Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols
Abstract
:1. Introduction

2. Asymmetric Wacker-Type Oxidative Heterocyclisations
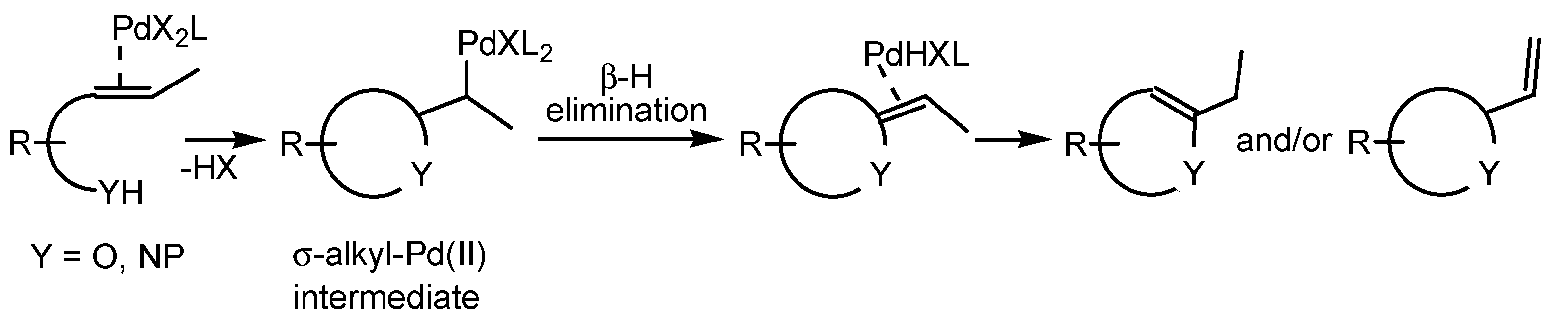
2.1. Oxidative Wacker Cyclisation of o-Allylphenols
