Novel Antimicrobial Peptide Dendrimers with Amphiphilic Surface and Their Interactions with Phospholipids — Insights from Mass Spectrometry
Abstract
:1. Introduction
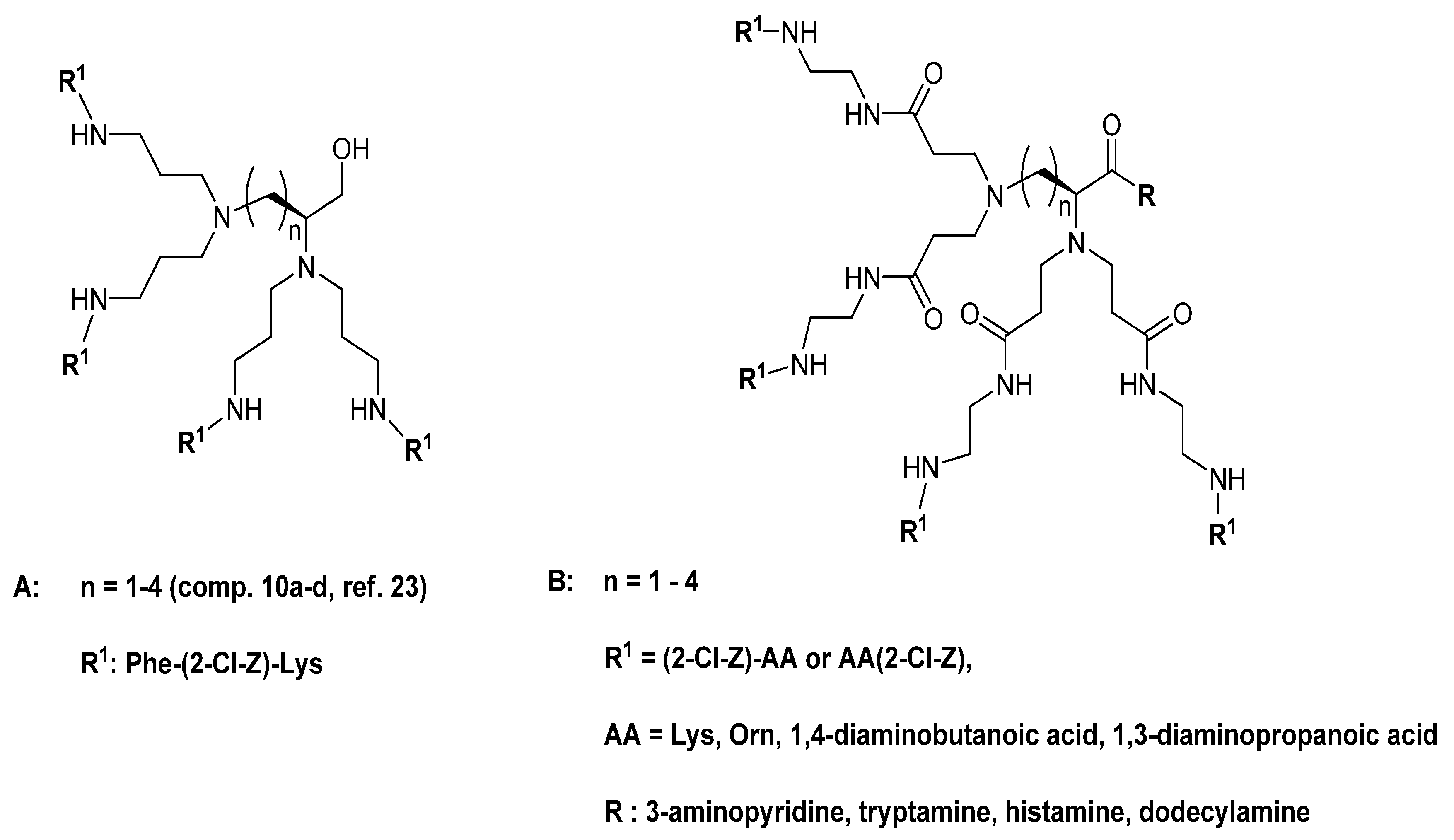
2. Results and Discussion
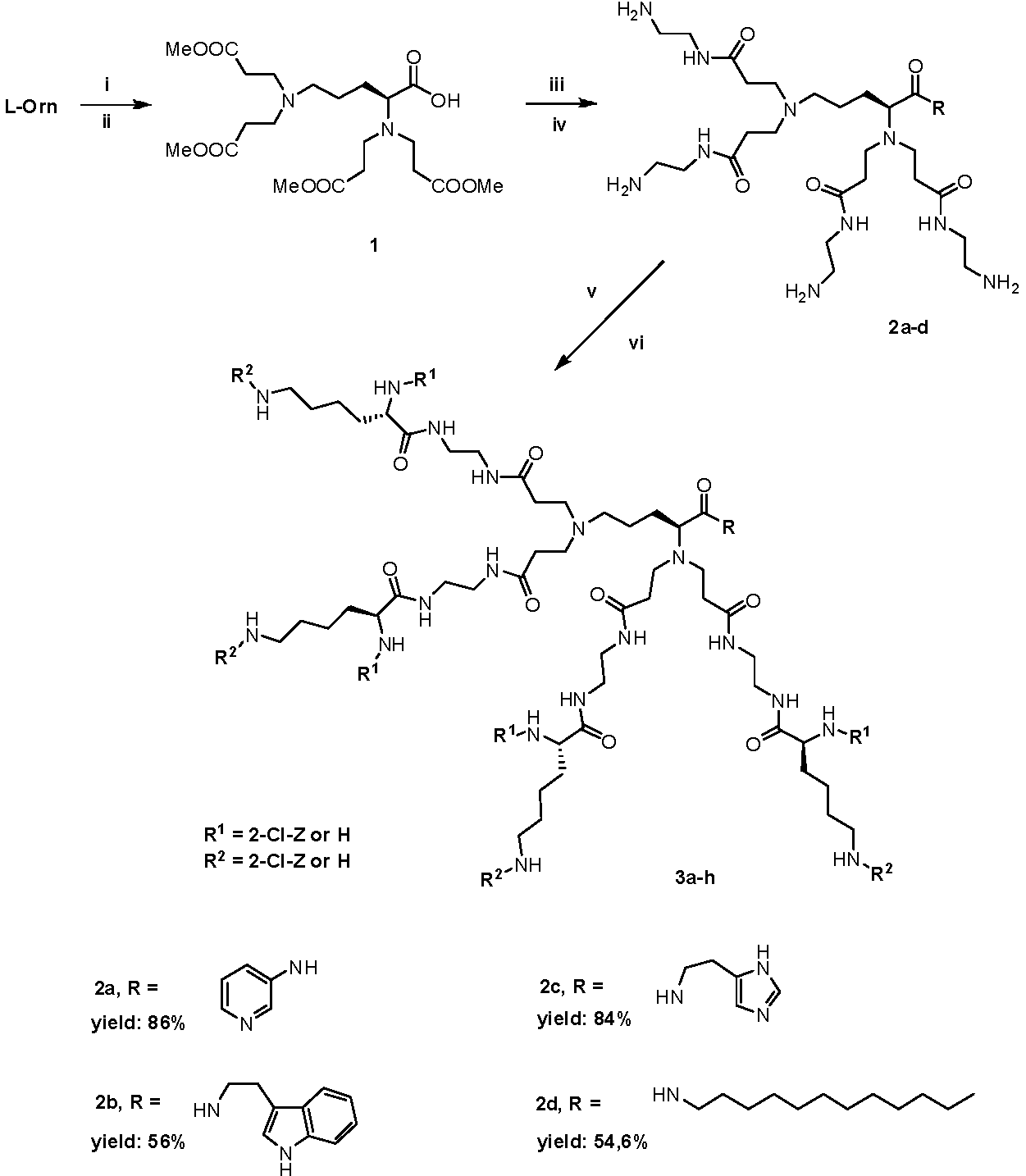
2.1. Synthesis
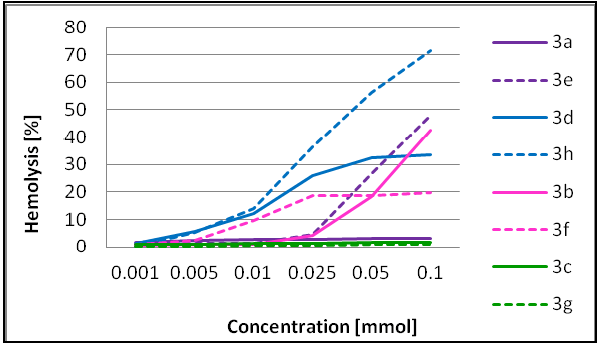
