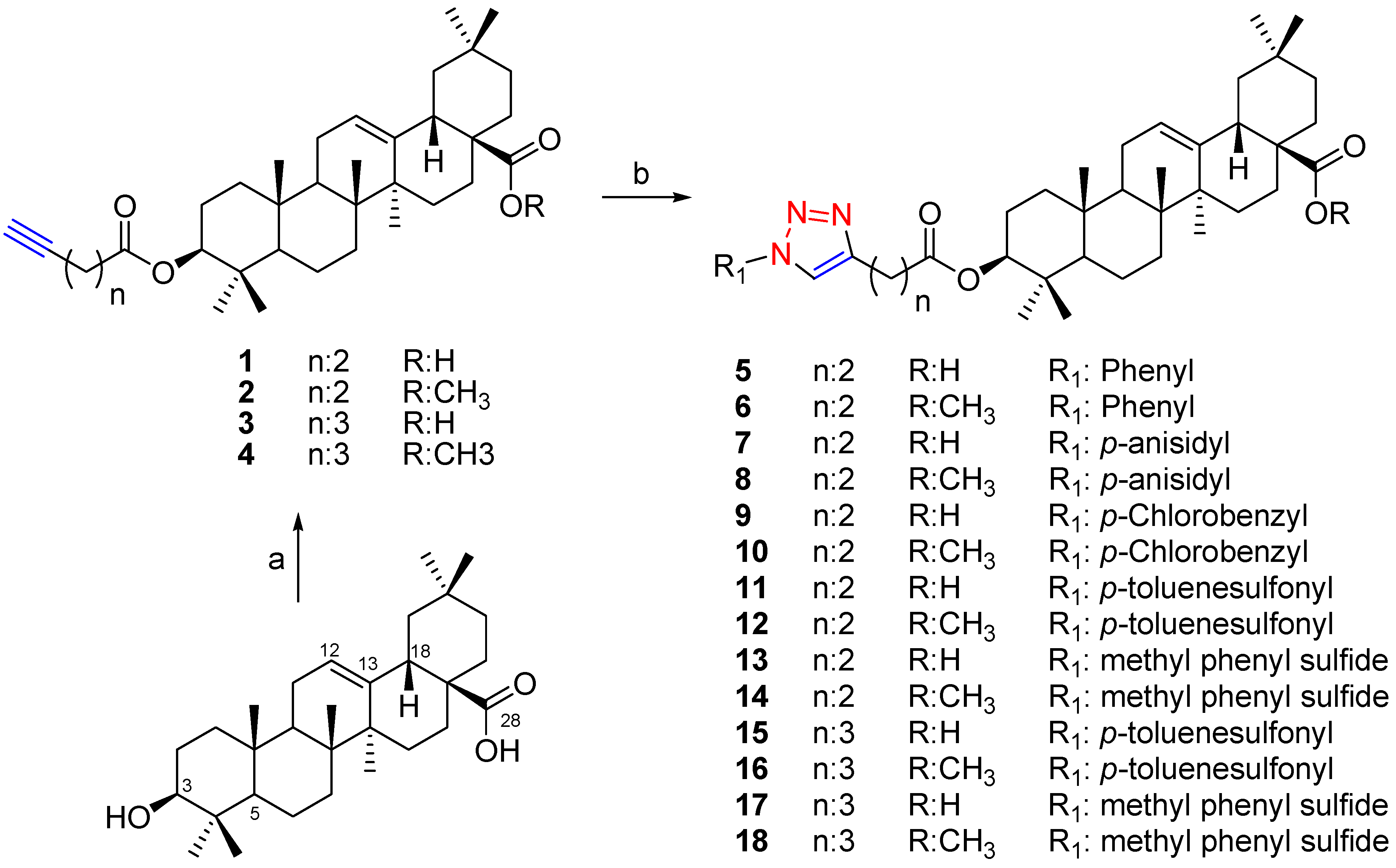
3.2.3. Preparation of Methyl Esters 2, 4, 6, 8, 10, 12, 14, 16 and 18
Methylation was performed using diazomethane in diethyl ether (Et2O). Methylation of 1 and 3 yielded the compounds 2 and 4, respectively. Methylation of the compounds 5, 7, 9, 11, 13, 15 and 17 afforded the corresponding methyl esters 6, 8, 10, 12, 14, 16 and 18.
