chem. 1998, 69, 349–477. [Google Scholar]
- Xu, X.; Qian, X.; Li, Z.; Song, G.; Chen, W. Synthesis and fungicidal activity of fluorine-containing phenylimino-thiazolidines derivatives. J. Fluorine Chem. 2005, 126, 297–300. [Google Scholar]
- Woltering, T.J.; Wostl, W.; Hilpert, H.; Rogers-Evans, M.; Pinard, E.; Maywega, A.; Göbel, M.; Banner, D.W.; Benz, J.; Travagli, M.; et al. BACE1 inhibitors: A head group scan on a series of amides. Bioorg. Med. Chem. Lett. 2013, 23, 4239–4243. [Google Scholar]
- Kai, H.; Morioka, Y.; Murashi, T.; Morita, K.; Shinonome, S.; Nakazato, H.; Kawamoto, K.; Hanasaki, K.; Takahashi, F.; Mihara, S.; et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 1: Discovery of CB2 receptor selective compounds. Bioorg. Med. Chem. Lett. 2007, 17, 4030–4034. [Google Scholar]
- Kai, H.; Morioka, Y.; Tomida, M.; Takahashi, T.; Hattori, M.; Hanasaki, K.; Koike, K.; Chiba, H.; Shinohara, S.; Kanemasa, T.; et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 2: Orally bioavailable compounds. Bioorg. Med. Chem. Lett. 2007, 17, 3925–3929. [Google Scholar]
- Kai, H.; Morioka, Y.; Koriyama, Y.; Okamoto, K.; Hasegawa, Y.; Hattori, M.; Koike, K.; Chiba, H.; Shinohara, S.; Iwamoto, Y.; et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 3: Synthesis and activity of isosteric analogs. Bioorg. Med. Chem. Lett. 2008, 18, 6444–6447. [Google Scholar]
- Blokhina, S.V.; Volkova, T.V.; Ol’khovich, M.V.; Sharapova, A.V.; Proshin, A.N.; Bachurin, S.O.; Perlovich, G.L. Synthesis, biological activity, distribution and membrane permeability of novel spiro-thiazines as potent neuroprotectors. Eur. J. Med. Chem. 2014, 77, 8–17. [Google Scholar]
- Szakonyi, Z.; Fülöp, F. Mild and efficient ring opening of monoterpene-fused β-lactam enantiomers. Synthesis of novel β-amino acid derivatives. Arkivoc 2003, 2003, 225–232. [Google Scholar]
- Gyónfalvi, S.; Szakonyi, Z.; Fülöp, F. Synthesis and transformation of novel cyclic β-amino acid derivatives from (+)-3-carene. Tetrahedron: Asymmetry 2003, 14, 3965–3972. [Google Scholar]
- Szakonyi, Z.; Martinek, T.A.; Sillanpää, R.; Fülöp, F. Regio- and stereoselective synthesis of constrained enantiomeric β-amino acid derivatives. Tetrahedron: Asymmetry 2008, 19, 2296–2303. [Google Scholar]
- Fülöp, F.; Szakonyi, Z. Chiral cyclic β-amino acids and their derivates, pharmaceutical compositions containing them and the use of such compounds. WO2008059299 A1, 22 May 2008. [Google Scholar]
- Szakonyi, Z.; Balázs, Á.; Martinek, T.A.; Fülöp, F. Stereoselective synthesis of pinane-based β- and γ-amino acids via conjugate addition of lithium amides and nitromethane. Tetrahedron: Asymmetry 2010, 21, 2498–2504. [Google Scholar]
- Fülöp, F.; Szakonyi, Z.; Pallai, P.V. 1,3-Heterocycles Condensed with Monoterpene Skeleton, Their Use and Pharmaceutical Compositions Comprising Such Compounds. WO 2010070365 A1, 24 June 2010. [Google Scholar]
- Sohár, P.; Stájer, G.; Szabó, A.; Fülöp, F.; Szúnyog, J.; Bernáth, G. Stereochemical studies. Part 89. Saturated heterocycles. Part 84. Preparation and nuclear magnetic resonance study of norbornane-norbornene-fused 2-phenylimino-1,3-oxazines and -thiazines. J. Chem. Soc. Perkin Trans. 2 1987. [Google Scholar] [CrossRef]
- Fülöp, F; Csirinyi, G.; Bernáth, G. Saturated heterocycles, 135. Cyclic amino alcohols and related compounds, 29. Synthesis of condensed-skeleton cis- and trans-2-phenylimino- and 2-methyliminotetrahydro-1,3-thiazines and 1,3-oxazines. Acta Chim. Hung. 1988, 125, 193–199. [Google Scholar]
- Kim, T.H.; Cha, M.-H. Efficient synthesis of 2-methylaminothiazolines via Mitsunobu reaction of N-(2-hydroxyethyl)-N'-methyl-thioureas. Tetrahedron Lett. 1999, 40, 3125–3128. [Google Scholar]
- Bernacki, A.L.; Zhu, L.; Hennings, D.D. A selective and convenient method for the synthesis of 2-phenylaminothiazolines. Org. Lett. 2010, 12, 5526–5529. [Google Scholar]
- Berényi, A.; Minorics, R.; Iványi, Z.; Ocsovszki, I.; Ducza, E.; Thole, H.; Messinger, J.; Wölfling, J.; Mótyán, G.; Mernyák, E.; et al. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 6, 55–63. [Google Scholar]
- Kanizsai, I.; Szakonyi, Z.; Sillanpää, R.; D’Hooghe, M.; de Kimpe, N.; Fülöp, F. Synthesis of chiral 1,5-disubstituted pyrrolidinones via electrophile-induced cyclization of 2-(3-butenyl)oxazolines derived from (1R,2S)- and (1S,2R)-norephedrine. Tetrahedron: Asymmetry 2006, 17, 2857–2863. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szakonyi, Z.; Zupkó, I.; Sillanpää, R.; Fülöp, F. Stereoselective Synthesis and Cytoselective Toxicity of Monoterpene-Fused 2-Imino-1,3-thiazines. Molecules 2014, 19, 15918-15937. https://doi.org/10.3390/molecules191015918
Szakonyi Z, Zupkó I, Sillanpää R, Fülöp F. Stereoselective Synthesis and Cytoselective Toxicity of Monoterpene-Fused 2-Imino-1,3-thiazines. Molecules. 2014; 19(10):15918-15937. https://doi.org/10.3390/molecules191015918
Chicago/Turabian StyleSzakonyi, Zsolt, István Zupkó, Reijo Sillanpää, and Ferenc Fülöp. 2014. "Stereoselective Synthesis and Cytoselective Toxicity of Monoterpene-Fused 2-Imino-1,3-thiazines" Molecules 19, no. 10: 15918-15937. https://doi.org/10.3390/molecules191015918
APA StyleSzakonyi, Z., Zupkó, I., Sillanpää, R., & Fülöp, F. (2014). Stereoselective Synthesis and Cytoselective Toxicity of Monoterpene-Fused 2-Imino-1,3-thiazines. Molecules, 19(10), 15918-15937. https://doi.org/10.3390/molecules191015918