2.2. Microbiological and Hemolysis Studies of Dendrimers
| Strain | 3a (3-AP) | 3b (TrpN) | 3c (HisN) | 3d (ddN) | 3e (3-AP) | 3f (TrpN) | 3g (HisN) | 3h (ddN) |
|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 2.85 | 1.87 | 24.7 | 1.85 | 16.6 | 0.93 | 51 | 3.7 |
| S. aureus ATCC 43300 | >252 | 12.1 | 141 | 0.46 | 142 | 20.4 | 16.4 | 1.85 |
| E. coli ATCC 25922 | >252 | 12.1 | 141 | 1.85 | 142 | 7.9 | 211 | 7.9 |
| P. aeruginosa ATCC 27853 | >252 | 51 | 141 | 7.8 | 142 | 60 | 211 | 32 |
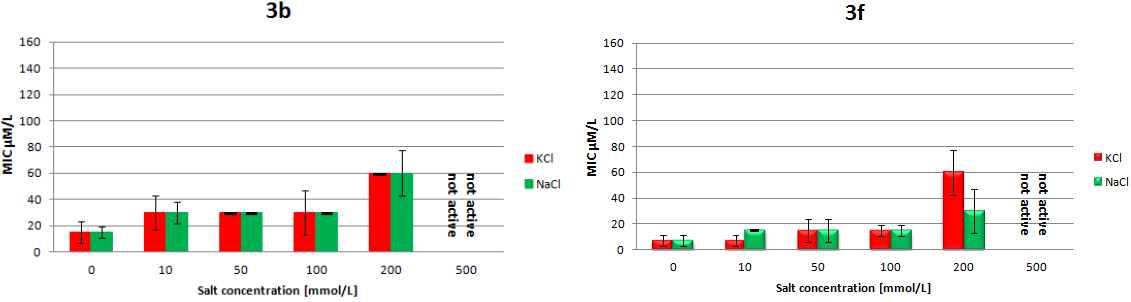
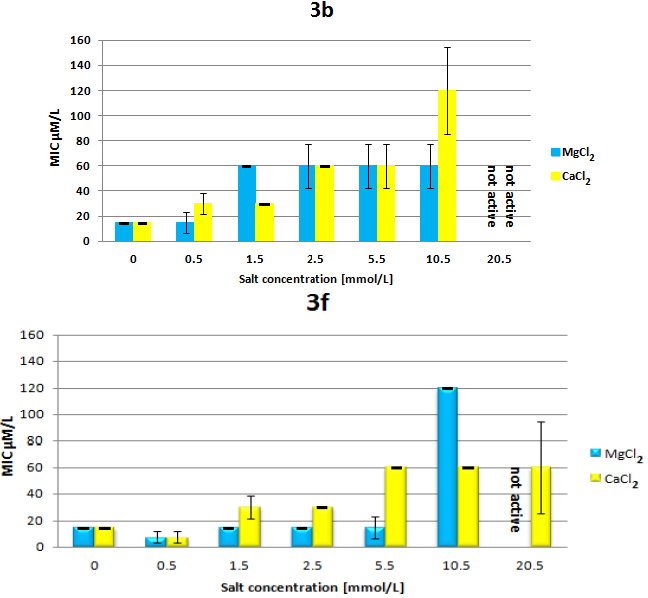
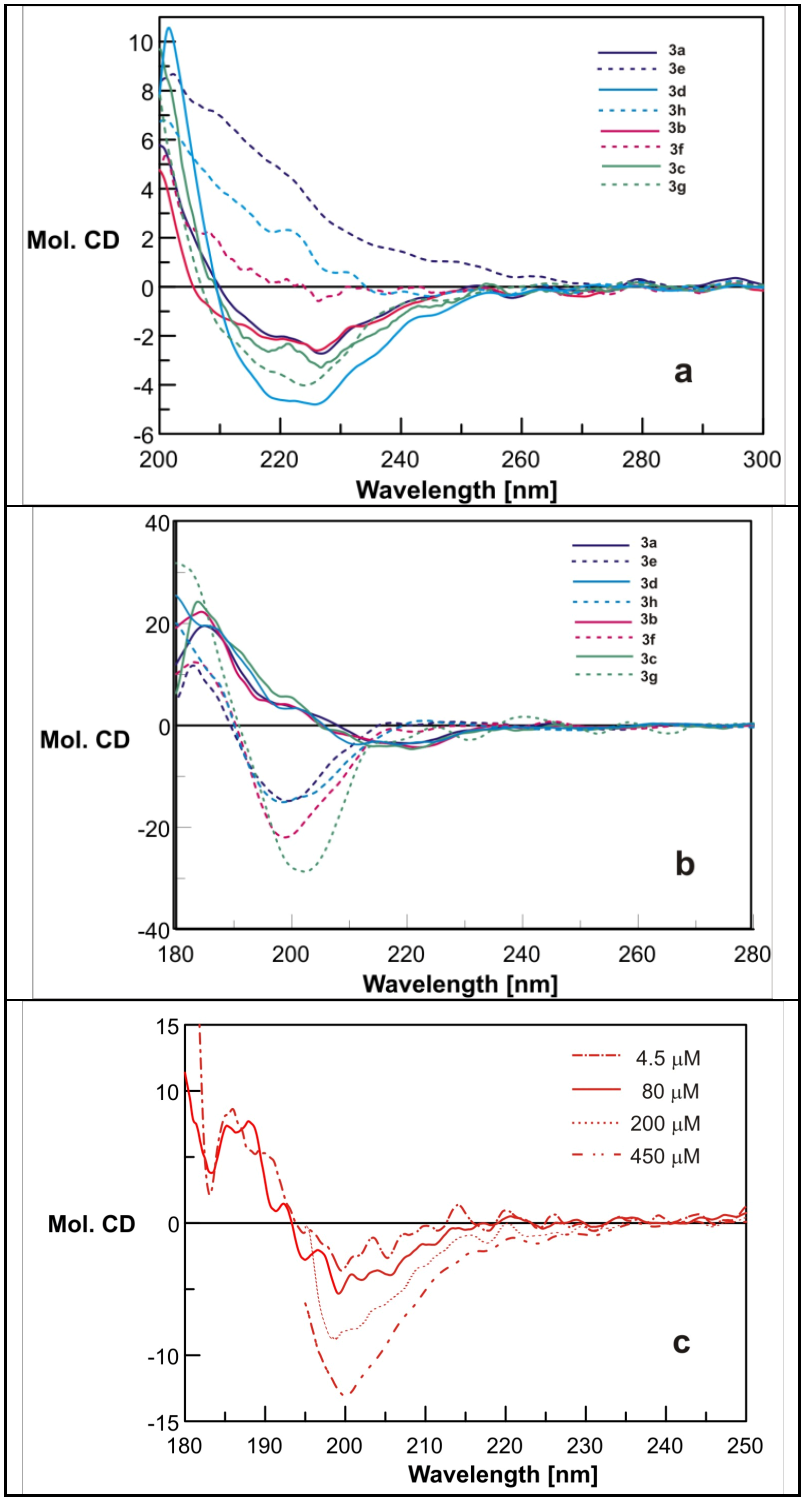
2.3. Circular Dichroism Spectroscopy Reveals Structure-Dependent Curve Evolution