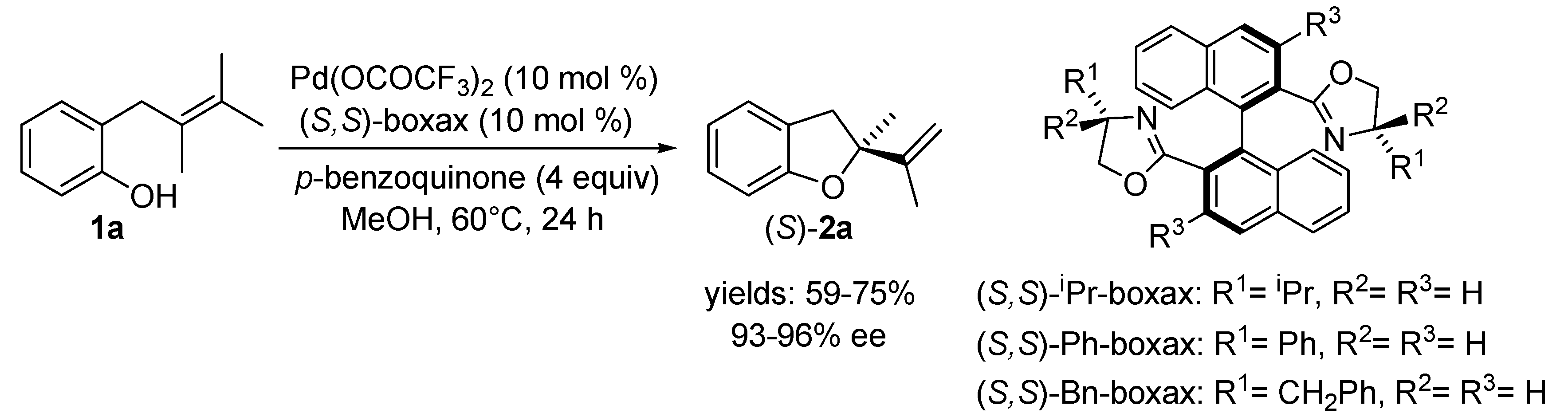
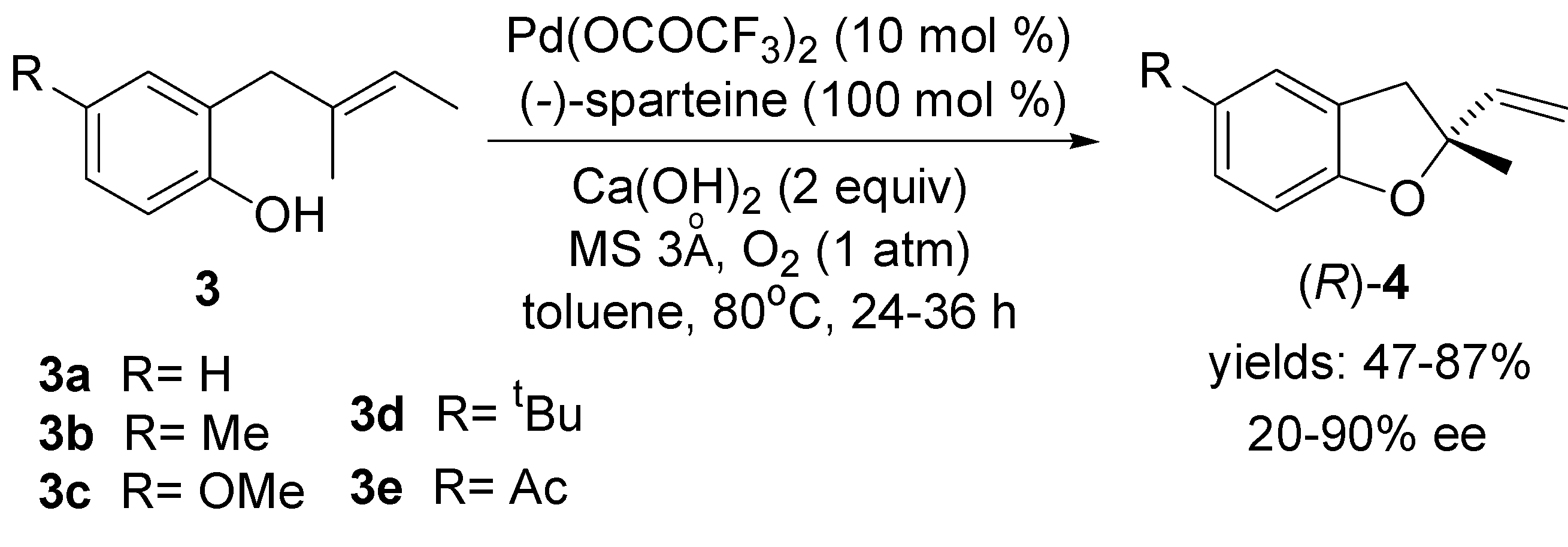
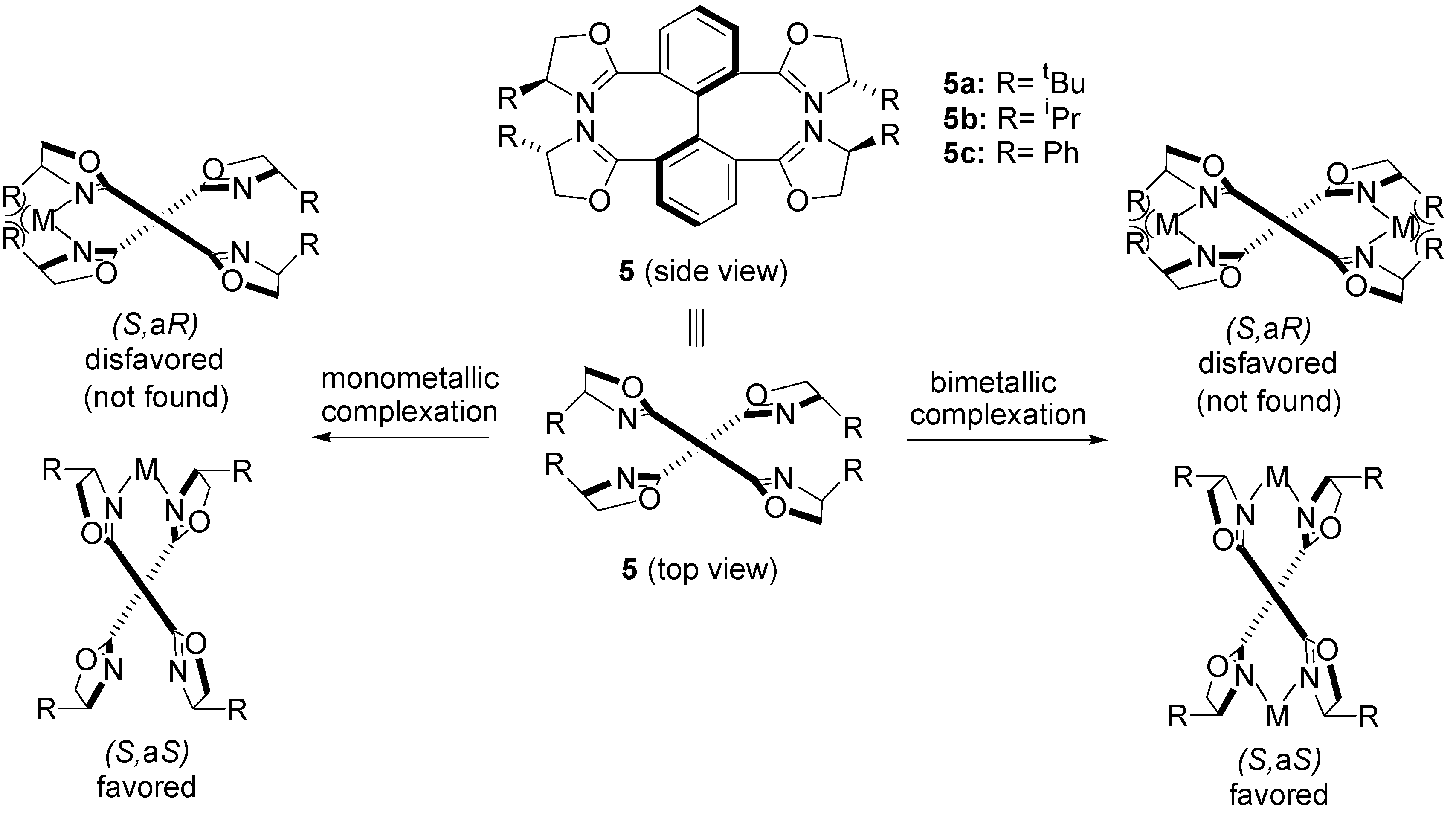