Compound (1). Oleanolic acid (OA) (170 mg, 0.373 mmol), DCC (77 mg, 0.373 mmol), a catalytic amount of DMAP and 4-pentynoic acid (88 mg, 0.373 mmol), in dry CH2Cl2 (20 mL), were stirred at room temperature for 2–4 h. The reaction mixture was worked-up as described in 3.2.1. The residue was purified by silica gel column chromatography, eluting with hexane/EtOAc (8:2), yielding 1 (94 mg, 47%): white solid; mp 235 °C; [α]20 D +65 (c 0.058, CHCl3); IR νmax (film) 3309, 2941, 2873, 1731, 1695, 1466, 1270, 760 cm−1; 1H-NMR (CDCl3): δ 5.25 (1H, brs, H-12), 4.52 (1H, t, J = 8.3 Hz, H-3α), 2.80 (1H, dd, J = 13.5; 3.6 Hz, H-18), 2.48–2.56 (4H, m, OCOCH2CH2), 1.96 (1H, brs, H-5'), 1.11 (3H, s), 0.92 (3H, s), 0.91 (3H, s), 0.89 (3H, s), 0.86 (3H, s), 0.84 (3H, s), 0.72 (3H, s); 13C-NMR (CDCl3): δ 184.39 (C-28), 171.52 (C-1'), 143.62 (C-13), 122.49 (C-12), 82.62 (C-4'), 81.33 (C-3), 69.07 (C-5'), 55.27 (C-5), 47.53, 46.53, 45.82, 41.52, 40.87, 39.26, 38.01, 37.73, 36.97, 33.85, 33.78, 33.07, 32.48, 30.66, 29.70, 28.05, 27.66, 25.93, 23.58, 23.53, 23.38, 22.83, 18.15, 17.19, 16.70, 15.36, 14.56; HREIMS m/z 536.3768 [M]+• (calcd for C35H52O4, 536.3866).
Compound (2). Compound 1 (50 mg, 0.093 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 47 mg (92%) of 2: white solid; mp 173 °C; [α]20 D +71 (c 0.046, CHCl3); IR νmax (film) 3306, 2945, 2873, 1731, 1461, 1262, 754 cm−1; 1H-NMR (CDCl3): δ 5.25 (1H, brs, H-12), 4.50 (1H, t, J = 8.1 Hz, H-3α), 3.59 (3H, s, OMe), 2.82 (1H, dd, J = 13.7; 3.8 Hz, H-18), 2.46–2.54 (4H, m, OCOCH2CH2), 1.94 (1H, brs, H-5'), 1.10 (3H, s), 0.90 (3H, s), 0.89 (3H, s), 0.87 (3H, s), 0.84 (3H, s), 0.83 (3H, s), 0.69 (3H, s); 13C-NMR (CDCl3): δ 178.23 (C-28), 171.43 (C-1'), 143.78 (C-13), 122.24 (C-12), 82.60 (C-4'), 81.30 (C-3), 69.06 (C-5'), 55.28 (C-5), 51.51 (OMe), 47.52, 46.69, 45.83, 41.62, 41.27, 39.26, 38.06, 37.71, 36.90, 33.83 (2C), 33.11, 32.58, 32.36, 30.68, 28.05, 27.68, 25.90, 23.64, 23.53, 23.39, 23.04, 18.20, 16.82, 16.73, 15.34, 14.51; HREIMS m/z 550.4324 [M]+• (calcd for C36H54O4, 550.4022).
Compound (3). Compound 3 was synthesized as described for compound 1, using OA and 5-hexynoic acid yielding 105 mg (51%) of 3: white solid; mp 212 °C; [α]20 D +66 (c 0.023, CHCl3); IR νmax (film) 3308, 2941, 2874, 1729, 1694, 1462, 1276, 757 cm−1; 1H-NMR (CDCl3): δ 5.27 (1H, brs, H-12), 4.50 (1H, t, J = 8.3 Hz, H-3α), 2.80 (1H, dd, J = 13.4; 3.5 Hz, H-18), 2.45 (2H, t, J = 7.5 Hz, H-2'), 2.26 (2H, dt, J = 7.0; 2.5 Hz, H-4'), 1.94 (1H, brs, H-6'), 1.83–1.88 (2H, m, H-3'), 1.12 (3H, s), 0.93 (3H, s), 0.92 (3H, s), 0.90 (3H, s), 0.86 (3H, s), 0.85 (3H, s), 0.74 (3H, s); 13C-NMR (CDCl3): δ 184.01 (C-28), 172.84 (C-1'), 143.59 (C-13), 122.52 (C-12), 83.23 (C-4'), 80.90 (C-3), 69.06 (C-5'), 55.26 (C-5), 47.53, 46.52, 45.81, 41.53, 40.89, 39.25, 38.02, 37.73, 36.97, 33.76, 33.42, 33.05, 32.49, 30.66, 29.69, 28.07, 27.65, 25.91, 23.79, 23.57, 23.38, 22.86, 18.15, 17.91, 17.15, 16.71, 15.36, 14.10; HREIMS m/z 550.4410 [M]+• (calcd for C36H54O4, 550.4022).