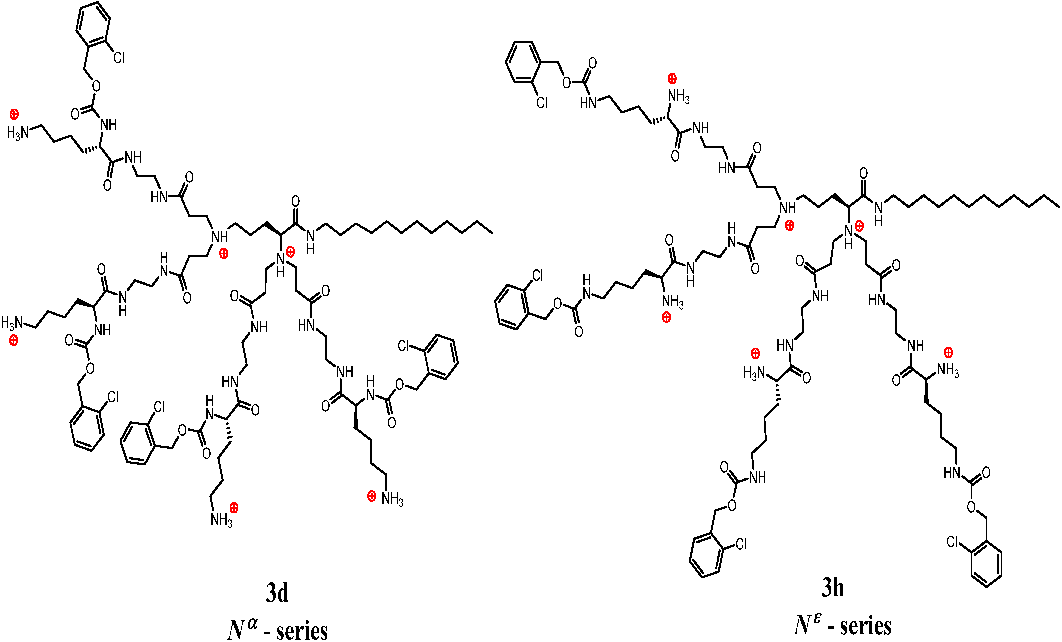
2.4. Mass Spectrometry Studies of Gas Phase Complexation of Dendrimers by Phospholipids

- -
- Dendrimers 3e and 3h of the Nε-series form less stable complexes with both phospholipids; however complexes with DMPG molecules, bearing formal negative charge are more stable than that with zwitterionic DMPC by 0.11 eV.
