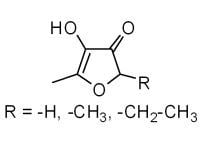
Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®)
Abstract
:1. Introduction
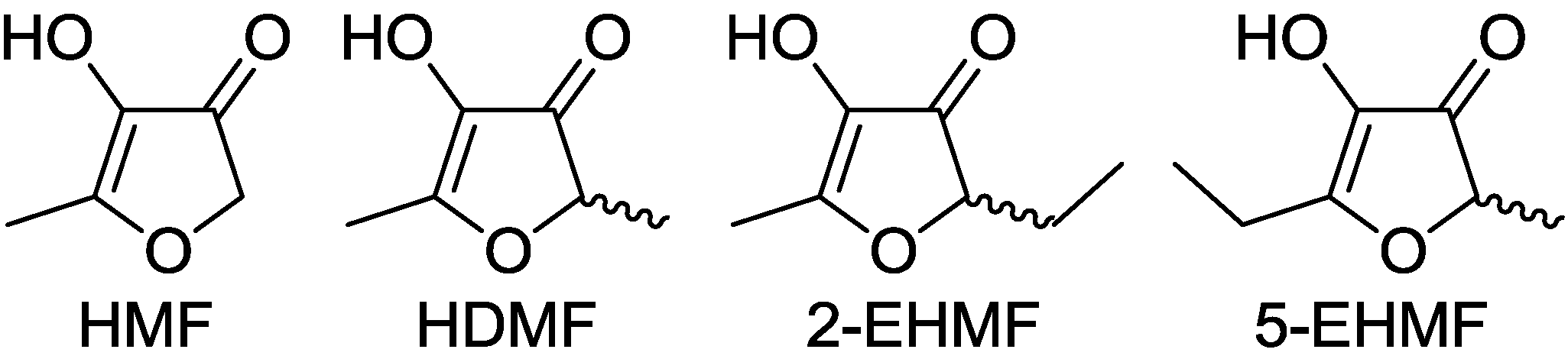
2. Properties

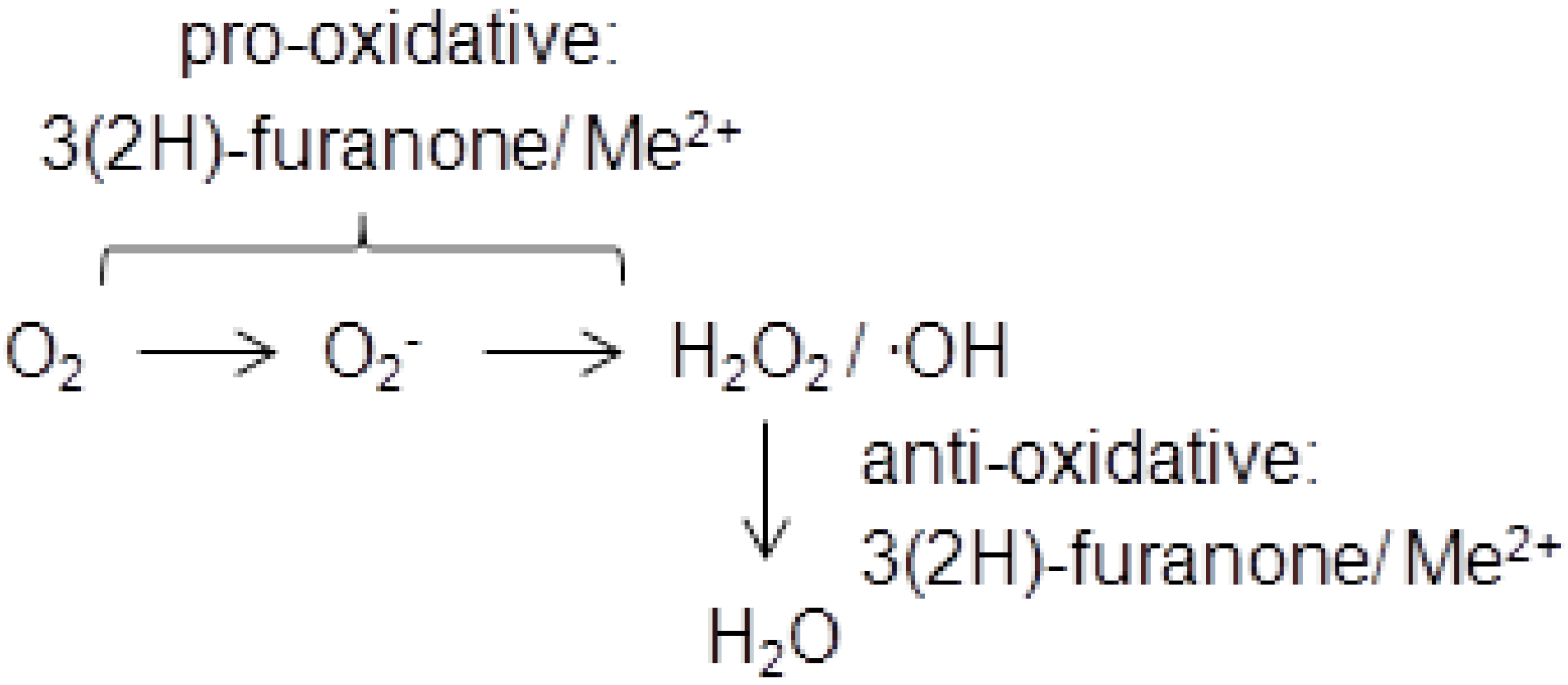
3. Physiological Activity and Toxicology
4. Occurrence
5. Microbial Formation
5.1. Yeast
5.2. Bacteria
6. Formation in Plants
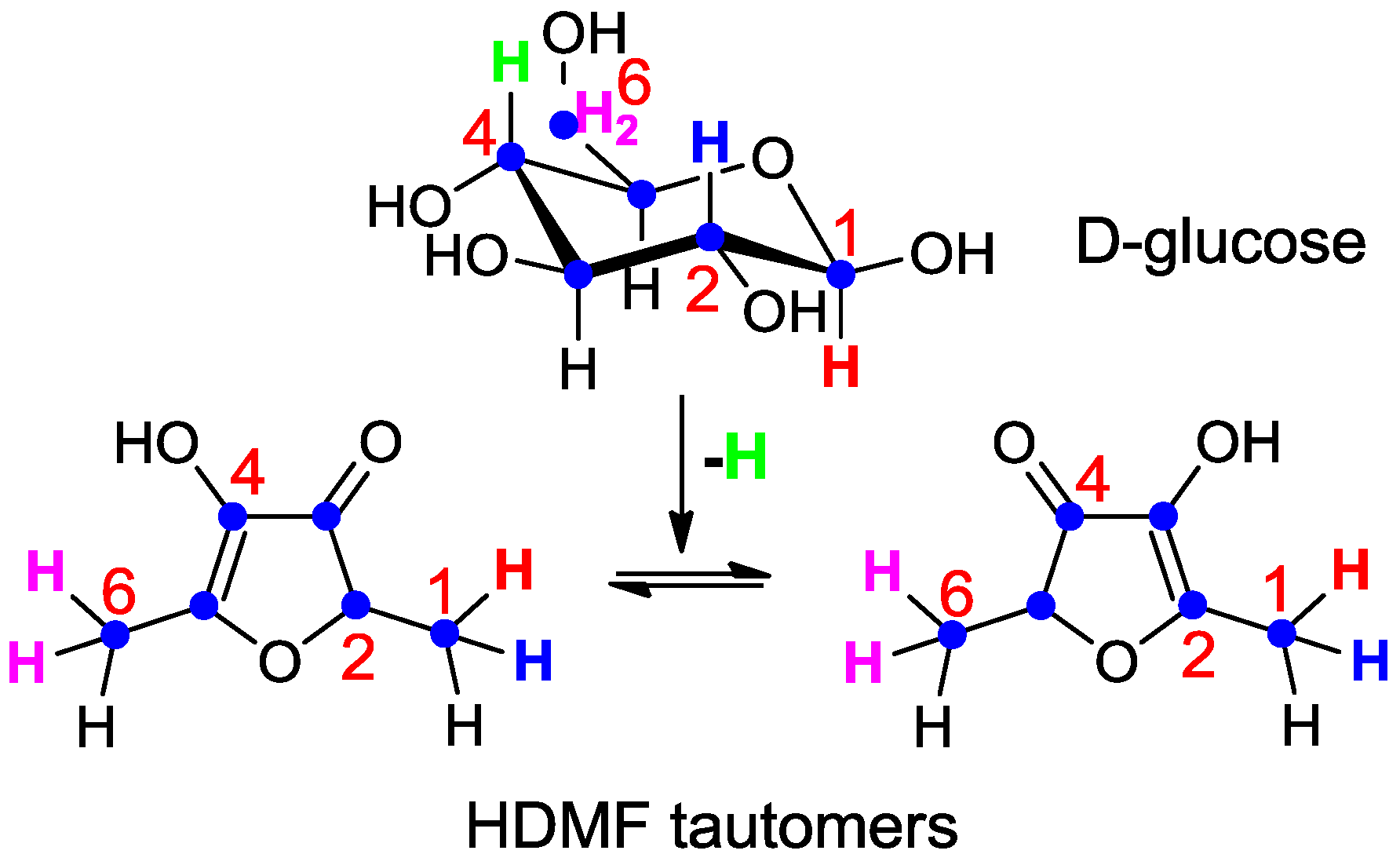

7. Conclusions
Conflicts of Interest
References
- Hodge, J.E. Novel reductones and methods of making them. U.S. Patent 2,936,308, 10 May 1960. [Google Scholar]
- Ledl, F.; Schleicher, E. New aspects of the Maillard reaction in foods and in the human body. Angew. Chem. Int. Ed. 1990, 29, 565–594. [Google Scholar] [CrossRef]
- Cerny, C. The aroma side of the Maillard reaction. Ann. N Y Acad. Sci. 2008, 1126, 66–71. [Google Scholar]
- Colin Slaughter, J.C. The naturally occurring furanones: Formation and function from pheromone to food. Biol. Rev. 1999, 74, 259–276. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Schwab, W.; Roscher, R. 4-Hydroxy-3(2H)-furanones: Natural and Maillard products. Rec. Res. Develop. Phytochem. 1997, 1, 643–673. [Google Scholar]
- Roscher, R.; Herderich, M.; Steffen, J.P.; Schreier, P.; Schwab, W. 2,5-Dimethyl-4-hydroxy-3[2H]-furanone 6'O-malonyl-β-D-glucopyranoside in strawberry fruit (Fragaria x ananassa, cv. Senga sengana). Phytochemistry 1996, 43, 155–160. [Google Scholar]
- Roscher, R.; Steffen, J.P.; Herderich, M.; Schwab, W.; Schreier, P. Synthesis of phenyl 6'-O-malonyl-β-D-glucopyranoside. Facile preparation of malonylated glycoconjugates. J. Agric. Food Chem. 1996, 44, 1626–1629. [Google Scholar] [CrossRef]
- Roscher, R.; Schwab, W.; Schreier, P. Stability of naturally occurring 2,5-dimethyl-4-hydroxy-3[2H]-furanone derivatives. Z. Lebensm. Unters. Forsch. 1997, 204, 438–441. [Google Scholar] [CrossRef]
- Emura, M.; Yaguchi, Y.; Nakahashi, A.; Sugimoto, D.; Miura, N.; Monde, K. Stereochemical studies of odorous 2-substituted-3(2H)-furanones by vibrational circular dichroism. J. Agric. Food Chem. 2009, 57, 9909–9915. [Google Scholar]
- Yaguchi, Y.; Nakahashi, A.; Miura, N.; Sugimoto, D.; Monde, K.; Emura, M. Stereochemical study of chiral tautomeric flavorous furanones by vibrational circular dichroism. Org. Lett. 2008, 10, 4883–4885. [Google Scholar] [CrossRef]
- Monde, K.; Nakahashi, A.; Miura, N.; Yaguchi, Y.; Sugimoto, D.; Emura, M. Stereochemical study of a novel tautomeric furanone: Homofuraneol. Chirality 2009, 21 (Suppl. 1), E110–5. [Google Scholar] [CrossRef]
- Roscher, R.; Schreier, P.; Schwab, W. Metabolism of 2,5-dimethyl-4-hydroxy-3(2H)-furanone in detached ripening strawberry fruits. J. Agric. Food Chem. 1997, 45, 3202–3205. [Google Scholar] [CrossRef]