Compound (4). Compound 3 (50 mg, 0.089 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 47 mg (93%) of 4: white solid; mp 176 °C; [α]20 D +60 (c 0.042, CHCl3); IR νmax (film) 3297, 2942, 2868, 1726, 1460, 1226, 754 cm−1; 1H-NMR (CDCl3): δ 5.27 (1H, brs, H-12), 4.50 (1H, t, J = 8.1 Hz, H-3α), 3.61 (3H, s, OMe), 2.84 (1H, dd, J = 13.8; 3.8 Hz, H-18), 2.44 (2H, t, J = 7.5 Hz, H-2'), 2.25 (2H, dt, J = 7.0; 2.5 Hz, H-4'), 1.96 (1H, brs, H-6'), 1.81–1.88 (2H, m, H-3'), 1.12 (3H, s), 0.92 (6H, s), 0.89 (3H, s), 0.85 (6H, s), 0.71 (3H, s); 13C-NMR (CDCl3): δ 178.25 (C-28), 172.78 (C-1'), 143.76 (C-13), 122.22 (C-12), 83.28 (C-4'), 80.87 (C-3), 69.02 (C-5'), 55.24 (C-5), 51.48 (OMe), 47.50, 46.67, 45.80, 41.59, 41.24, 39.24, 38.04, 37.69, 36.88, 33.81, 33.38, 33.06, 32.55, 32.33, 30.65, 28.04, 27.64, 25.86, 23.76, 23.60, 23.51, 23.36, 23.02, 18.17, 17.87, 16.79, 16.71, 15.31; HREIMS m/z 564.4164 [M]+• (calcd for C37H56O4, 564.4179).
Compound (5). Compound 1 (76 mg, 0.142 mmol) and azidobenzene (17 mg, 0.142 mmol), were dissolved in CH2Cl2/H2O (3 mL/3 mL) followed by the addition of 4 mg CuSO4.5H2O (0.014 mmol, dissolved in 200 μL of water) and 6 mg of sodium ascorbate (0.028 mmol, dissolved in 200 μL of water). The solution was stirred at room temperature for 24 h. The reaction mixture was worked-up as described in 3.2.2 and was purified by silica gel CC eluting with hexane/EtOAc (8:2), yielding 5 (72 mg, 77%). White solid; mp 240 °C; [α]20 D +53 (c 0.064, CHCl3); IR νmax (film) 3415, 2937, 2856, 1727, 1693, 1462, 1277, 761 cm−1; 1H-NMR (CDCl3): δ 7.79 (1H, s, H-5'), 7.68 (2H, d, J = 7.8 Hz, H-2'' and H-6''), 7.49 (2H, t, J = 7.8 Hz, H-3'' and H-5''), 7.40 (1H, t, J = 7.3 Hz, H-4''), 5.25 (1H, brs, H-12), 4.50 (1H, t, J = 8.3 Hz, H-3α), 3.12 (2H, t, J = 7.2 Hz, H-3'), 2.81 (1H, dd, J = 13.4; 3.5 Hz, H-18), 2.79 (2H, t, J = 7.0 Hz, H-2'), 1.10 (3H, s), 0.90 (6H, s), 0.88 (3H, s), 0.81 (3H, s), 0.79 (3H, s), 0.72 (3H, s); 13C-NMR (CDCl3): δ 183.97 (C-28), 172.58 (C-1'), 147.20 (C-4'), 143.68 (C-13), 137.20 (C-1''), 129.72 (2C, C-3'' and C-5''), 128.59 (C-4''), 122.43 (C-12), 120.44 (2C, C-2'' and C-6''), 119.55 (C-5'), 81.26 (C-3), 55.28 (C-5), 47.53, 46.50, 45.86, 41.53, 40.90, 39.25, 38.03, 37.72, 36.95, 34.02, 33.75, 33.08, 32.50, 32.46, 30.68, 27.99, 27.67, 25.92, 23.58, 23.59, 23.38, 22.85, 21.17, 18.15, 17.14, 16.70, 15.36; HREIMS m/z 656.4529 [M+H]+ (calcd for C41H58N3O4, 656.4427).
Compound (6). Compound 5 (50 mg, 0.076 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 44 mg (86%) of 6: white solid; mp 168 °C; [α]20 D +44 (c 0.050, CHCl3); IR νmax (film) 2944, 2876, 1730, 1463, 1260, 756 cm−1; 1H-NMR (CDCl3): δ 7.79 (1H, s, H-5'), 7.69 (2H, d, J = 7.7 Hz, H-2'' and H-6''), 7.51 (2H, t, J = 7.7 Hz, H-3'' and H-5''), 7.42 (1H, t, J = 7.3 Hz, H-4''), 5.27 (1H, brs, H-12), 4.51 (1H, t, J = 8.3 Hz, H-3α), 3.62 (3H, s, OMe), 3.13 (2H, t, J = 7.2 Hz, H-3'), 2.85 (1H, dd, J = 13.4; 3.5 Hz, H-18), 2.80 (2H, t, J = 7.0 Hz, H-2'), 1.12 (3H, s), 0.92 (3H, s), 0.91 (3H, s), 0.89 (3H, s), 0.83 (3H, s), 0.80 (3H, s), 0.71 (3H, s); 13C-NMR (CDCl3): δ 178.35 (C-28), 172.56 (C-1'), 147.24 (C-4'), 143.53 (C-13), 137.56 (C-1''), 129.73 (2C, C-3'' and C-5''), 128.58 (C-4''), 122.26 (C-12), 120.46 (2C, C-2'' and C-6''), 119.50 (C-5'), 81.26 (C-3), 55.30 (C-5), 51.56 (OMe), 47.54, 46.72, 45.84, 41.63, 41.28, 39.27, 38.08, 37.73, 36.91, 34.03, 33.84, 33.11, 32.56, 32.37, 30.70, 27.99, 27.67, 25.90, 23.64, 23.55, 23.40, 23.05, 21.20, 18.19, 16.82, 16.71, 15.34; HREIMS m/z 670.5248 [M+H]+ (calcd for C42H60N3O4, 670.5286).