- -
- DMPG molecules form complexes with similar gas phase stability with dendrimers of the Nα-series: 3a, 3c and 3d; however these complexes are more stable than DMPG complexes with 3e and 3h by ca. 0.1 eV. Similar differences in binding strength between DMPG and DMPC have been reported for peptide-phospholipid non-covalent complexes [40].
- -
- Compounds of the Nα- and Nε-series show different recognition patterns with DMPG vs. DMPC in the gas phase. This accounts for the significant difference in stability of complexes for a particular dendrimer, as evidenced by ∆CID50 values reported in Table 2. Moreover, a high ∆CID50 values correlate with a lower level of hemotoxicity (shown in Figure 4).
| Dendrimer | ΔCID50 (eV) | Hemotoxicity |
|---|---|---|
| 3h | 0.11 | high |
| 3e | 0.12 | high |
| 3c | 0.15 | low |
| 3a | 0.19 | low |
| 3d | 0.18 | low |
3. Experimental
3.1. General
3.2. Synthesis and Characterization
3.2.2. Preparation of (3-Aminopyridyl)-amide (1a)
3.2.3. General Procedure for Preparation of Tryptamide (1b), Histamide (1c) and Dodecylamide (1d)
3.2.4. General Procedure for Preparation of Core Molecules 2a–d
3.2.5. General Procedure for Preparation of Dendrimers 3a–3h
3.3. Antibacterial Susceptibility Testing
3.4. Hemolysis Assay

3.5. Circular Dichroism Spectroscopy
3.6. Mass Spectrometry Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Castonguay, A.; Ladd, E.; van de Ven, T.G.M.; Kakkar, A. Dendrimers as bactericides. New J. Chem. 2012, 36, 199–204. [Google Scholar]
- Medina, S.H.; El-Sayed, M.E.H. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009, 109, 3141–3157. [Google Scholar]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar]
- Salimpour Abkenar, S.; Mohammad Ali Malek, R. Preparation, characterization, and antimicrobial property of cotton cellulose fabric grafted with poly (propylene imine) dendrime. Cellulose 2012, 19, 1701–1714. [Google Scholar]
- Sadler, K.; Tam, J.P. Peptide dendrimers: Applications and synthesis. J. Biotechnol. 2002, 90, 195–229. [Google Scholar]
- Rasines, B.; Manuel Hernandez-Ros, J.; de las Cuevas, N.; Luis Copa-Patino, J.; Soliveri, J.; Angeles Munoz-Fernandez, M.; Gomez, R.; de la Mata, F.J. Water-stable ammonium-terminated carbosilane dendrimers as efficient antibacterial agents. Dalton Trans. 2009, 8704–8713. [Google Scholar]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.-M.; Bousmina, M.; et al. iological properties of new viologen-phosphorus dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar]
- Yang, H.; Lopina, S.T. Penicillin V-conjugated PEG-PAMAM star polymers. J. Biomater. Sci. Polym. Ed. 2003, 14, 1043–1056. [Google Scholar]
- Jones, C.F.; Campbell, R.A.; Franks, Z.; Gibson, C.C.; Thiagarajan, G.; Vieira-de-Abreu, A.; Sukavaneshvar, S.; Mohammad, S.F.; Li, D.Y.; Ghandehari, H.; et al. ationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012, 9, 1599–1611. [Google Scholar]
- Kim, Y.; Klutz, A.M.; Jacobson, K.A. Systematic investigation of polyamidoamine dendrimers surface-modified with poly(ethylene glycol) for drug delivery applications: Synthesis, characterization, and evaluation of cytotoxicit. Bioconjugate Chem. 2008, 19, 1660–1672. [Google Scholar]
- Kolhatkar, R.B.; Kitchens, K.M.; Swaan, P.W.; Ghandehari, H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjugate Chem. 2007, 18, 2054–2060. [Google Scholar]