- Kitao, S.; Matsudo, T.; Sasaki, T.; Koga, T.; Kawamura, M. Enzymatic synthesis of stable, odorless and powdered furanone glucosides by sucrose phosphorylase. Biosci. Biotechnol. Biochem. 2000, 64, 134–141. [Google Scholar] [CrossRef]
- Haleva-Toledo, E.; Naim, M.; Zehavi, U.; Rouseff, R.L. Effects of L-cysteine and N-acetyl-L-cysteine on 4-hydroxy-2,5-dimethyl-3(2H)-furanone (furaneol), 5-(hydroxymethyl)furfural, and 5-methylfurfural formation and browning in buffer solutions containing either rhamnose or glucose and arginine. J. Agric. Food Chem. 1999, 47, 4140–4145. [Google Scholar] [CrossRef]
- Illmann, S.; Davidek, T.; Gouézec, E.; Rytz, A.; Schuchmann, H.P.; Blank, I. Generation of 4-hydroxy-2,5-dimethyl-3(2H)-furanone from rhamnose as affected by reaction parameters: Experimental design approach. J. Agric. Food Chem. 2009, 57, 2889–2895. [Google Scholar] [CrossRef]
- Yaylayan, V.A.; Machiels, D.; Istasse, L. Thermal decomposition of specifically phosphorylated D-glucoses and their role in the control of the Maillard reaction. J. Agric. Food Chem. 2003, 51, 3358–3366. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Formation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone through methylglyoxal: A Maillard reaction intermediate. J. Agric. Food Chem. 2008, 56, 7405–7409. [Google Scholar] [CrossRef]
- Li, X.; Hiramoto, K.; Yoshida, M.; Kato, T.; Kikugawa, K. Identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF) and 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF) with DNA breaking activity in soy sauce. Food Chem. Toxicol. 1998, 36, 305–314. [Google Scholar] [CrossRef]
- Murakami, K.; Haneda, M.; Makino, T.; Yoshino, M. Prooxidant action of furanone compounds: implication of reactive oxygen species in the metal-dependent strand breaks and the formation of 8-hydroxy-2'-deoxyguanosine in DNA. Food Chem. Toxicol. 2007, 45, 1258–1262. [Google Scholar] [CrossRef]
- Liu, T.T.; Yang, T.S. Effects of water-soluble natural antioxidants on photosensitized oxidation of conjugated linoleic acid in an oil-in-water emulsion system. J. Food Sci. 2008, 73, C256–C261. [Google Scholar] [CrossRef]
- Yamashita, N.; Murata, M.; Inoue, S.; Hiraku, Y.; Yoshinaga, T.; Kawanishi, S. Superoxide formation and DNA damage induced by a fragrant furanone in the presence of copper(II). Mutat. Res. 1998, 397, 191–201. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamakoshi, J.; Saito, M.; Kasai, K.; Matsudo, T.; Koga, T.; Mori, K. Antioxidative activities of 4-hydroxy-3(2H)-furanones and their anti-cataract effect on spontaneous cataract rat (ICR/f). Biosci. Biotechnol. Biochem. 1998, 62, 1865–1869. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamakoshi, J.; Saito, M.; Kasai, K.; Matsudo, T.; Kikuchi, M.; Koga, T.; Mori, K. Synthesis of 4-hydroxy-3(2H)-furanone acyl derivatives and their anti-cataract effect on spontaneous cataract rats (ICR/f). Biosci. Biotechnol. Biochem. 1998, 62, 2145–2154. [Google Scholar] [CrossRef]
