The Development of Aromas in Ruminant Meat
Abstract
:1. Introduction
2. Lipid Oxidation
2.1. Mechanisms of Reaction
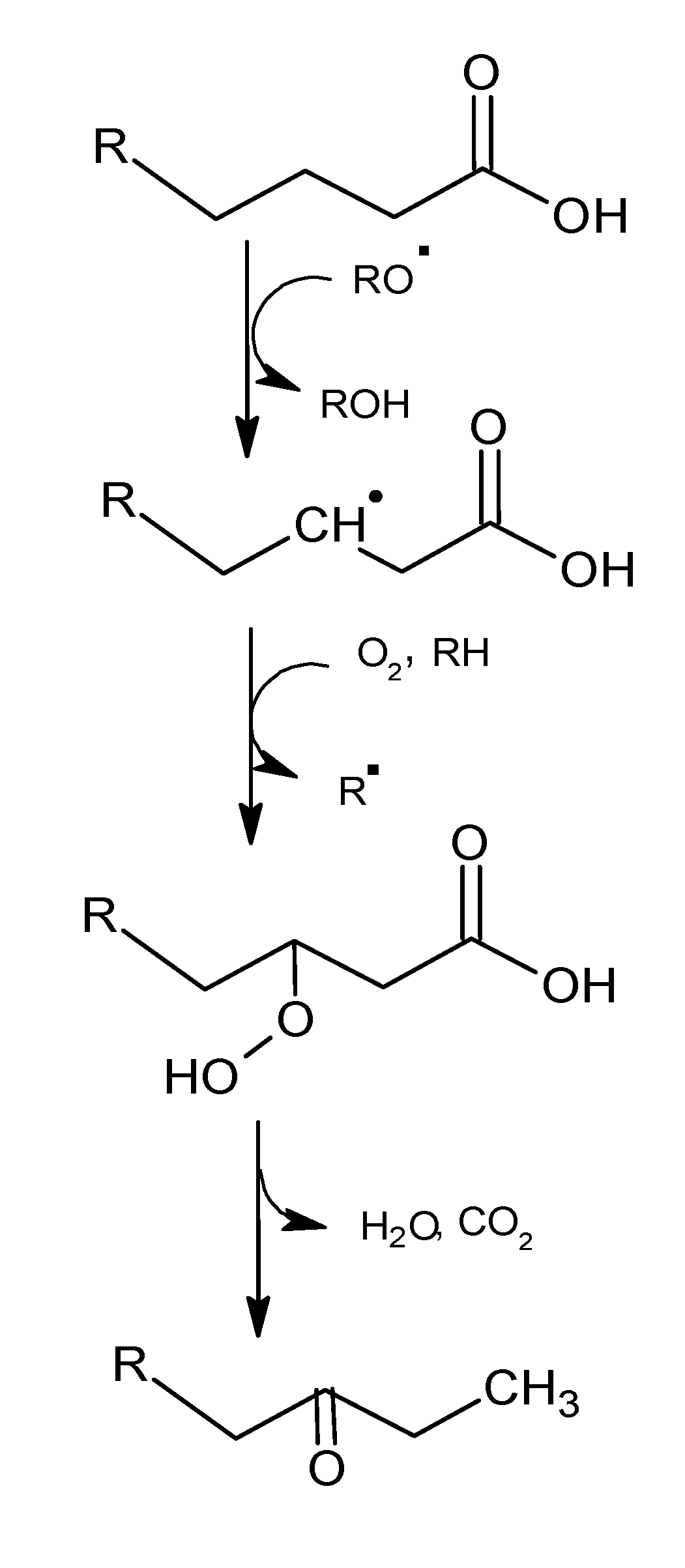
2.1.1. Primary Phase
2.1.2. Secondary Phase

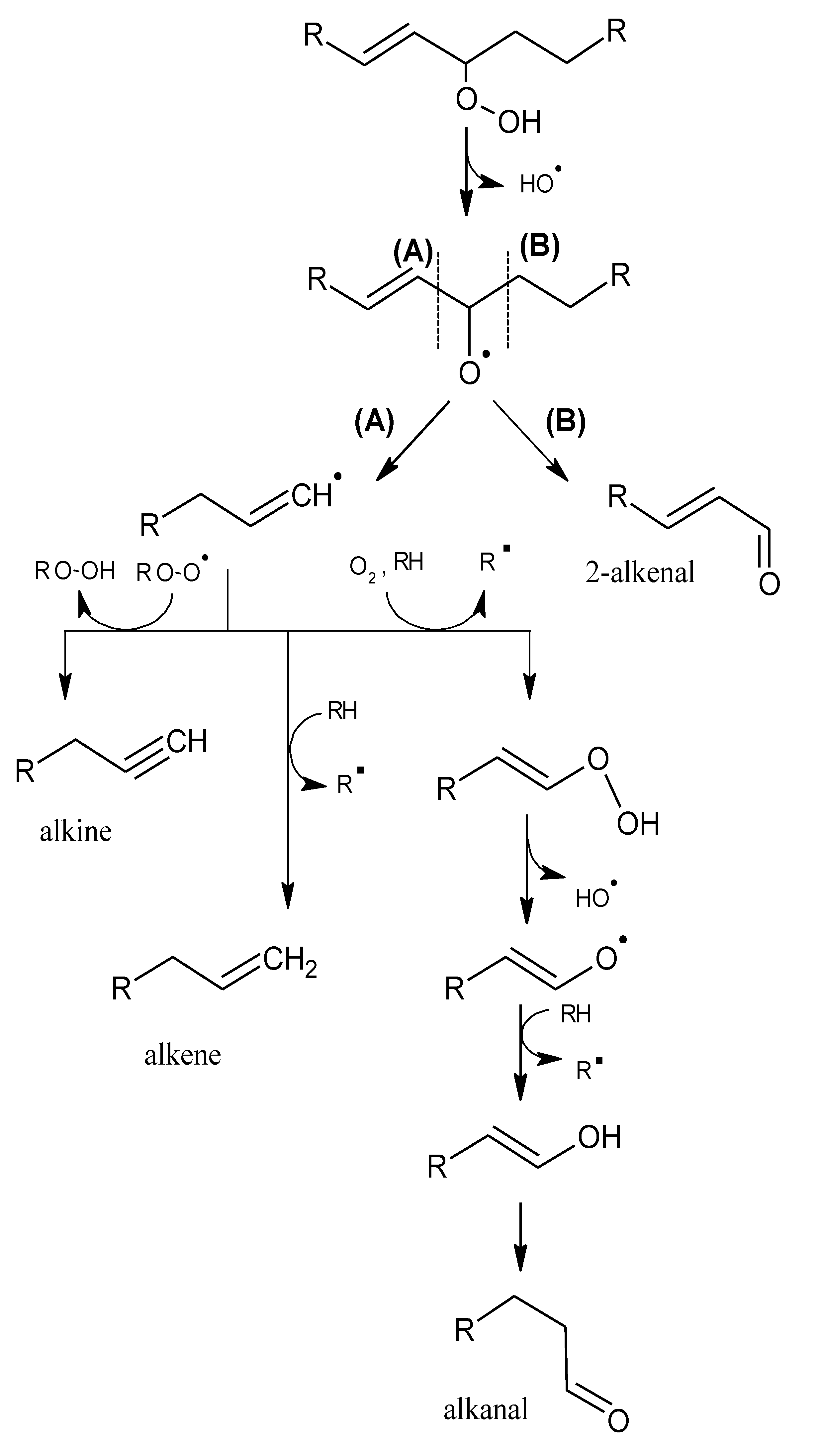
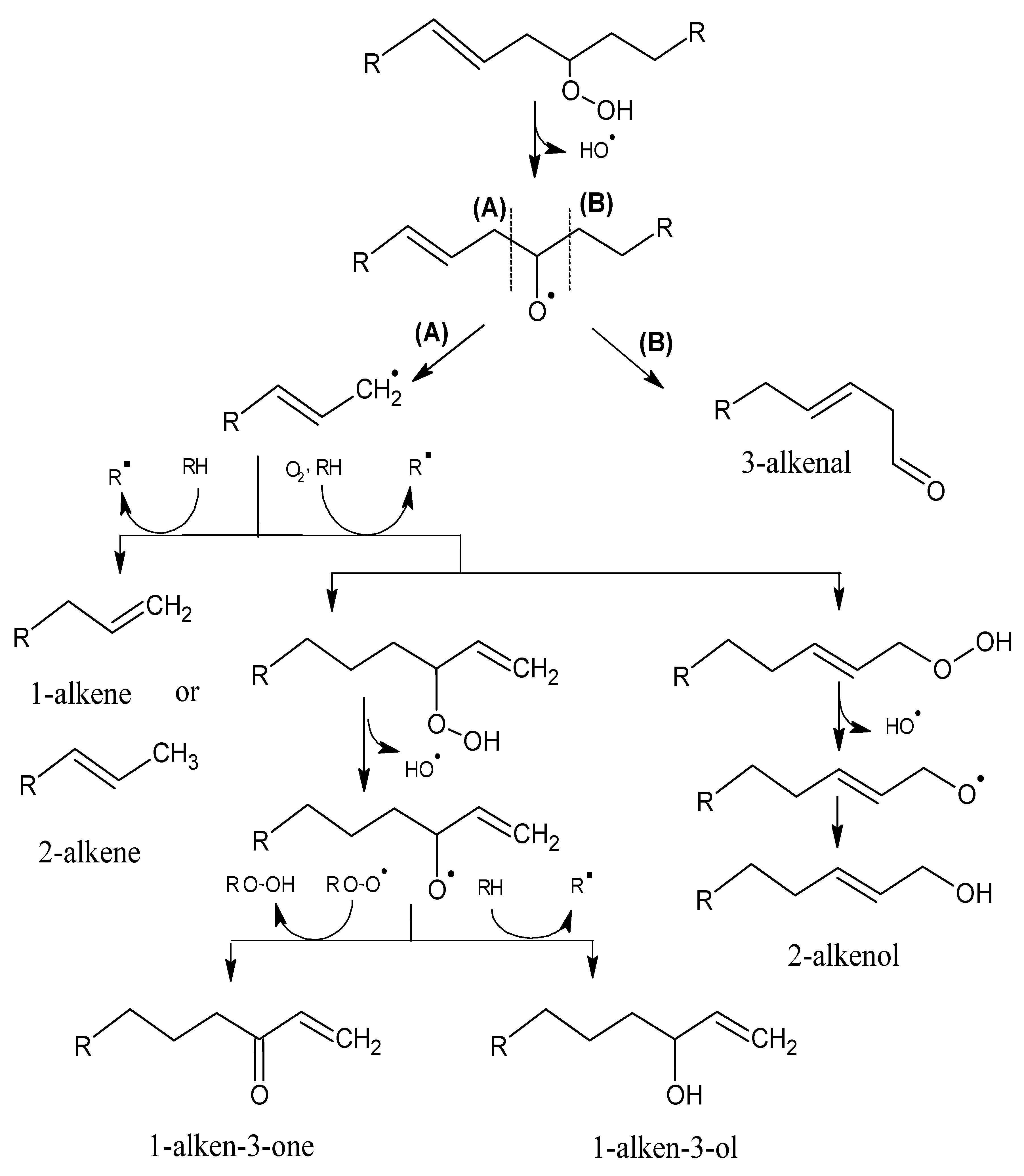
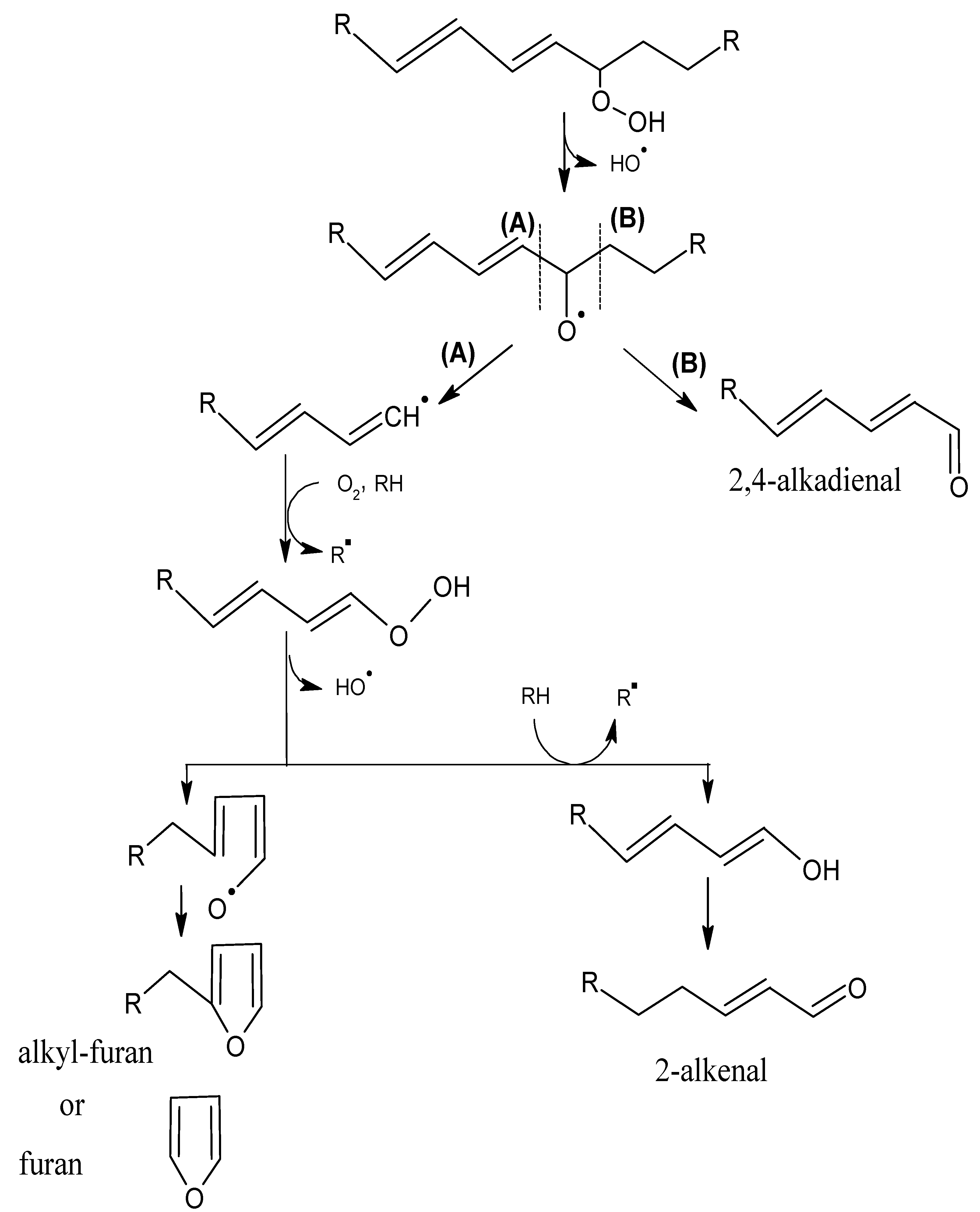
| Homologous series | Number of carbons | Major compounds |
|---|---|---|
| Aldehydes | 3–17 | hexanal heptanal octanal |
| Methyl ketones | 3–17 | 2-heptanone 2-nonanone 2-decanone |
| Acids | 2–12 | hexanoic acid pentanoic acid butanoic acid |
| Hydrocarbons | 4–17 | heptadecane nonane decane |
| γ-Lactones | 4–14 | γ-butyrolactone γ-pentalactone γ-heptalactone |
| Alcohols | 4–14 | octanol nonanol decanol |
2.2. Aroma Compounds Derived from Lipid Oxidation
| Neutral lipids | Polar lipids | |||||
|---|---|---|---|---|---|---|
| Fatty acid | Pigs | Sheep | Cattle | Pigs | Sheep | Cattle |
| 14:00 | 1.6 | 3.0 | 2.7 | 0.3 | 0.4 | 0.2 |
| 16:00 | 23.8 | 25.6 | 27.4 | 16.6 | 15.0 | 14.6 |
| 16:1 n - 7 | 2.6 | 2.2 | 3.5 | 0.8 | 1.5 | 0.8 |
| 18:00 | 15.6 | 13.6 | 15.5 | 12.1 | 10.4 | 11.0 |
| 18:1 n - 9 | 36.2 | 43.8 | 35.2 | 9.4 | 22.1 | 15.8 |
| 18:2 n - 6 | 12.0 | 1.5 | 2.3 | 31.4 | 12.4 | 22.0 |
| 18:3 n - 3 | 1.0 | 1.2 | 0.3 | 0.6 | 4.6 | 0.7 |
| 20:4 n - 6 | 0.2 | nd | nd | 10.5 | 5.9 | 10.0 |
| 20:5 n - 3 | nd | nd | nd | 1.0 | 4.1 | 0.8 |
2.3. Rancidity in Raw Meat
2.4. Lipid Oxidation during Cooking
2.5. Unpleasant Aromas in Meat after Reheating
2.6. Phospholipids and Triacylglycerols in the Flavour of Meat
3. Thiamine Degradation
| Reference | [31] | [37] | [20] | [59,60] | [61] | [62] | [34] a | [34] b | [6] | [63] | [64] | Origin probable e | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of meat | Beef | Beef | Beef | Beef | Beef | Beef | Beef | Sheep | Lamb | Lamb | |||||
| Cooking method | Boiled | Boiled | Fried | Roasted | Fried | Stewed (juice) | Fried patties | Raw | Boiled | Grilled | Grilled | ||||
| Extraction method c | SD dc | SD ac | DHS dc | DHS dc | DHS ac | SHS ac | SD ac | SD ac | SD | SD ac | DHS ac | Mouthspace | DHS dc | ||
| GC-O analysis d | DA | DA, CA | DA | DF | DF | DA | DA | DA | MF | MF | MF | ||||
| hexanal | 2 | 2 | 3 | LO | |||||||||||
| heptanal | 3 | LO | |||||||||||||
| octanal | 3 | 3 | LO | ||||||||||||
| nonanal | 2 | 4 | LO | ||||||||||||
| decanal | 1 | LO | |||||||||||||
| (E)-2-heptenal | 2 | LO | |||||||||||||
| (E)-2-nonenal | 1 | 1 | 1 | 3 | 4 | 2 | 2 | 3 | 2 | 2 | LO | ||||
| (Z)-2-nonenal | 4 | 3 | LO | ||||||||||||
| (Z)-3-nonenal | 3 | 4 | LO | ||||||||||||
| (E)-2-decenal | 4 | LO | |||||||||||||
| (Z)-2-decenal | 3 | 1 | LO | ||||||||||||
| (E,E)-2,4-heptadienal | 1 | LO | |||||||||||||
| (E,E)-2,4-nonadienal | 4 | 4 | 3 | LO | |||||||||||
| (E,E)-2,4-decadienal | 1 | 2 | 2 | 2 | 2 | 2 | 4 | 1 | 2 | LO | |||||
| 4,5-epoxy-(E)-2-decenal | 4 | 1 | 1 | LO | |||||||||||
| ethanal acetaldehyde | 1 | SR | |||||||||||||
| methional | 1 | 3 | 1 | 1 | 2 | 1 | 1 | 1 | SR | ||||||
| 3-methylbutanal | 1 | 2 | SR | ||||||||||||
| phenylacetaldehyde | 2 | SR | |||||||||||||
| 12-methyltridecanal | 3 | 1 | B | ||||||||||||
| 1-octen-3-one | 2 | 1 | 4 | 4 | 3 | 2 | LO | ||||||||
| 1-nonen-3-one | 4 | LO | |||||||||||||
| 3-mercapto-2-pentanone | 2 | TD | |||||||||||||
| 2,3-butanodione | 2 | 1 | 2 | B, MR | |||||||||||