Compound (7). Compound 7 was synthesized as described for compound 5, using compound 1 (100 mg, 0.187 mmol) and 4-azidoanisole (28 mg, 0.187 mmol) yielding 68 mg (53%) of 3: white solid; mp 210 °C; [α]20 D +35 (c 0.045, CHCl3); IR νmax (film) 3416, 2946, 2877, 1732, 1695, 1460, 1255, 747 cm−1; 1H-NMR (CDCl3): δ 7.70 (1H, s, H-5'), 7.57 (2H, d, J = 8.7 Hz, H-2'' and H-6''), 6.98 (2H, d, J = 8.7 Hz, H-3'' and H-5''), 5.25 (1H, brs, H-12), 4.50 (1H, t, J = 8.3 Hz, H-3α), 3.85 (3H, s, PhOMe), 3.11 (2H, t, J = 7.2 Hz, H-3'), 2.80 (1H, dd, J = 13.4; 3.5 Hz, H-18), 2.78 (2H, t, J = 7.0 Hz, H-2'), 1.11 (3H, s), 0.91 (6H, s), 0.87 (3H, s), 0.82 (3H, s), 0.79 (3H, s), 0.73 (3H, s); 13C-NMR (CDCl3): δ 183.70 (C-28), 172.60 (C-1'), 159.67 (C-4''), 146.99 (C-4'), 143.68 (C-13), 130.97 (C-1''), 122.45 (C-12), 122.10 (2C, C-2'' and C-6''), 119.75 (C-5'), 114.64 (2C, C-3'' and C-5''), 81.22 (C-3), 55.33 (OMe), 55.25 (C-5), 47.52, 46.50, 45.84, 41.54, 40.92, 39.25, 38.05, 37.73, 36.95, 34.07, 33.77, 33.01, 32.47, 32.30, 30.67, 27.93, 27.69, 25.83, 23.65, 23.53, 23.38, 23.20, 21.18, 18.13, 16.76, 16.64, 15.29; HREIMS m/z 686.4309 [M+H]+ (calcd for C42H60N3O5, 686.4533).
Compound (8). Compound 7 (40 mg, 0.058 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 36 mg (89%) of 8: white solid; mp 151 °C; [α]20 D +47 (c 0.015, CHCl3); IR νmax (film) 2941, 2872, 1722, 1460, 1252, 755 cm−1; 1H-NMR (CDCl3): δ 7.70 (1H, s, H-5'), 7.58 (2H, d, J = 8.9 Hz, H-2'' and H-6''), 6.99 (2H, d, J = 8.9 Hz, H-3'' and H-5''), 5.27 (1H, brs, H-12), 4.50 (1H, t, J = 8.3 Hz, H-3α), 3.86 (3H, s, PhOMe), 3.61 (3H, s, OMe), 3.11 (2H, t, J = 7.2 Hz, H-3'), 2.84 (1H, dd, J = 13.4; 3.5 Hz, H-18), 2.78 (2H, t, J = 7.0 Hz, H-2'), 1.11 (3H, s), 0.91 (6H, s), 0.89 (3H, s), 0.82 (3H, s), 0.79 (3H, s), 0.71 (3H, s); 13C-NMR (CDCl3): δ 178.26 (C-28), 172.51 (C-1'), 159.63 (C-4''), 146.97 (C-4'), 143.77 (C-13), 130.62 (C-1''), 122.21 (C-12), 122.05 (2C, C-2'' and C-6''), 119.63 (C-5'), 114.68 (2C, C-3'' and C-5''), 81.19 (C-3), 55.58 (PhOMe), 55.27 (C-5), 51.50 (OMe), 47.51, 46.67, 45.81, 41.59, 41.25, 39.24, 38.05, 37.69, 36.89, 34.03, 33.81, 33.07, 32.54, 32.33, 30.66, 27.95, 27.64, 25.86, 23.60, 23.52, 23.36, 23.02, 21.17, 18.16, 16.78, 16.67, 15.31; HREIMS m/z 700.4257 [M+H]+ (calcd for C43H62N3O5, 700.4689).