- Roscher, R.; Koch, H.; Herderich, M.; Schreier, P.; Schwab, W. Identification of 2,5-dimethyl-4-hydroxy-3-[2H]-furanone β-D-glucuronide as the major metabolite of a strawberry flavour constituent in humans. Food Chem. Toxic. 1997, 35, 777–782. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kato, T.; Takahashi, Y.; Yugi, K.; Kikugawa, K. Absorption and induction of micronucleated peripheral reticulocytes in mice after oral administration of fragrant hydroxyfuranones generated in the Maillard reaction. Mutat. Res. 1998, 415, 79–83. [Google Scholar] [CrossRef]
- Stadler, N.C.; Somoza, V.; Schwab, W. Absorption of 3(2H)-furanones by human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2009, 57, 3949–3954. [Google Scholar] [CrossRef]
- Mi, H.; Hiramoto, K.; Kujirai, K.; Ando, K.; Ikarashi, Y.; Kikugawa, K. Effect of food reductones, 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF) and hydroxyhydroquinone (HHQ), on lipid peroxidation and type IV and I allergy responses of mous. J. Agric. Food Chem. 2001, 49, 4950–4955. [Google Scholar] [CrossRef]
- Ando, K.; Ogawa, K.; Li, X.; Kikugawa, K. Inhibition of iron ion-induced oxidative damage of erythrocyte membranes and low density lipoprotein by a Maillard product.; 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF). Biol. Pharm. Bull. 2000, 23, 689–694. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lee, J.; Huh, S.; Boo, Y.C.; Hyun, C.G.; Kim, Y.S.; Park, D. Mechanisms of melanogenesis inhibition by 2,5-dimethyl-4-hydroxy-3(2H)-furanone. Br. J. Dermatol. 2007, 157, 242–248. [Google Scholar] [CrossRef]
- Sung, W.S.; Jung, H.J.; Park, K.; Kim, H.S.; Lee, I.S.; Lee, D.G. 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF).; Antimicrobial compound with cell cycle arrest in nosocomial pathogens. Life Sci. 2007, 80, 586–591. [Google Scholar] [CrossRef]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Morales, M.L.; Ferreira, V.; Hernández-Orte, P. Glycosidically bound aroma compounds and impact odorants of four strawberry varieties. J. Agric. Food Chem. 2012, 60, 6095–6102. [Google Scholar] [CrossRef]
- Du, X.; Finn, C.; Qian, M.C. Distribution of volatile composition in 'Marion' (Rubus species hyb) blackberry pedigree. J. Agric. Food Chem. 2010, 58, 1860–1869. [Google Scholar] [CrossRef]
- Du, X.; Finn, C.E.; Qian, M.C. Bound volatile precursors in genotypes in the pedigree of 'Marion' blackberry (Rubus sp.). J. Agric. Food Chem. 2010, 58, 3694–3699. [Google Scholar] [CrossRef]