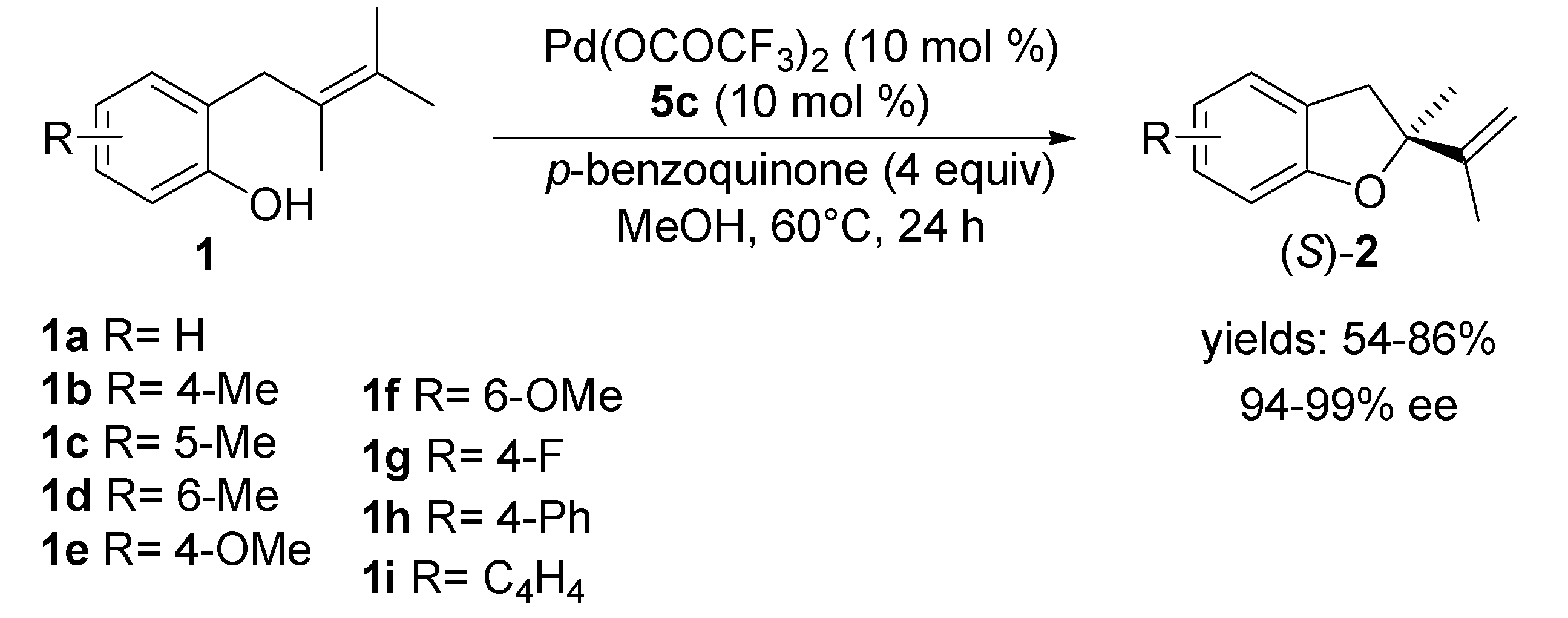
2.2. Oxidative Wacker Cyclisation of Alkeneols
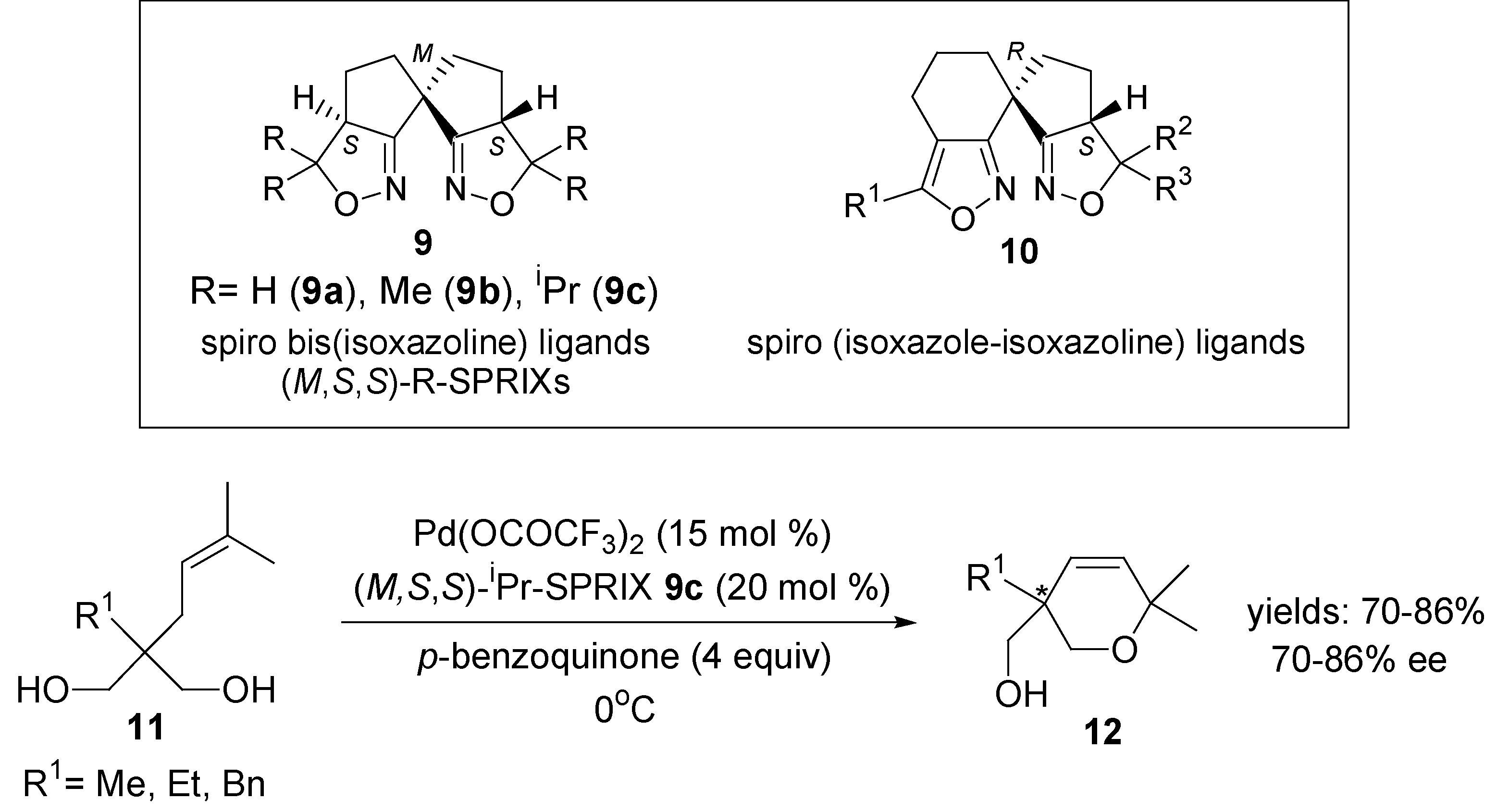