| 2,3-pentanodione | 3 | MR | |||||||||||||
| (Z)-1,5-octadien-3-one | 2 | 2 | LO | ||||||||||||
| β-ionone | 1 | CD | |||||||||||||

4. Strecker Reaction
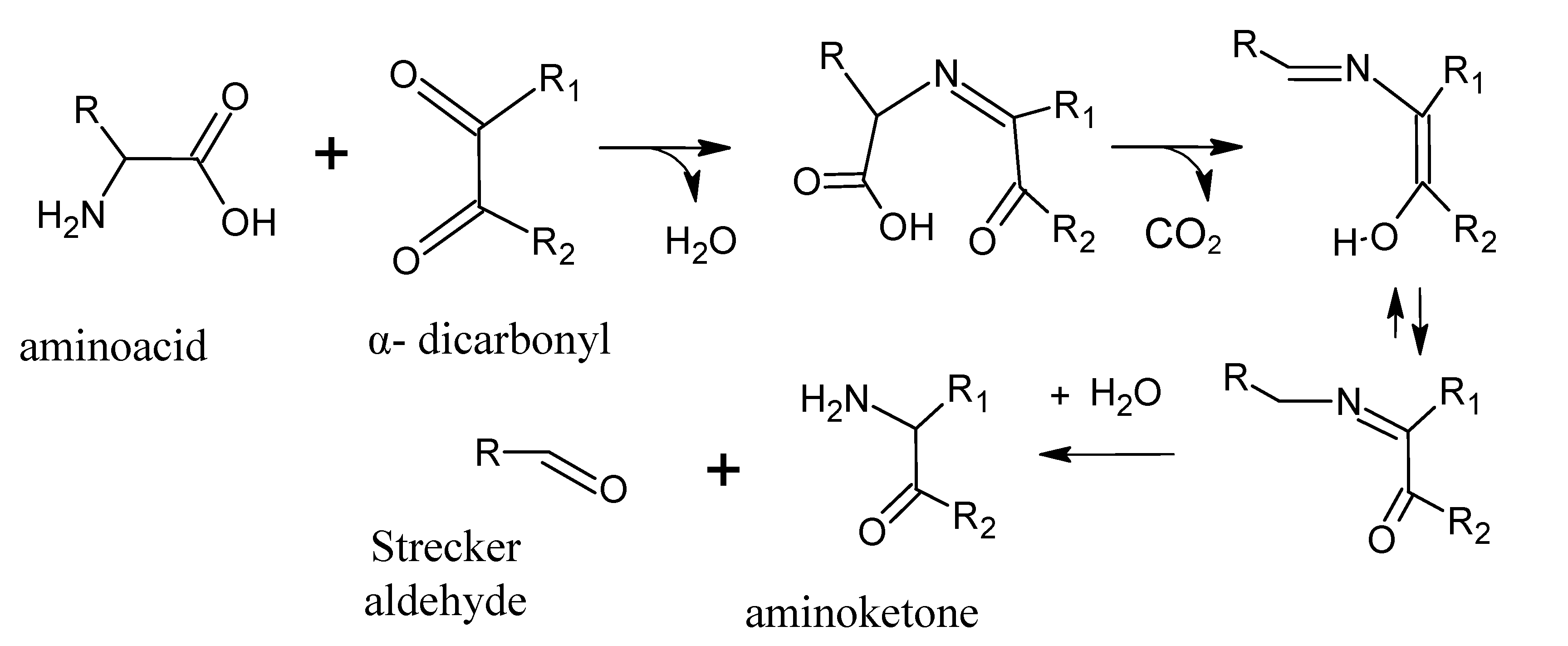
| Strecker aldehyde | Structure | Amino acid precursor | Odour threshold |
|---|---|---|---|
| ethanal = acetaldehyde |  | α-alanine/cysteine | 25 |
| propanal |  | α-aminobutyric | - |
| 2-methylpropanal |  | valine | 2 |
| 3-methylbutanal |  | leucine | 3 |
| 2-methylbutanal |  | isoleucine | 4 |
| methional |  | methionine | 0.2 |
| 2-phenyletanal = phenylacetaldehyde |  | phenylalanine | 4 |

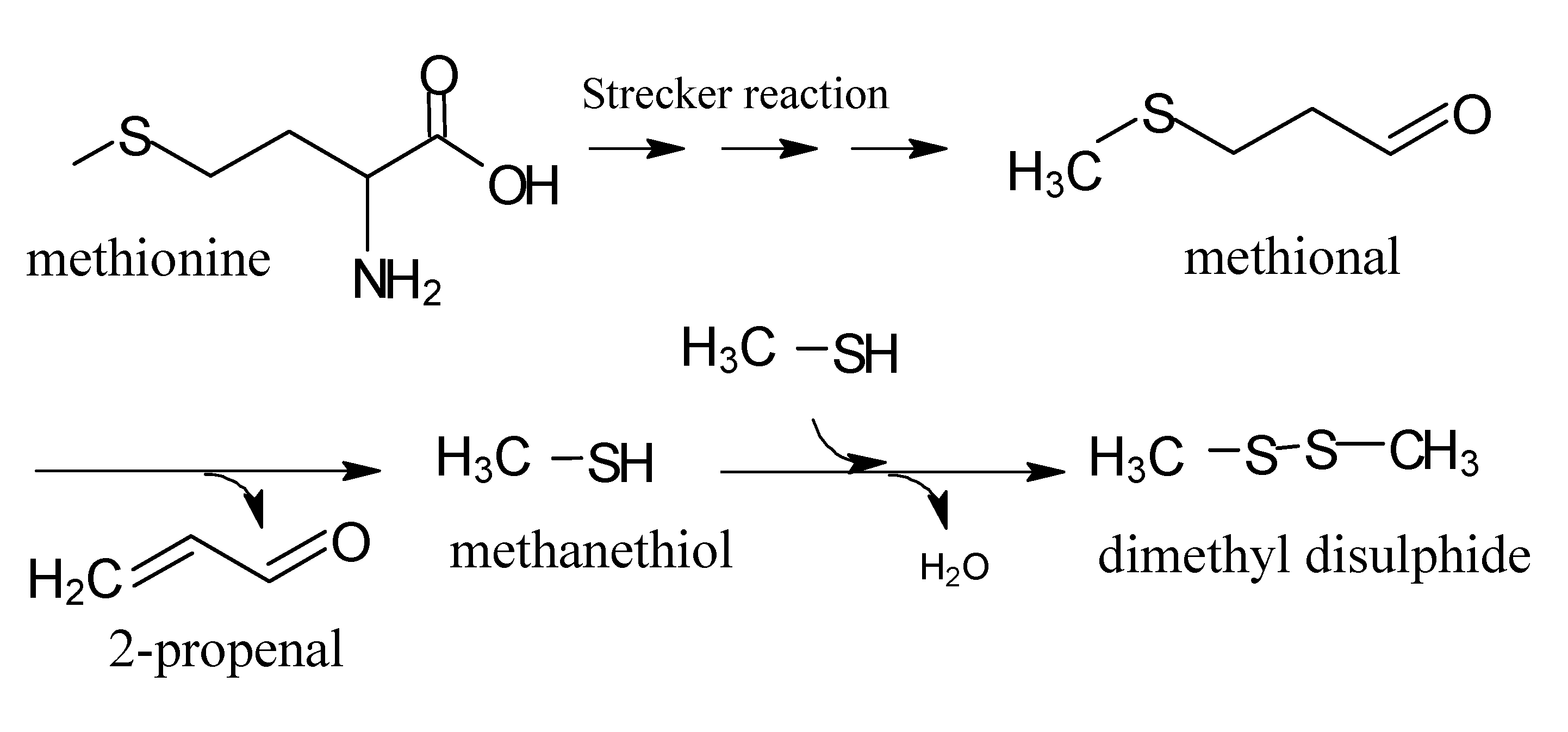
5. Maillard Reaction
5.1. Mechanisms of Reaction
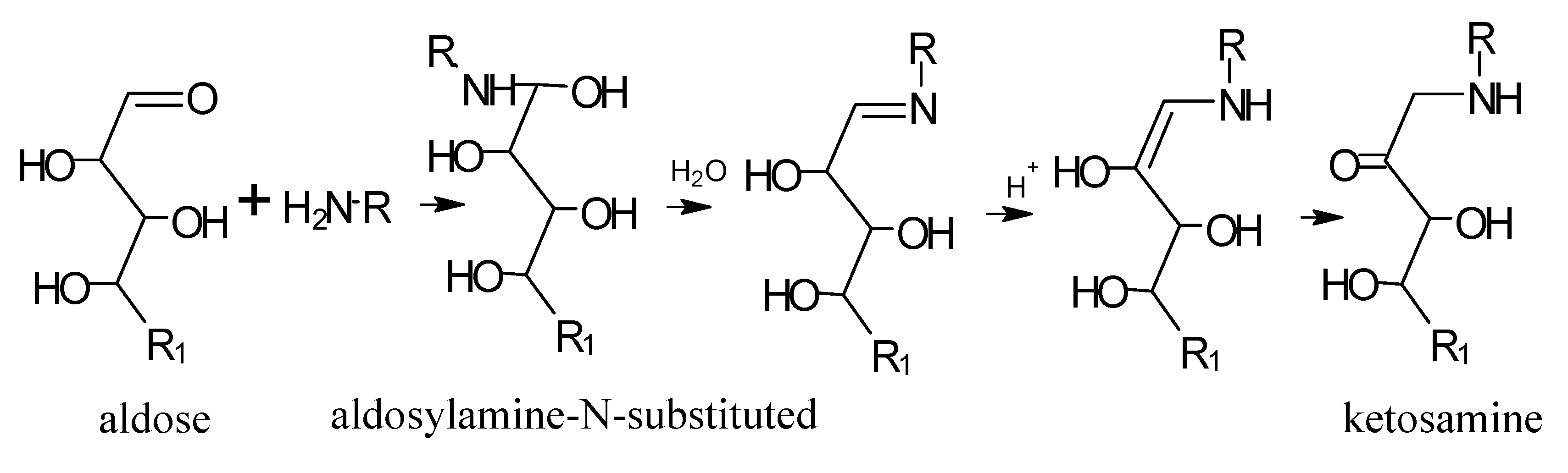

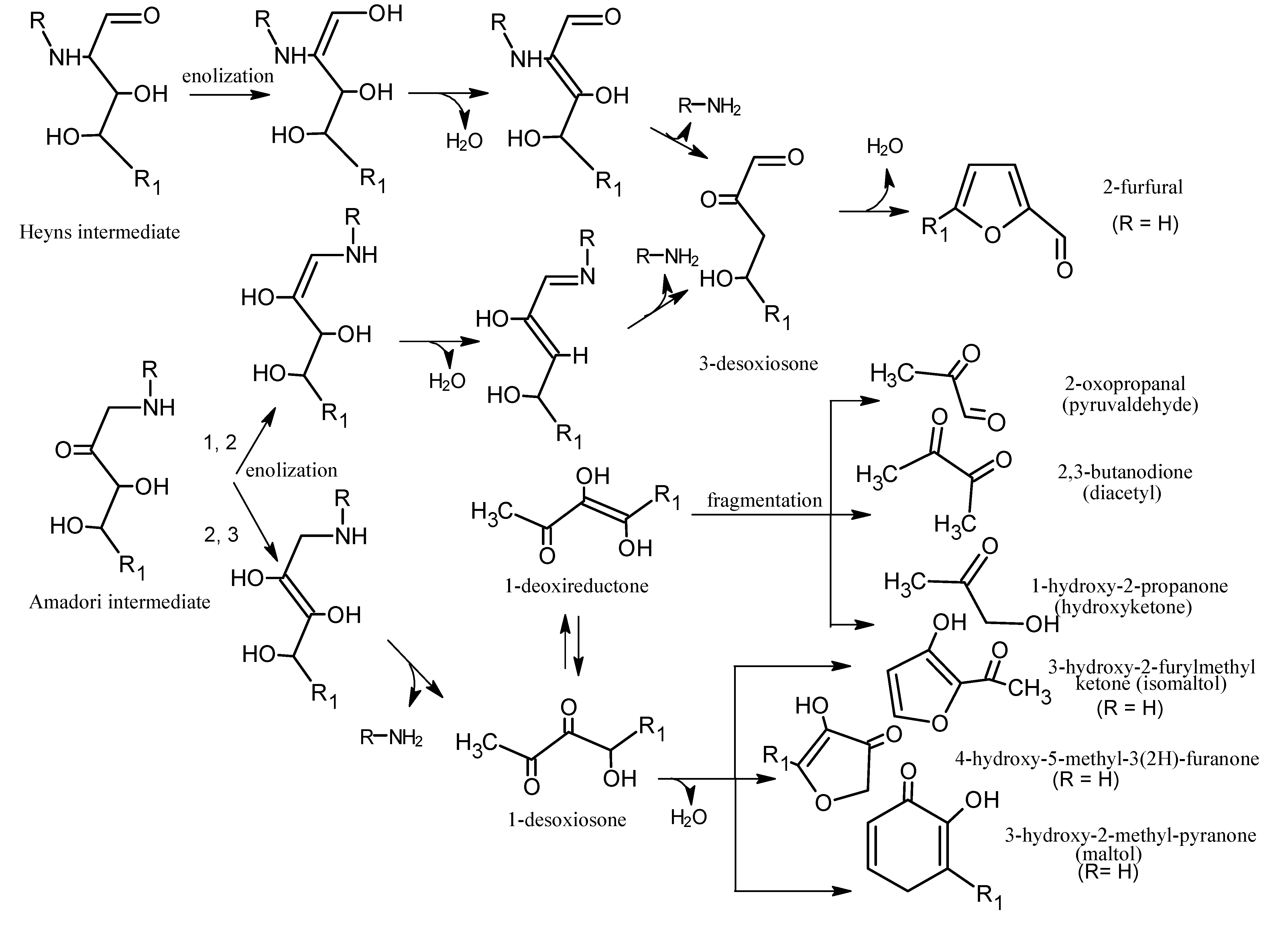
6. Aroma Compounds of Cooked Meat

6.1. Branched-Chain Fatty Acids
6.2. Carbonyls
6.3. Indoles, Phenols, Terpenes and Lactones
6.4. Pyrazines

6.5. Sulphur Compounds
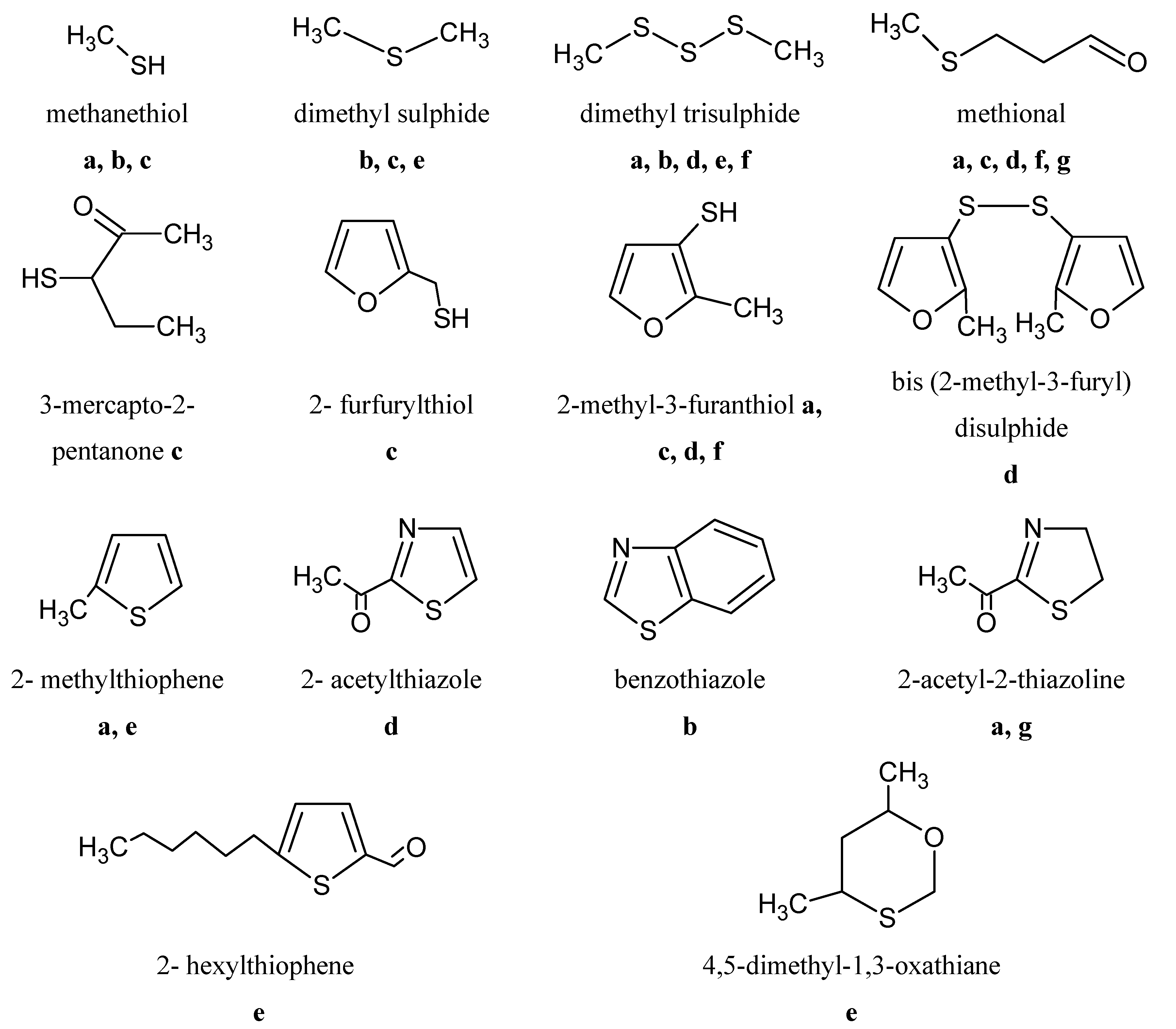
7. Conclusions
Conflicts of Interest
References
- Matsuishi, M.; Igeta, M.; Takeda, S.; Okitani, A. Sensory factors contributing to the identification of the animal species of meat. J. Food Sci. 2004, 69, S218–S220. [Google Scholar] [CrossRef]
- Rødbotten, M.; Kubberød, E.; Lea, P.; Ueland, Ø. A sensory map of the meat universe. Sensory profile of meat from 15 species. Meat Sci. 2004, 68, 137–144. [Google Scholar] [CrossRef]
- Stelzleni, A.M.; Johnson, D.D. Effect of days on concentrate feed on sensory off-flavor score, off-flavor descriptor and fatty acid profiles for selected muscles from cull beef cows. Meat Sci. 2008, 79, 382–393. [Google Scholar] [CrossRef]
- Font i Furnols, M.; Realini, C.E.; Guerrero, L.; Oliver, M.A.; Sañudo, C.; Campo, M.M.; Nute, G.R.; Cañeque, V.; Álvarez, I.; San Julián, R.; et al. Acceptability of lamb fed on pasture, concentrate or combinations of both systems by european consumers. Meat Sci. 2009, 81, 196–202. [Google Scholar] [CrossRef]
- Watkins, P.J.; Frank, D.; Singh, T.K.; Young, O.A.; Warner, R.D. Sheepmeat flavor and the effect of different feeding systems: A review. J. Agric. Food Chem. 2013, 61, 3561–3579. [Google Scholar] [CrossRef]
- Rota, V.; Schieberle, P. Changes in key odorants of sheep meat induced by cooking. In Food Lipids; American Chemical Society: Washington, DC, USA, 2005; Volume 920, pp. 73–83. [Google Scholar]
- Imafidon, G.I.; Spanier, A.M. Unraveling the secret of meat flavor. Trends Food Sci. Tech. 1994, 5, 315–321. [Google Scholar] [CrossRef]
- Vasta, V.; Priolo, A. Ruminant fat volatiles as affected by diet. A review. Meat Sci. 2006, 73, 218–228. [Google Scholar] [CrossRef]
- Whitfield, F.B. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Buckley, D.J.; Morrissey, P.A.; Gray, J.I. Influence of dietary vitamin E on the oxidative stability and quality of pig meat. J. Anim. Sci. 1995, 73, 3122–3130. [Google Scholar]
- Gray, J.I.; Gomaa, E.A.; Buckley, D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996, 43, 111–123. [Google Scholar]
- Baron, C.P.; Andersen, H.J. Myoglobin-induced lipid oxidation. A review. J. Agric. Food Chem. 2002, 50, 3887–3897. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids and meat quality: Lipolysis, oxidation, Maillard reaction and flavour. Sci. Aliment. 1999, 19, 439–458. [Google Scholar]