Compound (9). Compound 9 was synthesized as described for compound 5, using compound 1 (120 mg, 0.224 mmol) and 1-azido-4-chlorobenzene (34 mg, 0.224 mmol) yielding 84 mg (54%) of 9: white solid; mp 184 °C; [α]20 D +46 (c 0.052, CHCl3); IR νmax (film) 3432, 2942, 2880, 1720, 1688, 1460, 1271, 756 cm−1; 1H-NMR (CDCl3): δ 7.78 (1H, s, H-5'), 7.64 (2H, d, J = 8.6 Hz, H-2'' and H-6''), 7.46 (2H, d, J = 8.6 Hz, H-3'' and H-5''), 5.25 (1H, brs, H-12), 4.49 (1H, t, J = 8.3 Hz, H-3α), 3.13 (2H, t, J = 7.0 Hz, H-3'), 2.80 (1H, dd, J = 13.4; 3.5 Hz, H-18), 2.78 (2H, t, J = 6.9 Hz, H-2'), 1.10 (3H, s), 0.90 (6H, s), 0.88 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.72 (3H, s); 13C-NMR (CDCl3): δ 184.16 (C-28), 172.53 (C-1'), 147.51 (C-4'), 143.65 (C-13), 135.60 (C-1''), 134.28 (C-4''), 129.89 (2C, C-3'' and C-5''), 122.46 (C-12), 121.54 (2C, C-2'' and C-6''), 119.49 (C-5'), 81.29 (C-3), 55.28 (C-5), 47.53, 46.51, 45.85, 41.51, 40.88, 39.24, 38.02, 37.72, 36.96, 33.92, 33.78, 33.08, 32.47, 32.34, 30.68, 27.99, 27.65, 25.92, 23.60, 23.58, 23.39, 22.87, 21.13, 18.21, 17.17, 16.70, 15.36; HREIMS m/z 690.4133 [M+H]+ (calcd for C41H57ClN3O4, 690.4037).
Compound (10). Compound 9 (50 mg, 0.073 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 45 mg (88%) of 10: white solid; mp 206 °C; [α]20 D +53 (c 0.015, CHCl3); IR νmax (film) 2949, 2873, 1725, 1461, 1257, 760 cm−1; 1H-NMR (CDCl3): δ 7.77 (1H, s, H-5'), 7.62 (2H, d, J = 8.7 Hz, H-2'' and H-6''), 7.43 (2H, d, J = 8.7 Hz, H-3'' and H-5''), 5.23 (1H, brs, H-12), 4.46 (1H, t, J = 8.1 Hz, H-3α), 3.58 (3H, s, OMe), 3.08 (2H, t, J = 7.2 Hz, H-3'), 2.81 (1H, dd, J = 13.6; 3.4 Hz, H-18), 2.75 (2H, t, J = 7.2 Hz, H-2'), 1.08 (3H, s), 0.88 (3H, s), 0.87 (3H, s), 0.85 (3H, s), 0.78 (3H, s), 0.75 (3H, s), 0.67 (3H, s); 13C-NMR (CDCl3): δ 178.21 (C-28), 172.39 (C-1'), 147.42 (C-4'), 143.76 (C-13), 135.59 (C-1''), 134.16 (C-4''), 129.83 (2C, C-3'' and C-5''), 122.20 (C-12), 121.46 (2C, C-2'' and C-6''), 119.40 (C-5'), 81.20 (C-3), 55.26 (C-5), 51.50 (OMe), 47.50, 46.65, 45.80, 41.57, 41.24, 39.22, 38.04, 37.68, 36.87, 33.87, 33.81, 33.11, 32.53, 32.33, 30.66, 27.96, 27.64, 25.88, 23.63, 23.53, 23.36, 23.00, 21.13, 18.16, 16.79, 16.70, 15.32; HREIMS m/z 704.4243 [M+H]+ (calcd for C42H59ClN3O4, 704.4194).
Compound (11). Compound 11 was synthesized as described for compound 5, using compound 1 (120 mg, 0.224 mmol) and p-toluenesulfonyl azide (48 mg, 0.224 mmol) yielding 91 mg (55%) of 11: white solid; mp 163 °C; [α]20 D +39 (c 0.054, CHCl3); IR νmax (film) 3420, 2946, 2871, 1730, 1693, 1460, 1272, 759 cm−1; 1H-NMR (CDCl3): δ 7.96 (2H, d, J = 8.3 Hz, H-2'' and H-6''), 7.91 (1H, s, H-5'), 7.36 (2H, d, J = 8.3 Hz, H-3'' and H-5''), 5.26 (1H, brs, H-12), 4.47 (1H, t, J = 8.3 Hz, H-3α), 3.03 (2H, t, J = 7.2 Hz, H-3'), 2.81 (1H, dd, J = 13.5; 3.1 Hz, H-18), 2.70 (2H, t, J = 7.2 Hz, H-2'), 2.43 (3H, s, PhMe), 1.11 (3H, s), 0.91 (3H, s), 0.90 (3H, s), 0.89 (3H, s), 0.77 (3H, s), 0.73 (6H, s); 13C-NMR (CDCl3): δ 184.33 (C-28), 172.05 (C-1'), 147.19 (C-4'), 146.37 (C-4''), 143.64 (C-13), 133.17 (C-1''), 130.43 (2C, C-2'' and C-6''), 128.62 (2C, C-3'' and C-5''), 122.47 (C-12), 121.02 (C-5'), 81.36 (C-3), 55.23 (C-5), 47.53, 46.53, 45.82, 41.51, 40.88, 39.25, 38.03, 37.67, 36.95, 33.78, 33.49, 33.10, 32.46, 32.30, 30.68, 27.95, 27.65, 25.94, 23.60, 23.49, 23.37, 23.14, 21.87, 20.91, 18.13, 17.17, 16.65, 15.37; HREIMS m/z 734.4814 [M+H]+ (calcd for C42H60N3O6S, 734.4203).