- Buttery, R.G.; Takeoka, G.R.; Naim, M.; Rabinowitch, H.; Nam, Y. Analysis of Furaneol in tomato using dynamic headspace sampling with sodium sulfate. J. Agric. Food Chem. 2001, 49, 4349–4351. [Google Scholar] [CrossRef]
- Garcia, C.V.; Quek, S.Y.; Stevenson, R.J.; Winz, R.A. Characterization of the bound volatile extract from baby kiwi (Actinidia arguta). J. Agric. Food Chem. 2011, 59, 8358–8365. [Google Scholar] [CrossRef]
- Ong, P.K.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoshida, K.; Muto, T.; Fujita, A.; Watanabe, N. Changes in the volatile compounds and in the chemical and physical properties of snake fruit (Salacca edulis Reinw) Cv. Pondoh during maturation. J. Agric. Food Chem. 2002, 50, 7627–7633. [Google Scholar] [CrossRef]
- Tokitomo, Y.; Steinhaus, M.; Büttner, A.; Schieberle, P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci. Biotechnol. Biochem. 2005, 69, 1323–1330. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Sun, G.M.; Liu, Y.G.; Lv, L.L.; Yang, W.X.; Zhao, W.F.; Wei, C.B. Aroma volatile compounds from two fresh pineapple varieties in china. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar]
- Mayer, F.; Takeoka, G.R.; Buttery, R.G.; Whitehand, L.C.; Naim, M.; Rabinowitch, H.D. Studies on the aroma of five fresh tomato cultivars and the precursors of cis-and trans-4,5-epoxy-(E)-2-decenals and methional. J. Agric. Food Chem. 2008, 56, 3749–3757. [Google Scholar]
- De la Peña Moreno, F.; Blanch, G.P.; Flores, G.; Ruiz Del Castillo, M.L. Development of a method based on on-line reversed phase liquid chromatography and gas chromatography coupled by means of an adsorption-desorption interface for the analysis of selected chiral volatile compounds in methyl jasmonate treated strawberries. J. Chromatogr. A 2010, 1217, 1083–1038. [Google Scholar] [CrossRef]
- Ferreira, V.; Jarauta, I.; López, R.; Cacho, J. Quantitative determination of sotolon, maltol and free furaneol in wine by solid-phase extraction and gas chromatography-ion-trap mass spectrometry. J. Chromatogr. A 2003, 1010, 95–103. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Lisanti, M.T.; Moio, L. Determination of furaneol (4-hydroxy-2,5-dimethyl-3(2H)-furanone) in some wines from Italian native grapes by Gas-Chromatography-SIM/MASS spectrometry. Ann. Chim. 2005, 95, 415–419. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Muiño, I.; Cadahía, E. Characterization of volatile constituents in commercial oak wood chips. J. Agric. Food Chem. 2010, 58, 9587–9596. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.F.; Ferreira, V. Identification and quantification of impact odorants of aged red wines from Rioja. GC-olfactometry, quantitative GC-MS, and odor evaluation of HPLC fractions. J. Agric. Food Chem. 2001, 49, 2924–2929. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Botelho, G.; Mendes-Faia, A.; Clímaco, M.C. Characterisation of free and glycosidically bound odourant compounds of Aragonez clonal musts by GC-O. Anal. Chim. Acta. 2010, 657, 198–203. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Baumes, R. Identification of impact odorants in Bordeaux red grape juice, in the commercial yeast used for its fermentation, and in the produced wine. J. Agric. Food Chem. 2000, 48, 400–406. [Google Scholar] [CrossRef]
- Bailly, S.; Jerkovic, V.; Meurée, A.; Timmermans, A.; Collin, S. Fate of key odorants in Sauternes wines through aging. J. Agric. Food Chem. 2009, 57, 8557–8563. [Google Scholar] [CrossRef]
- Ferreira, V.; Aznar, M.; López, R.; Cacho, J. Quantitative gas chromatography-olfactometry carried out at different dilutions of an extract. Key differences in the odor profiles of four high-quality Spanish aged red wines. J. Agric. Food Chem. 2001, 49, 4814–4824. [Google Scholar]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Bingham, P.M.; Stevens-Tuttle, D.; Lavin, E.; Acree, T. Odorants in breast milk. Arch. Pediatr. Adolesc. Med. 2003, 157, 1031. [Google Scholar] [CrossRef]
- Karagül-Yüceer, Y.; Drake, M.A.; Cadwallader, K.R. Aroma-active components of nonfat dry milk. J. Agric. Food Chem. 2001, 49, 2948–2953. [Google Scholar] [CrossRef]
- Mahajan, S.S.; Goddik, L.; Qian, M.C. Aroma compounds in sweet whey powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef]
- Counet, C.; Callemien, D.; Ouwerx, C.; Collin, S. Use of gas chromatography-olfactometry to identify key odorant compounds in dark chocolate. Comparison of samples before and after conching. J. Agric. Food Chem. 2002, 50, 2385–2391. [Google Scholar] [CrossRef]
- Drake, M.A.; Miracle, R.E.; McMahon, D.J. Impact of fat reduction on flavor and flavor chemistry of Cheddar cheeses. J. Dairy Sci. 2010, 93, 5069–5081. [Google Scholar] [CrossRef]
- Steinhaus, P.; Schieberle, P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef]
- Wu, Y.F.; Cadwallader, K.R. Characterization of the aroma of a meatlike process flavoring from soybean-based enzyme-hydrolyzed vegetable protein. J. Agric. Food Chem. 2002, 50, 2900–2907. [Google Scholar] [CrossRef]
- Bravo, A.; Herrera, J.C.; Scherer, E.; Ju-Nam, Y.; Rübsam, H.; Madrid, J.; Zufall, C.; Rangel-Aldao, R. Formation of alpha-dicarbonyl compounds in beer during storage of Pilsner. J. Agric. Food Chem. 2008, 56, 4134–4144. [Google Scholar] [CrossRef]
- Song, H.; Cadwallader, K.R. Aroma components of American country ham. J. Food Sci. 2008, 73, C29–C35. [Google Scholar] [CrossRef]