- Ladikos, D.; Lougovois, V. Lipid oxidation in muscle foods: A review. Food Chem. 1990, 35, 295–314. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin, Germany, 2009. [Google Scholar]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef]
- Porter, N.; Caldwell, S.; Mills, K. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Mottram, D.S. Some aspects of the chemistry of meat flavour. In Flavor of Meat and Meat Products, 1st ed.; Shahidi, F., Ed.; Blackie Academic & Professional: Glasgow, UK, 1994; pp. 210–230. [Google Scholar]
- Drumm, T.D.; Spanier, A.M. Changes in the content of lipid autoxidation and sulfur-containing compounds in cooked beef during storage. J. Agric. Food Chem. 1991, 39, 336–343. [Google Scholar] [CrossRef]
- Specht, K.; Baltes, W. Identification of volatile flavor compounds with high aroma values from shallow-fried beef. J. Agric. Food Chem. 1994, 42, 2246–2253. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Lorenz, S.; Buettner, A.; Ender, K.; Nürnberg, G.; Papstein, H.-J.; Schieberle, P.; Nürnberg, K. Influence of keeping system on the fatty acid composition in the longissimus muscle of bulls and odorants formed after pressure-cooking. Eur. Food Res. Technol. 2002, 214, 112–118. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Buckley, D.J.; Sheehy, P.J.A.; Monahan, F.J. Vitamin E and meat quality. Proc. Nutr. Soc. 1994, 53, 289–295. [Google Scholar] [CrossRef]
- Gill, C.O. Extending the storage life of raw chilled meats. Meat Sci. 1996, 43, 99–109. [Google Scholar] [CrossRef]
- Renerre, M. Oxidative processes and myoglobin. In Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; Decker, E.A., Faustman, C., López-Bote, C.J., Eds.; Wiley: New York, NY, USA, 2000; pp. 113–133. [Google Scholar]
- Vieira, C.; Diaz, M.T.; Martínez, B.; García-Cachán, M.D. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef]
- Mottram, D.S. Lipid oxidation and flavour in meat and meat products. Food Sci. Technol. Today 1987, 1, 159–162. [Google Scholar]
- Zakrys, P.I.; Hogan, S.A.; O’Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648–655. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Gasser, U.; Grosch, W. Identification of volatile flavour compounds with high aroma values from cooked beef. Z. Lebensm. Unters. Forsch. 1988, 186, 489–494. [Google Scholar]
- Mottram, D.S. The effect of cooking conditions on the formation of volatile heterocyclic compounds in pork. J. Sci. Food Agr. 1985, 36, 377–382. [Google Scholar] [CrossRef]
- Brewer, M.S.; Vega, J.D. Detectable odor thresholds of selected lipid oxidation compounds in a meat model system. J. Food Sci. 1995, 60, 592–595. [Google Scholar] [CrossRef]
- Kerler, J.; Grosch, W. Odorants contributing to warmed-over flavor (WOF) of refrigerated cooked beef. J. Food Sci. 1996, 61, 1271–1275. [Google Scholar] [CrossRef]
- Yang, A.; Brewster, M.J.; Beilken, S.L.; Lanari, M.C.; Taylor, D.G.; Tume, R.K. Warmed-over flavor and lipid stability of beef: Effects of prior nutrition. J. Food Sci. 2002, 67, 3309–3313. [Google Scholar] [CrossRef]
- Mottram, D.S.; Edwards, R.A. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agric. 1983, 34, 517–522. [Google Scholar] [CrossRef]
- Kerscher, R.; Grosch, W. Comparative evaluation of potent odorants of boiled beef by aroma extract dilution and concentration analysis. Z. Lebensm. Unters. Forsch. A 1997, 204, 3–6. [Google Scholar] [CrossRef]