- Zhu, J.M.; Marchant, R.E. Dendritic saccharide surfactant polymers as antifouling interface materials to reduce platelet adhesion. Biomacromolecules 2006, 7, 1036–1041. [Google Scholar]
- Janiszewska, J.; Swieton, J.; Lipkowski, A.W.; Urbanczyk-Lipkowska, Z. Low molecular mass peptide dendrimers that express antimicrobial properties. Bioorganic Med. Chem. Lett. 2003, 13, 3711–3713. [Google Scholar]
- Janiszewska, J.; Urbanczyk-Lipkowska, Z. Amphiphilic dendrimeric peptides as model non-sequential pharmacophores with antimicrobial properties. J. Mol. Microbiol. Biotechnol. 2007, 13, 220–225. [Google Scholar]
- Janiszewska, J.; Urbanczyk-Lipkowska, Z. Synthesis, antimicrobial activity and structural studies of low molecular mass lysine dendrimers. Acta Biochim. Pol. 2006, 53, 77–82. [Google Scholar]
- Janiszewska, J.; Sowinska, M.; Rajnisz, A.; Solecka, J.; Lacka, I.; Milewski, S.; Urbanczyk-Lipkowska, Z. Novel dendrimeric lipopeptides with antifungal activity. Bioorganic Med. Chem. Lett. 2012, 22, 5330–5330. [Google Scholar]
- Afacan, N.J.; Yeung, A.T.Y.; Pena, O.M.; Hancock, R.E.W. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar]
- Hou, S.; Zhou, C.; Liu, Z.; Young, A.W.; Shi, Z.; Ren, D.; Kallenbach, N.R. Antimicrobial dendrimer active against escherichia coli biofilms. Bioorganic Med. Chem. Lett. 2009, 19, 5478–5481. [Google Scholar]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar]
- Maisuria, B.B.; Actis, M.L.; Hardrict, S.N.; Falkinham, J.O. Comparing micellar, hemolytic, and antibacterial properties of di- and tricarboxyl dendritic amphiphil. Bioorganic Med. Chem. 2011, 19, 2918–2926. [Google Scholar]
- Pan, J.; Guo, L.; Ouyang, L.; Yin, D.; Zhao, Y. Synthesis, antibacterial activity and cytotoxicity of novel Janus peptide dendrimers. Synlett 2012, 23, 1937–1940. [Google Scholar]
- Polcyn, P.; Jurczak, M.; Rajnisz, A.; Solecka, J.; Urbanczyk-Lipkowska, Z. Design of antimicrobially active small amphiphilic peptide dendrimers. Molecules 2009, 14, 3881–3905. [Google Scholar]
- Buhleier, E.; Wehner, W.; Vogtle, F. Cascade-chain-like and nonskid-chain-like syntheses of molecular cavity topologies. Synth. Stuttg. 1978, 2, 155–158. [Google Scholar]
- Hong, S.P.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.Y.; Balogh, L.; Orr, B.G.; Baker, J.R.; Holl, M.M.B. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjugate Chem. 2004, 15, 774–782. [Google Scholar]
- Chen, C.Z.S.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar]
- Smith, P.E.S.; Brender, J.R.; Duerr, U.H.N.; Xu, J.; Mullen, D.G.; Holl, M.M.B.; Ramamoorthy, A. Solid-state nmr reveals the hydrophobic-core location of poly(amidoamine) dendrimers in biomembranes. J. Am. Chem. Soc. 2010, 132, 8087–8097. [Google Scholar]
- Scorciapino, M.A.; Pirri, G.; Vargiu, A.V.; Ruggerone, P.; Giuliani, A.; Casu, M.; Buerck, J.; Wadhwani, P.; Ulrich, A.S.; Rinaldi, A.C. A novel dendrimeric peptide with antimicrobial properties: Structure-function analysis of sb056. Biophys. J. 2012, 102, 1039–1048. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar]
- Tam, J.P.; Lu, Y.A.; Yang, J.L. Antimicrobial dendrimeric peptides. Eur. J. Biochem. 2002, 269, 923–932. [Google Scholar]