- Elmore, J.S.; Papantoniou, E.; Mottram, D.S. A comparison of headspace entrainment on Tenax with solid phase microextraction for the analysis of the aroma volatiles of cooked beef. Adv. Exp. Med. Biol. 2001, 488, 125–132. [Google Scholar] [CrossRef]
- Sugawara, E.; Ohata, M.; Kanazawa, T.; Kubota, K.; Sakurai, Y. Effects of the amino-carbonyl reaction of ribose and glycine on the formation of the 2(or 5)-ethyl-5(or 2)-methyl-4-hydroxy-3(2H)-furanone aroma component specific to miso by halo-tolerant yeast. Biosci. Biotechnol. Biochem. 2007, 71, 1761–1763. [Google Scholar] [CrossRef]
- Ohata, M.; Kohama, K.; Morimitsu, Y.; Kubota, K.; Sugawara, E. The formation mechanism by yeast of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone in Miso. Biosci. Biotechnol. Biochem. 2007, 71, 407–413. [Google Scholar] [CrossRef]
- Hauck, T.; Landmann, C.; Brühlmann, F.; Schwab, W. Formation of 5-methyl-4-hydroxy-3[2H]-furanone in cytosolic extracts obtained from Zygosaccharomyces rouxii. J. Agric. Food Chem. 2003, 51, 1410–1414. [Google Scholar] [CrossRef]
- Hauck, T.; Hübner, Y.; Brühlmann, F.; Schwab, W. Alternative pathway for the formation of 4,5-dihydroxy-2,3-pentanedione, the proposed precursor of 4-hydroxy-5-methyl-3(2H)-furanone as well as autoinducer-2, and its detection as natural constituent of tomato fruit. Biochem. Biophys. Acta 2003, 1623, 109–119. [Google Scholar] [CrossRef]
- Winzer, K.; Hardie, K.R.; Burgess, N.; Doherty, N.; Kirke, D.; Holden, M.T.; Linforth, R.; Cornell, K.A.; Taylor, A.J.; Hill, P.J.; Williams, P. LuxS: Its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 2002, 148, 909–922. [Google Scholar]
- Tavender, T.J.; Halliday, N.M.; Hardie, K.R.; Winzer, K. LuxS-independent formation of AI-2 from ribulose-5-phosphate. BMC Microbiol. 2008, 8, 98. [Google Scholar] [CrossRef]
- Dahlen, T.; Hauck, T.; Wein, M.; Schwab, W. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone as a secondary metabolite from D-fructose-1,6-diphosphate metabolism by Zygosaccharomyces rouxii. J. Biosci. Bioeng. 2001, 91, 352–358. [Google Scholar]
- Hauck, T.; Brühlmann, F.; Schwab, W. 4-Hydroxy-2,5-dimethyl-3(2H)-furanone formation by Zygosaccharomyces rouxii: Effect of the medium. J. Agric. Food Chem. 2003, 51, 4753–4756. [Google Scholar] [CrossRef]
- Hauck, T.; Brühlmann, F.; Schwab, W. Formation of 4-hydroxy-2,5-dimethyl-3[2H]-furanone by Zygosaccharomyces rouxii: Identification of an intermediate. Appl. Environ. Microbiol. 2003, 69, 3911–3918. [Google Scholar] [CrossRef]
- Hauck, T.; Landmann, C.; Raab, T.; Brühlmann, F.; Schwab, W. Chemical formation of 4-hydroxy-2,5-dimethyl-3[2H]-furanone from D-fructose-1,6-diphosphate. Carbohydr. Res. 2002, 337, 1184–1190. [Google Scholar]