- Kerscher, R.; Nurnberg, K.; Voigt, J.; Schieberle, P.; Grosch, W. Occurrence of 12-methyltridecanal in microorganisms and physiological samples isolated from beef. J. Agric. Food Chem. 2000, 48, 2387–2390. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Lane, G.A.; Tavendale, M.H.; Barry, T.N.; McNabb, W.C. Pastoral flavour in meat products from ruminants fed fresh forages and its amelioration by forage condensed tannins. Anim. Feed Sci. Technol. 2008, 146, 193–221. [Google Scholar] [CrossRef]
- Vasta, V.; Ratel, J.; Engel, E. Mass spectrometry analysis of volatile compounds in raw meat for the authentication of the feeding background of farm animals. J. Agric. Food Chem. 2007, 55, 4630–4639. [Google Scholar] [CrossRef]
- Riéra, C.; Gouézec, E.; Matthey-Doret, W.; Robert, F.; Blank, I. The role of lipids in aroma/food matrix interactions in complex liquid model systems. Dev. Food Sci. 2006, 43, 409–412. [Google Scholar] [CrossRef]
- Young, O.A.; Reid, D.H.; Smith, M.E.; Braggins, T.J. Sheepmeat odour and flavour. In Flavor of Meat and Meat Products; Shahidi, F., Ed.; Blackie Academic & Professional: London, UK, 1994; pp. 71–97. [Google Scholar]
- Brennand, C.P.; Lindsay, R.C. Distribution of volatile branched-chain fatty acids in various lamb tissues. Meat Sci. 1992, 31, 411–421. [Google Scholar] [CrossRef]
- Young, O.A.; Berdagué, J.L.; Viallon, C.; Rousset-Akrim, S.; Theriez, M. Fat-borne volatiles and sheepmeat odour. Meat Sci. 1997, 45, 183–200. [Google Scholar] [CrossRef]
- Wong, E.; Nixon, L.N.; Johnson, C.B. Volatile medium chain fatty acids and mutton flavor. J. Agric. Food Chem. 1975, 23, 495–498. [Google Scholar] [CrossRef]
- Macleod, G. The flavour of beef. In Flavor of Meat and Meat Products; Shahidi, F., Ed.; Blackie Academic & Professional: Glasgow, UK, 1994; pp. 4–37. [Google Scholar]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Kerscher, R.; Grosch, W. Quantification of 2-methyl-3-furanthiol, 2-furfurylthiol, 3-mercapto-2-pentanone, and 2-mercapto-3-pentanone in heated meat. J. Agric. Food Chem. 1998, 46, 1954–1958. [Google Scholar] [CrossRef]
- Güntert, M.; Brüning, J.; Emberger, R.; Köpsel, M.; Kuhn, W.; Thielmann, T.; Werkhoff, P. Identification and formation of some selected sulfur-containing flavor compounds in various meat model systems. J. Agric. Food Chem. 1990, 38, 2027–2041. [Google Scholar] [CrossRef]
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The influence of cooking and fat trimming on the actual nutrient intake from meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef]
- Dawson, K.R.; Unklesbay, N.F.; Hedrick, H.B. HPLC determination of riboflavin, niacin, and thiamin in beef, pork, and lamb after alternate heat-processing methods. J. Agric. Food Chem. 1988, 36, 1176–1179. [Google Scholar] [CrossRef]
- Badiani, A.; Nanni, N.; Gatta, P.P.; Bitossi, F.; Tolomelli, B.; Manfredini, M. Nutrient content and retention in selected roasted cuts from 3-month-old ram lambs. Food Chem. 1998, 61, 89–100. [Google Scholar] [CrossRef]
- Aliani, M.; Farmer, L.J. Precursors of chicken flavor. I. Determination of some flavor precursors in chicken muscle. J. Agric. Food Chem. 2005, 53, 6067–6072. [Google Scholar] [CrossRef]