Compound (12). Compound 11 (60 mg, 0.082 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 53 mg (87%) of 12: white solid; mp 154 °C; [α]20 D +35 (c 0.053, CHCl3); IR νmax (film) 2946, 2876, 1726, 1467, 1260, 757 cm−1; 1H-NMR (CDCl3): δ 7.94 (2H, d, J = 8.3 Hz, H-2'' and H-6''), 7.90 (1H, s, H-5'), 7.34 (2H, d, J = 8.3 Hz, H-3'' and H-5''), 5.25 (1H, brs, H-12), 4.44 (1H, t, J = 8.2 Hz, H-3α), 3.60 (3H, s, OMe), 3.01 (2H, t, J = 7.2 Hz, H-3'), 2.83 (1H, dd, J = 13.4; 3.4 Hz, H-18), 2.68 (2H, t, J = 7.0 Hz, H-2'), 2.41 (3H, s, PhMe), 1.10 (3H, s), 0.90 (3H, s), 0.87 (6H, s), 0.74 (3H, s), 0.70 (3H, s), 0.69 (3H, s); 13C-NMR (CDCl3): δ 178.26 (C-28), 171.97 (C-1'), 147.14 (C-4'), 146.36 (C-4''), 143.81 (C-13), 133.19 (C-1''), 130.40 (2C, C-2'' and C-6''), 128.60 (2C, C-3'' and C-5''), 122.21 (C-12), 121.00 (C-5'), 81.31 (C-3), 55.23 (C-5), 51.54 (OMe), 47.51, 46.68, 45.81, 41.60, 41.26, 39.25, 38.03, 37.63, 36.87, 33.83, 33.46, 33.11, 32.54, 32.35, 30.69, 27.91, 27.66, 25.90, 23.64, 23.46, 23.39, 23.03, 21.84, 20.91, 18.17, 16.80, 16.66, 15.33; HREIMS m/z 748.4274 [M+H]+ (calcd for C43H62N3O6S, 748.4359).
Compound (13). Compound 13 was synthesized as described for compound 5, using compound 1 (120 mg, 0.224 mmol) and azidomethyl phenyl sulfide (37 mg, 0.224 mmol) yielding 98 mg (62%) of 13: white solid; mp 170 °C; [α]20 D +13 (c 0.055, CHCl3); IR νmax (film) 3426, 2939, 2871, 1723, 1691, 1461, 1276, 756 cm−1; 1H-NMR (CDCl3): δ 7.37 (1H, s, H-5'), 7.29 (5H, s, Ph), 5.57 (2H, s CH2S), 5.25 (1H, brs, H-12), 4.47 (1H, t, J = 8.3 Hz, H-3α), 3.00 (2H, t, J = 7.3 Hz, H-3'), 2.81 (1H, dd, J = 13.5; 3.5 Hz, H-18), 2.68 (2H, t, J = 7.5 Hz, H-2'), 1.11 (3H, s), 0.91 (3H, s), 0.90 (3H, s), 0.89 (3H, s), 0.80 (3H, s), 0.78 (3H, s), 0.73 (3H, s); 13C-NMR (CDCl3): δ 184.07 (C-28), 172.42 (C-1'), 147.12 (C-4'), 143.66 (C-13), 132.09 (C-1''), 132.02 (2C, C-2'' and C-6''), 129.46 (2C, C-3'' and C-5''), 128.57 (C-4''), 122.47 (C-12), 120.70 (C-5'), 81.17 (C-3), 55.28 (C-5), 53.65 (CH2S), 47.54, 46.52, 45.85, 41.53, 40.89, 39.26, 38.02, 37.71, 36.96, 33.98, 33.79, 33.07, 32.49, 32.44, 30.67, 28.02, 27.66, 25.91, 23.59, 23.52, 23.38, 22.86, 21.19, 18.15, 17.17, 16.70, 15.35; HREIMS m/z 702.3102 [M+H]+ (calcd for C42H60N3O4S, 702.4304).