- Raab, T.; Hauck, T.; Knecht, A.; Schmitt, U.; Holzgrabe, U.; Schwab, W. Tautomerism of 4-hydroxy-2,5-dimethyl-3(2H)-furanone: Evidence for its enantioselective biosynthesis. Chirality 2003, 15, 573–578. [Google Scholar] [CrossRef]
- Raab, T.; Schmitt, U.; Hauck, T.; Knecht, A.; Holzgrabe, U.; Schwab, W. Capillary electrophoreticresolution of the enantiomers of 2,5-dimethyl-4-hydroxy-3(2H)-furanone, the key flavor compounds in strawberry fruits. Chromatographia 2003, 57, 501–504. [Google Scholar] [CrossRef]
- Roscher, R.; Hilkert, A.; Gessner, M.; Schindler, E.; Schreier, P.; Schwab, W. L-Rhamnose: Progenitor of 2,5-dimethyl-4-hydroxy-3[2H]-furanone formation by Pichia capsulata? Z. Lebensm. Unters. Forsch. 1997, 204, 198–201. [Google Scholar] [CrossRef]
- Hayashida, Y.; Hatano, M.; Tamura, Y.; Kakimoto, M.; Nishimura, K.; Igoshi, K.; Kobayashi, H.; Kuriyama, H. 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF) production in simple media by lactic acid bacterium, Lactococcus lactis subsp. cremoris IFO 3427. J. Biosci. Bioeng. 2001, 91, 97–99. [Google Scholar]
- Roscher, R.; Bringmann, G.; Schreier, P.; Schwab, W. Radiotracer studies on the formation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone in detached ripening strawberry fruits. J. Agric. Food Chem. 1998, 46, 1488–1493. [Google Scholar] [CrossRef]
- Pérez, A.G.; Olías, R.; Olías, J.M.; Sanz, C. Biosynthesis of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and derivatives in in vitro grown strawberries. J. Agric. Food Chem. 1999, 47, 655–658. [Google Scholar] [CrossRef]
- Roscher, R.; Schreier, P.; Schwab, W. A facile synthesis of 2,5-dimethyl-4-hydroxy-3(2H)-furanone [2- (or 5-) methyl 14C] (Furaneol [2- (or 5-) methyl 14C]). J. Label. Comp. Radiopharm. 1997, 39, 493–499. [Google Scholar] [CrossRef]
- Lavid, N.; Schwab, W.; Kafkas, E.; Koch-Dean, M.; Bar, E.; Larkov, O.; Ravid, U.; Lewinsohn, E. Aroma biosynthesis in strawberry: S-Adenosylmethionine:Furaneol O-methyltransferase in ripening fruits. J. Agric. Food Chem. 2002, 50, 4025–4030. [Google Scholar] [CrossRef]
- Wein, M.; Lavid, N.; Lunkenbein, S.; Lewinsohn, E.; Schwab, W.; Kaldenhoff, R. Isolation, cloning and expression of a multifunctional O-methyltransferase capable of forming 2,5-dimethyl-4-methoxy-3(2H)-furanone, one of the key aroma compounds in strawberry fruits. Plant J. 2002, 31, 755–765. [Google Scholar] [CrossRef]
- Lunkenbein, S.; Salentijn, E.M.J.; Coiner, H.A.; Boone, M.J.; Krens, F.A.; Schwab, W. Up- and down-regulation of Fragaria x ananassa O-methyltransferase: Impacts on furanone and phenylpropanoid metabolism. J. Exp. Bot. 2006, 57, 2445–2453. [Google Scholar] [CrossRef]
- Zorrilla-Fontanesi, Y.; Rambla, J.L.; Cabeza, A.; Medina, J.J.; Sánchez-Sevilla, J.F.; Valpuesta, V.; Botella, M.A.; Granell, A.; Amaya, I. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiol. 2012, 159, 851–870. [Google Scholar] [CrossRef]