- Lassen, A.; Kall, M.; Hansen, K.; Ovesen, L. A comparison of the retention of vitamins B1, B2 and B6, and cooking yield in pork loin with conventional and enhanced meal-service systems. Eur. Food Res. Technol. 2002, 215, 194–199. [Google Scholar] [CrossRef]
- Cerny, C.; Briffod, M. Effect of pH on the Maillard reaction of [13C5]xylose, cysteine, and thiamin. J. Agric. Food Chem. 2007, 55, 1552–1556. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Cliff, M.A.; Li-Chan, E.C.Y. Odour-active components of simulated beef flavour analysed by solid phase microextraction and gas chromatography-mass spectrometry and –olfactometry. Food Res. Int. 2006, 39, 294–308. [Google Scholar] [CrossRef]
- Cerny, C.; Guntz-Dubini, R. Identification of 5-hydroxy-3-mercapto-2-pentanone in the Maillard reaction of thiamine, cysteine, and xylose. J. Agric. Food Chem. 2008, 56, 10679–10682. [Google Scholar] [CrossRef]
- Mottram, D.S.; Madruga, M.S.; Whitfield, F.B. Some novel meatlike aroma compounds from the reactions of alkanediones with hydrogen sulfide and furanthiols. J. Agric. Food Chem. 1995, 43, 189–193. [Google Scholar] [CrossRef]
- Rochat, S.; Chaintreau, A. Carbonyl odorants contributing to the in-oven roast beef top note. J. Agric. Food Chem. 2005, 53, 9578–9585. [Google Scholar] [CrossRef]
- Rochat, S.; Laumer, J.-Y.D.S.; Chaintreau, A. Analysis of sulfur compounds from the in-oven roast beef aroma by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2007, 1147, 85–94. [Google Scholar]
- Machiels, D.; Istasse, L.; van Ruth, S.M. Gas chromatography-olfactometry analysis of beef meat originating from differently fed Belgian Blue, Limousin and Aberdeen Angus bulls. Food Chem. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. Identification of the character impact odorants of stewed beef juice by instrumental analyses and sensory studies. J. Agric. Food Chem. 1994, 42, 2862–2866. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Gas chromatographic-olfactometric characterisation of headspace and mouthspace key aroma compounds in fresh and frozen lamb meat. Food Chem. 2011, 129, 1909–1918. [Google Scholar] [CrossRef]
- Farmer, L.J.; Hagan, T.D.J. Precursors of Flavour in Cooked Beef; ICoMST: Roma, Italy, 2002; pp. 322–323. [Google Scholar]
- Aliani, M.; Farmer, L.J. Precursors of chicken flavor. II. Identification of key flavor precursors using sensory methods. J. Agric. Food Chem. 2005, 53, 6455–6462. [Google Scholar] [CrossRef]
- Fillion, L.; Henry, C.J. Nutrient losses and gains during frying: A review. Int. J. Food Sci. Nutr. 1998, 49, 157–168. [Google Scholar] [CrossRef]
- Meinert, L.; Tikk, K.; Tikk, M.; Brockhoff, P.B.; Bredie, W.L.P.; Bjergegaard, C.; Aaslyng, M.D. Flavour development in pork. Influence of flavour precursor concentrations in longissimus dorsi from pigs with different raw meat qualities. Meat Sci. 2009, 81, 255–262. [Google Scholar] [CrossRef]
- Yaylayan, V.A. Recent advances in the chemistry of Strecker degradation and Amadori rearrangement: Implications to aroma and color formation. Food Sci. Technol. Res. 2003, 9, 1–6. [Google Scholar] [CrossRef]
- Albrecht, E.; Teuscher, F.; Ender, K.; Wegner, J. Growth and breed-related changes of marbling characteristics in cattle. J. Anim. Sci. 2006, 84, 1067–1075. [Google Scholar]