Compound (14). Compound 13 (60 mg, 0.086 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 55 mg (90%) of 14: white solid; mp 195 °C; [α]20 D +17 (c 0.102, CHCl3); IR νmax (film) 2947, 2875, 1731, 1460, 1257, 763 cm−1; 1H-NMR (CDCl3): δ 7.37 (1H, s, H-5'), 7.28 (5H, s, Ph), 5.56 (2H, s CH2S), 5.26 (1H, brs, H-12), 4.46 (1H, t, J = 8.1 Hz, H-3α), 3.60 (3H, s, OMe), 2.99 (2H, t, J = 7.3 Hz, H-3'), 2.84 (1H, dd, J = 13.7; 3.9 Hz, H-18), 2.68 (2H, t, J = 7.6 Hz, H-2'), 1.10 (3H, s), 0.90 (3H, s), 0.89 (3H, s), 0.88 (3H, s), 0.80 (3H, s), 0.76 (3H, s), 0.70 (3H, s); 13C-NMR (CDCl3): δ 178.30 (C-28), 172.39 (C-1'), 147.12 (C-4'), 143.80 (C-13), 132.08 (C-1''), 132.00 (2C, C-2'' and C-6''), 129.45 (2C, C-3'' and C-5''), 128.56 (C-4''), 122.34 (C-12), 120.69 (C-5'), 81.18 (C-3), 55.29 (C-5), 53.63 (CH2S), 51.54 (OMe), 47.53, 46.70, 45.83, 41.61, 40.27, 39.25, 38.06, 37.70, 36.90, 33.96, 33.83, 33.11, 32.56, 32.36, 30.69, 28.01, 27.66, 25.90, 23.64, 23.52, 23.39, 23.04, 21.19, 18.18, 16.81, 16.71, 15.33; HREIMS m/z 716.4378 [M+H]+ (calcd for C43H62N3O4S, 716.4461).
Compound (15). Compound 15 was synthesized as described for compound 5, using compound 3 (120 mg, 0.218 mmol) and p-toluenesulfonyl azide (32 mg, 0.218 mmol) yielding 76 mg (47%) of 15: white solid; mp 186 °C; [α]20 D +51 (c 0.048, CHCl3); IR νmax (film) 3430, 2943, 2873, 1729, 1689, 1461, 1272, 757 cm−1; 1H-NMR (CDCl3): δ 7.94 (2H, d, J = 8.2 Hz, H-2'' and H-6''), 7.87 (1H, s, H-5'), 7.34 (2H, d, J = 8.1 Hz, H-3'' and H-5''), 5.23 (1H, brs, H-12), 4.47 (1H, t, J = 8.1 Hz, H-3α), 2.79 (1H, dd, J = 13.4; 3.0 Hz, H-18), 2.73 (2H, t, J = 7.5 Hz, H-4'), 2.40 (3H, s, PhMe), 2.32 (2H, t, J = 7.3 Hz, H-2'), 1.96 (2H, m, H-3'), 1.09 (3H, s), 0.90 (3H, s), 0.89 (3H, s), 0.87 (3H, s), 0.81 (6H, s), 0.71 (3H, s); 13C-NMR (CDCl3): δ 184.46 (C-28), 172.82 (C-1'), 147.19 (C-4'), 147.12 (C-4''), 143.63 (C-13), 133.14 (C-1''), 130.44 (2C, C-2'' and C-6''), 128.57 (2C, C-3'' and C-5''), 122.47 (C-12), 120.71 (C-5'), 80.98 (C-3), 55.24 (C-5), 47.51, 46.50, 45.82, 41.48, 40.84, 39.23, 38.03, 37.70, 36.96, 33.81, 33.08, 32.47, 32.33, 31.60, 30.65, 28.10, 27.66, 25.94, 24.65, 24.26, 23.66, 23.59, 23.38, 22.82, 21.84, 18.15, 17.19, 16.75, 15.37; HREIMS m/z 748.4327 [M+H]+ (calcd for C43H62N3O6S, 748.4359).
Compound (16). Compound 15 (50 mg, 0.067 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 44 mg (86%) of 16: white solid; mp 165 °C; [α]20 D +32 (c 0.079, CHCl3); IR νmax (film) 2943, 2871, 1728, 1459, 1254, 757 cm−1; 1H-NMR (CDCl3): δ 7.96 (2H, d, J = 8.1 Hz, H-2'' and H-6''), 7.87 (1H, s, H-5'), 7.36 (2H, d, J = 8.1 Hz, H-3'' and H-5''), 5.26 (1H, brs, H-12), 4.48 (1H, t, J = 8.3 Hz, H-3α), 3.60 (3H, s, OMe), 2.84 (1H, dd, J = 13.5; 3.2 Hz, H-18), 2.74 (2H, t, J = 7.5 Hz, H-4'), 2.43 (3H, s, PhMe), 2.33 (2H, t, J = 7.3 Hz, H-2'), 1.98 (2H, m, H-3'), 1.11 (3H, s), 0.90 (6H, s), 0.88 (3H, s), 0.82 (6H, s), 0.70 (3H, s); 13C-NMR (CDCl3): δ 178.27 (C-28), 172.74 (C-1'), 147.16 (C-4'), 147.11 (C-4''), 143.80 (C-13), 133.19 (C-1''), 130.42 (2C, C-2'' and C-6''), 128.60 (2C, C-3'' and C-5''), 122.34 (C-12), 120.62 (C-5'), 80.99 (C-3), 55.27 (C-5), 51.55 (OMe), 47.53, 46.69, 45.83, 41.61, 41.27, 39.26, 38.06, 37.70, 36.91, 33.81, 33.11, 32.57, 32.37, 31.58, 30.69, 28.10, 27.66, 25.91, 24.68, 24.27, 23.64, 23.56, 23.39, 23.04, 21.84, 18.20, 16.82, 16.77, 15.35; HREIMS m/z 762.4427 [M+H]+ (calcd for C44H64N3O6S, 762.4516).