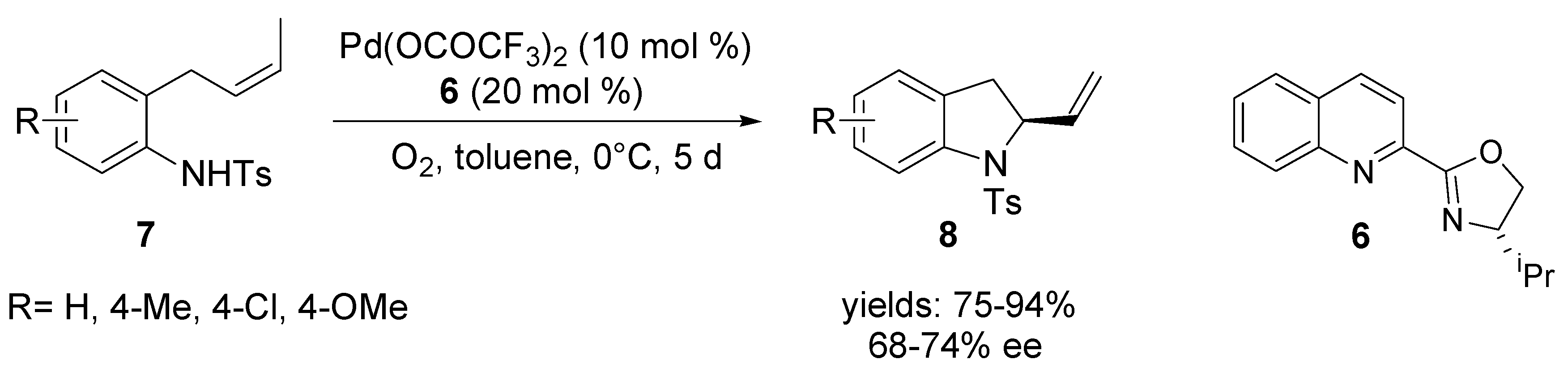
3. Asymmetric Domino Wacker-Type Cyclisation/Coupling Reactions