- Schwab, W. Application of stable isotope ratio analysis explaining the bioformation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone in plants by a biological Maillard reaction. J. Agric. Food Chem. 1998, 46, 2266–2269. [Google Scholar] [CrossRef]
- Wein, M.; Lewinsohn, E.; Schwab, W. Metabolic fate of isotopes during the biological transformation of carbohydrates to 2,5-dimethyl-4-hydroxy-3(2H)-furanone in strawberry fruits. J. Agric. Food Chem. 2001, 49, 2427–2432. [Google Scholar] [CrossRef]
- Raab, T.; López-Ráez, J.A.; Klein, D.; Caballero, J.L.; Moyano, E.; Schwab, W.; Muñoz-Blanco, J. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone encodes an enone oxidoreductase. Plant Cell 2006, 18, 1023–1037. [Google Scholar] [CrossRef]
- Klein, D.; Fink, B.; Arold, B.; Eisenreich, W.; Schwab, W. Functional characterization of enone oxidoreductases from strawberry and tomato fruit. J. Agric. Food Chem. 2007, 55, 6705–6711. [Google Scholar] [CrossRef]
- Durchschein, K.; Wallner, S.; Macheroux, P.; Schwab, W.; Winkler, T.; Kreis, W.; Faber, K. Nicotinamide-dependent ene reductases as alternative biocatalysts for the reduction of activated alkenes. Eur. J. Org. Chem. 2012, 26, 4963–4968. [Google Scholar]
- Schiefner, A.; Sinz, Q.; Neumaier, I.; Schwab, W.; Skerra, A. Structural basis for the enzymatic formation of the key strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone. J. Biol. Chem. 2013, 288, 16815–16826. [Google Scholar] [CrossRef]
- Verginer, M.; Siegmund, B.; Cardinale, M.; Müller, H.; Choi, Y.; Míguez, C.B.; Leitner, E.; Berg, G. Monitoring the plant epiphyte Methylobacterium extorquens DSM 21961 by real-time PCR and its influence on the strawberry flavor. FEMS Microbiol Ecol. 2010, 74, 136–145. [Google Scholar] [CrossRef]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P.; et al. The genome of woodland strawberry (Fragaria vesca). Nat. Genetics 2011, 43, 109–116. [Google Scholar] [CrossRef]
- Wolf, K.; van der Toorn, A.; Hartmann, K.; Schreiber, L.; Schwab, W.; Haase, A.; Bringmann, G. Metabolite monitoring in plants with double-quantum filtered chemical shift imaging. J. Exp. Bot. 2001, 51, 2109–2117. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schwab, W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules 2013, 18, 6936-6951. https://doi.org/10.3390/molecules18066936
Schwab W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules. 2013; 18(6):6936-6951. https://doi.org/10.3390/molecules18066936
Chicago/Turabian StyleSchwab, Wilfried. 2013. "Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®)" Molecules 18, no. 6: 6936-6951. https://doi.org/10.3390/molecules18066936