Compound (17). Compound 17 was synthesized as described for compound 5, using compound 3 (120 mg, 0.218 mmol) and azidomethyl phenyl sulfide (36 mg, 0.218 mmol) yielding 87 mg (56%) of 17: white solid; mp 198 °C; [α]20 D +43 (c 0.011, CHCl3); IR νmax (film) 3426, 2940, 2876, 1723, 1692, 1461, 1270, 754 cm−1; 1H-NMR (CDCl3): δ 7.30 (1H, s, H-5'), 7.29 (5H, s, Ph), 5.57 (2H, s CH2S), 5.25 (1H, brs, H-12), 4.49 (1H, t, J = 8.1 Hz, H-3α), 2.80 (1H, dd, J = 13.4; 3.2 Hz, H-18), 2.71 (2H, t, J = 7.5 Hz, H-4'), 2.31 (2H, t, J = 7.3 Hz, H-2'), 1.95 (2H, m, H-3'), 1.11 (3H, s), 0.91 (6H, s), 0.88 (3H, s), 0.84 (3H, s), 0.82 (3H, s), 0.73 (3H, s); 13C-NMR (CDCl3): δ 184.15 (C-28), 173.06 (C-1'), 147.75 (C-4'), 143.69 (C-13), 132.28 (2C, C-2'' and C-6''), 131.95 (C-1''), 129.47 (2C, C-3'' and C-5''), 128.66 (C-4''), 122.44 (C-12), 120.55 (C-5'), 80.88 (C-3), 55.27 (C-5), 53.72 (CH2S), 47.55, 46.52, 45.86, 41.53, 40.89, 39.26, 38.03, 37.73, 36.98, 33.92, 33.76, 33.10, 32.52, 32.41, 30.68, 28.12, 27.67, 25.95, 24.92, 24.73, 23.62, 23.57, 23.41, 22.89, 18.22, 17.18, 16.79, 15.39; HREIMS m/z 716.4423 [M+H]+ (calcd for C43H62N3O4S, 716.4461).
Compound (18). Compound 17 (50 mg, 0.068 mmol), was methylated with a solution of CH2N2 in ethyl ether, yielding 45 mg (91%) of 18: white solid; mp 166 °C; [α]20 D +39 (c 0.016, CHCl3); IR νmax (film) 2946, 2870, 1723, 1461, 1259, 755 cm−1; 1H-NMR (CDCl3): δ 7.28 (1H, s, H-5'), 7.26 (5H, s, Ph), 5.54 (2H, s CH2S), 5.24 (1H, brs, H-12), 4.46 (1H, t, J = 8.1 Hz, H-3α), 3.58 (3H, s, OMe), 2.82 (1H, dd, J = 13.4; 3.2 Hz, H-18), 2.69 (2H, t, J = 7.5 Hz, H-4'), 2.29 (2H, t, J = 7.3 Hz, H-2'), 1.93 (2H, m, H-3'), 1.09 (3H, s), 0.88 (6H, s), 0.86 (3H, s), 0.81 (3H, s), 0.80 (3H, s), 0.68 (3H, s); 13C-NMR (CDCl3): δ 178.23 (C-28), 172.95 (C-1'), 147.71 (C-4'), 143.77 (C-13), 132.24 (2C, C-2'' and C-6''), 131.98 (C-1''), 129.42 (2C, C-3'' and C-5''), 128.61 (C-4''), 122.23 (C-12), 120.48 (C-5'), 80.82 (C-3), 55.26 (C-5), 53.64 (CH2S), 51.51 (OMe), 47.51, 46.67, 45.81, 41.59, 41.25, 39.24, 38.06, 37.70, 36.90, 33.89, 33.83, 33.12, 32.56, 32.35, 30.68, 28.10, 27.65, 25.90, 24.93, 24.71, 23.65, 23.57, 23.38, 23.03, 18.19, 16.81 (2C), 15.36; HREIMS m/z 730.4572 [M+H]+ (calcd for C44H64N3O4S, 730.4617).