- Machiels, D.; van Ruth, S.M.; Posthumus, M.A.; Istasse, L. Gas chromatography-olfactometry analysis of the volatile compounds of two commercial Irish beef meats. Talanta 2003, 60, 755–764. [Google Scholar] [CrossRef]
- Hofmann, T.; Schieberle, P. Formation of aroma-active Strecker-aldehydes by a direct oxidative degradation of Amadori compounds. J. Agric. Food Chem. 2000, 48, 4301–4305. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Hofmann, T.; Munch, P.; Schieberle, P. Quantitative model studies on the formation of aroma-active aldehydes and acids by Strecker-type reactions. J. Agric. Food Chem. 2000, 48, 434–440. [Google Scholar] [CrossRef]
- Kerler, J.; Grosch, W. Character impact odorants of boiled chicken: Changes during refrigerated storage and reheating. Z. Lebensm. Unters. Forsch. A 1997, 205, 232–238. [Google Scholar] [CrossRef]
- Young, O.A.; Lane, G.A.; Priolo, A.; Fraser, K. Pastoral and species flavour in lambs raised on pasture, lucerne or maize. J. Sci. Food Agric. 2003, 83, 93–104. [Google Scholar]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar]
- Søndergaard, A.K.; Stahnke, L.H. Growth and aroma production by Staphylococcus xylosus, S. carnosus and S. equorum—A comparative study in model systems. Int. J. Food Microbiol. 2002, 75, 99–109. [Google Scholar] [CrossRef]
- Bailey, M.E. Maillard reactions and meat flavour development. In Flavor of Meat and Meat Products, 1st ed.; Shahidi, F., Ed.; Blackie Academic & Professional: Glasgow, UK, 1994; pp. 153–173. [Google Scholar]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Tech. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Fisher, C.; Scott, T.R. Food Flavours. Biology and Chemistry; The Royal Society of Chemistry: Cambridge, UK, 1997. [Google Scholar]
- van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of lipid composition on meat-like model systems containing cysteine, ribose, and polyunsaturated fatty acids. J. Agric. Food Chem. 2002, 50, 1126–1132. [Google Scholar] [CrossRef]
- Christlbauer, M.; Schieberle, P. Characterization of the key aroma compounds in beef and pork vegetable gravies á la chef by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2009, 57, 9114–9122. [Google Scholar] [CrossRef]
- Laing, D.G.; Legha, P.K.; Jinks, A.L.; Hutchinson, I. Relationship between molecular structure, concentration and odor qualities of oxygenated aliphatic molecules. Chem. Senses 2003, 28, 57–69. [Google Scholar]
- Ebeler, S.E. Sensory analysis and analytical flavor chemistry: Missing links. In Handbook of Flavor Characterization; Deibler, K.D., Delwiche, J., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 41–50. [Google Scholar]
- Prescott, J.; Young, O.; O’Neill, L. The impact of variations in flavour compounds on meat acceptability: A comparison of Japanese and New Zealand consumers. Food Qual. Prefer. 2001, 12, 257–264. [Google Scholar] [CrossRef]
- Sutherland, M.M.; Ames, J.M. The effect of castration on the headspace aroma components of cooked lamb. J. Sci. Food Agric. 1995, 69, 403–413. [Google Scholar] [CrossRef]
- Berthelot, V.; Pierzynowski, S.G.; Sauvant, D.; Kristensen, N.B. Hepatic metabolism of propionate and methylmalonate in growing lambs. Livest. Prod. Sci. 2002, 74, 33–43. [Google Scholar]
- Braggins, T.J. Effect of stress-related changes in sheepmeat ultimate ph on cooked odor and flavor. J. Agric. Food Chem. 1996, 44, 2352–2360. [Google Scholar] [CrossRef]