- Liu, S.P.; Zhou, L.; Lakshminarayanan, R.; Beuerman, R.W. Multivalent antimicrobial peptides as therapeutics: Design principles and structural diversities. Int. J. Pept. Res. Ther. 2010, 16, 199–213. [Google Scholar]
- Crespo, L.; Sanclimens, G.; Montaner, B.; Perez-Tomas, R.; Royo, M.; Pons, M.; Albericio, F.; Giralt, E. Peptide dendrimers based on polyproline helices. J. Am. Chem. Soc. 2002, 124, 8876–8883. [Google Scholar]
- Javor, S.; Natalello, A.; Doglia, S.M.; Reymond, J.-L. Alpha-helix stabilization within a peptide dendrimer. J. Am. Chem. Soc. 2008, 130, 17248–17249. [Google Scholar]
- Hofstadler, S.A.; Sannes-Lowery, K.A. Applications of esi-ms in drug discovery: Interrogation of noncovalent complexes. Nature Rev. Drug Discov. 2006, 5, 585–595. [Google Scholar]
- Schalley, C.A. Molecular recognition and supramolecular chemistry in the gas phase. Mass Spectrom. Rev. 2001, 20, 253–309. [Google Scholar]
- Loo, J.A. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom. Rev. 1997, 16, 1–23. [Google Scholar]
- Hilton, G.R.; Benesch, J.L.P. Two decades of studying non-covalent biomolecular assemblies by means of electrospray ionization mass spectrometry. J. R. Soc. Interface 2012, 9, 801–816. [Google Scholar]
- Sanderson, J.M. Peptide lipid interactions: Insights and perspectives. Org. Biomol. Chem. 2005, 3, 201–212. [Google Scholar]
- Klajnert, B.; Janiszewska, J.; Urbanczyk-Lipkowska, Z.; Bryszewska, A.; Epand, R.M. DSC studies on interactions between low molecular mass peptide dendrimers and model lipid membranes. Int. J. Pharm. 2006, 327, 145–152. [Google Scholar]
- Li, Y.; Heitz, F.; Le Grimellec, C.; Cole, R.B. Fusion peptide-phospholipid noncovalent interaction as observed by nanoelectrospray FTICR-MS. Anal. Chem. 2005, 77, 1556–1565. [Google Scholar]
- Clinical and Laboratory Standards Institute, Method for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard-8th; CLSI: Wayne, PA, USA, 2009.
- Knopik-Skrocka, A.; Bielawski, J. Differences in amphotericin B-induced hemolysis between human erythrocytes obtained from male and female donors. Biol. Lett. 2005, 42, 49–60. [Google Scholar]
- Sample Availability: Samples of the compounds 3g and 3h are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Polcyn, P.; Zielinska, P.; Zimnicka, M.; Troć, A.; Kalicki, P.; Solecka, J.; Laskowska, A.; Urbanczyk-Lipkowska, Z. Novel Antimicrobial Peptide Dendrimers with Amphiphilic Surface and Their Interactions with Phospholipids — Insights from Mass Spectrometry. Molecules 2013, 18, 7120-7144. https://doi.org/10.3390/molecules18067120
Polcyn P, Zielinska P, Zimnicka M, Troć A, Kalicki P, Solecka J, Laskowska A, Urbanczyk-Lipkowska Z. Novel Antimicrobial Peptide Dendrimers with Amphiphilic Surface and Their Interactions with Phospholipids — Insights from Mass Spectrometry. Molecules. 2013; 18(6):7120-7144. https://doi.org/10.3390/molecules18067120
Chicago/Turabian StylePolcyn, Piotr, Paulina Zielinska, Magdalena Zimnicka, Anna Troć, Przemysław Kalicki, Jolanta Solecka, Anna Laskowska, and Zofia Urbanczyk-Lipkowska. 2013. "Novel Antimicrobial Peptide Dendrimers with Amphiphilic Surface and Their Interactions with Phospholipids — Insights from Mass Spectrometry" Molecules 18, no. 6: 7120-7144. https://doi.org/10.3390/molecules18067120