- Madruga, M.S.; Stephen Elmore, J.; Dodson, A.T.; Mottram, D.S. Volatile flavour profile of goat meat extracted by three widely used techniques. Food Chem. 2009, 115, 1081–1087. [Google Scholar] [CrossRef]
- Madruga, M.S.; Arruda, S.G.B.; Narain, N.; Souza, J.G. Castration and slaughter age effects on panel assessment and aroma compounds of the “Mestiço” goat meat. Meat Sci. 2000, 56, 117–125. [Google Scholar] [CrossRef]
- Priolo, A.; Micol, D.; Agabriel, J. Effects of grass feeding systems on ruminant meat colour and flavour. A review. Anim. Res. 2001, 50, 185–200. [Google Scholar] [CrossRef]
- Ha, J.K.; Lindsay, R.C. Volatile alkylphenols and thiophenol in species-related characterizing flavors of red meats. J. Food Sci. 1991, 56, 1197–1202. [Google Scholar] [CrossRef]
- Priolo, A.; Cornu, A.; Prache, S.; Krogmann, M.; Kondjoyan, N.; Micol, D.; Berdagué, J.L. Fat volatiles tracers of grass feeding in sheep. Meat Sci. 2004, 66, 475–481. [Google Scholar] [CrossRef]
- Engel, E.; Ratel, J. Correction of the data generated by mass spectrometry analyses of biological tissues: Application to food authentication. J. Chromatogr. A 2007, 1154, 331–341. [Google Scholar]
- Larick, D.K.; Hedrick, H.B.; Bailey, M.E.; Williams, J.E.; Hancock, D.L.; Garner, G.B.; Morrow, R.E. Flavor constituents of beef as influenced by forage- and grain-feeding. J. Food Sci. 1987, 52, 245–251. [Google Scholar] [CrossRef]
- Elmore, J.S.; Warren, H.E.; Mottram, D.S.; Scollan, N.D.; Enser, M.; Richardson, R.I.; Wood, J.D. A comparison of the aroma volatiles and fatty acid compositions of grilled beef muscle from Aberdeen Angus and Holstein-Friesian steers fed diets based on silage or concentrates. Meat Sci. 2004, 68, 27–33. [Google Scholar]
- Warren, H.E.; Scollan, N.D.; Nute, G.R.; Hughes, S.I.; Wood, J.D.; Richardson, R.I. Effects of breed and a concentrate or grass silage diet on beef quality in cattle of 3 ages. II: Meat stability and flavour. Meat Sci. 2008, 78, 270–278. [Google Scholar] [CrossRef]
- Thorkelsson, G.; Jonsdottir, R.; Hilmarsson, O.T.; Olafsdottir, A.; Martinsdottir, E. The Influence of Grazing Time on Angelica Archangelica on Volatile Compounds and Sensory Quality of Meat from Pasture Lambs; ICoMST: Copenhague, Denmark, 2009. [Google Scholar]
- Urbach, G. Effect of feed on flavor in dairy foods. J. Dairy Sci. 1990, 73, 3639–3650. [Google Scholar] [CrossRef]
- Bendall, J.G. Aroma compounds of fresh milk from New Zealand cows fed different diets. J. Agric. Food Chem. 2001, 49, 4825–4832. [Google Scholar] [CrossRef]
- Sebastián, I.; Viallon-Fernandez, C.; Berge, P.; Berdagué, J.-L. Analysis of the volatile fraction of lamb fat tissue: Influence of the type of feeding. Sci. Aliment. 2003, 23, 497–511. [Google Scholar] [CrossRef]
- Sivadier, G.; Ratel, J.; Bouvier, F.; Engel, E. Authentication of meat products: Determination of animal feeding by parallel GC-MS analysis of three adipose tissues. J. Agric. Food Chem. 2008, 56, 9803–9812. [Google Scholar] [CrossRef]
- Cerny, C.; Grosch, W. Evaluation of potent odorants in roasted beef by aroma extract dilution analysis. Z. Lebensm. Unters. Forsch. A 1992, 194, 322–325. [Google Scholar] [CrossRef]
- Madruga, M.S.; Mottram, D.S. The effect of ph on the formation of Maillard-derived aroma volatiles using a cooked meat system. J. Sci. Food Agr. 1995, 68, 305–310. [Google Scholar] [CrossRef]
- Adams, A.; Polizzi, V.; van Boekel, M.; De Kimpe, N. Formation of pyrazines and a novel pyrrole in Maillard model systems of 1,3-dihydroxyacetone and 2-oxopropanal. J. Agric. Food Chem. 2008, 56, 2147–2153. [Google Scholar] [CrossRef]
- Adams, A.; Kimpe, N.D. Formation of pyrazines from ascorbic acid and amino acids under dry-roasting conditions. Food Chem. 2009, 115, 1417–1423. [Google Scholar] [CrossRef]
- Shibamoto, T.; Akiyama, T.; Sakaguchi, M.; Enomoto, Y.; Masuda, H. A study of pyrazine formation. J. Agric. Food Chem. 1979, 27, 1027–1031. [Google Scholar] [CrossRef]
- Chen, Y.; Xing, J.; Chin, C.-K.; Ho, C.-T. Effect of urea on volatile generation from Maillard reaction of cysteine and ribose. J. Agric. Food Chem. 2000, 48, 3512–3516. [Google Scholar] [CrossRef]
- Parker, J.K.; Arkoudi, A.; Mottram, D.S.; Dodson, A.T. Aroma formation in beef muscle and beef liver. Dev. Food Sci. 2006, 43, 335–338. [Google Scholar]
- Macy, R.L.; Naumann, H.D.; Bailey, M.E. Water-soluble flavor and odor precursors of meat. II. Effects of heating on amino nitrogen constituents and carbohydrates in lyophilized diffusates from aqueous extracts of beef, pork, and lamb. J. Food Sci. 1964, 29, 142–148. [Google Scholar] [CrossRef]
- Hudson, J.E.; Loxley, R.A. The effect of pentose sugars on the aroma and flavour of mutton. Food Technol. Aus. 1983, 35, 174–175. [Google Scholar]
- Young, O.A.; Cummings, T.L. Effect of xylose on sheepmeat flavors in casserole-style cooking. J. Food Sci. 2008, 73, S308–S313. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-soluble precursors of beef flavour: I. Effect of diet and breed. Meat Sci. 2008, 79, 124–130. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-soluble precursors of beef flavour. Part II: Effect of post-mortem conditioning. Meat Sci. 2008, 79, 270–277. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef]
- Farmer, L.J.; Kennedy, J.; Hagan, T. Contribution of Aqueous Precursors to the Odour and Flavour of Cooked Meats; ICoMST: Copenhague, Denmark, 2009. [Google Scholar]
- Hwang, H.-I.; Hartman, T.G.; Ho, C.-T. Relative reactivities of amino acids in pyrazine formation. J. Agric. Food Chem. 1995, 43, 179–184. [Google Scholar] [CrossRef]
- Shu, C.-K. Pyrazine formation from serine and threonine. J. Agric. Food Chem. 1999, 47, 4332–4335. [Google Scholar] [CrossRef]
- Blanco, D.; Barbieri, G.; Dellapina, G.; Bolzoni, L. Pyrazines produced by bacteria responsible for the potato-like off-odour isolated from raw ham. Microbiol. Aliments Nutr. 1994, 12, 413–422. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Resconi, V.C.; Escudero, A.; Campo, M.M. The Development of Aromas in Ruminant Meat. Molecules 2013, 18, 6748-6781. https://doi.org/10.3390/molecules18066748
Resconi VC, Escudero A, Campo MM. The Development of Aromas in Ruminant Meat. Molecules. 2013; 18(6):6748-6781. https://doi.org/10.3390/molecules18066748
Chicago/Turabian StyleResconi, Virginia C., Ana Escudero, and María M. Campo. 2013. "The Development of Aromas in Ruminant Meat" Molecules 18, no. 6: 6748-6781. https://doi.org/10.3390/molecules18066748
APA StyleResconi, V. C., Escudero, A., & Campo, M. M. (2013). The Development of Aromas in Ruminant Meat. Molecules, 18(6), 6748-6781. https://doi.org/10.3390/molecules18066748