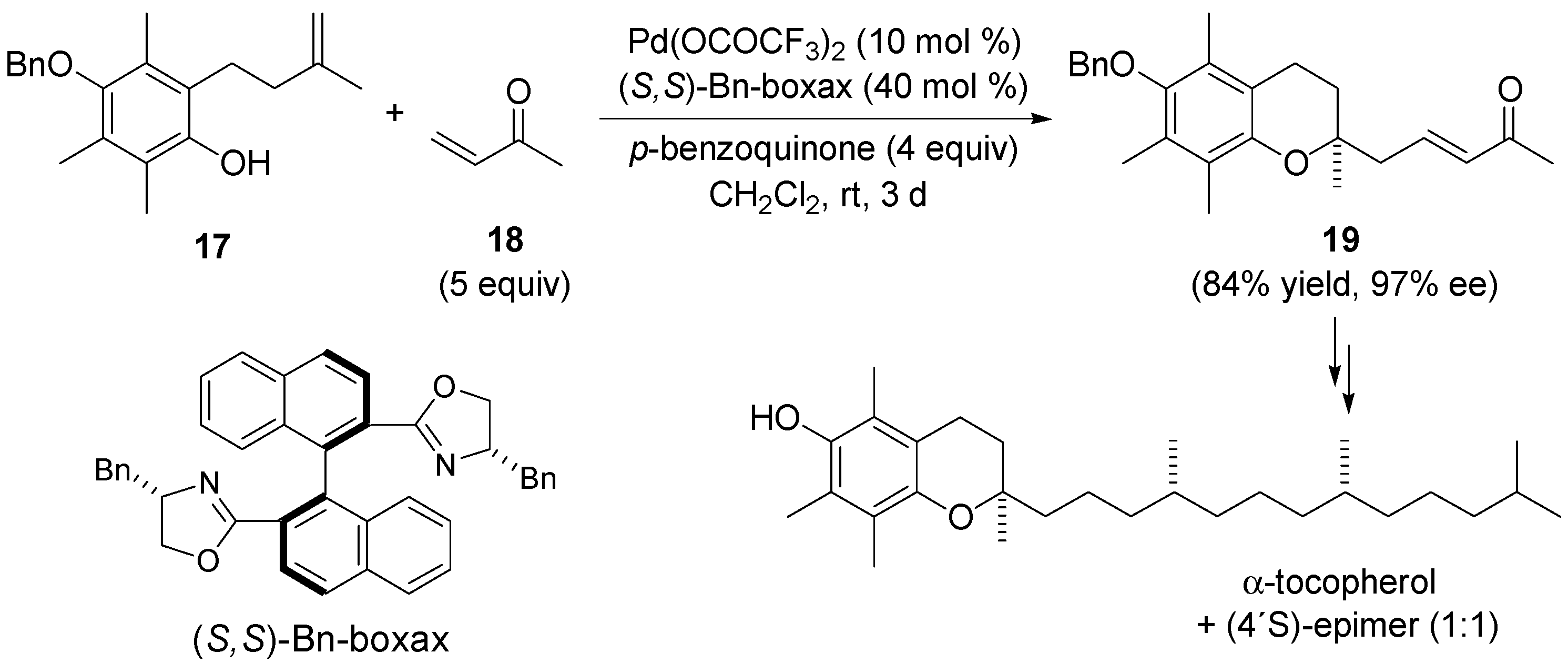
3.1. Coupling with Alkenes via Heck Vinylation
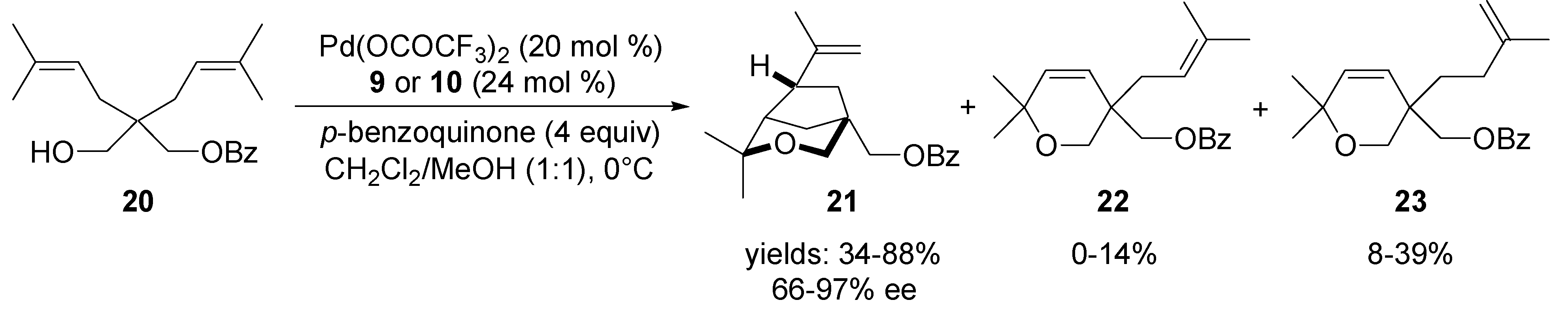
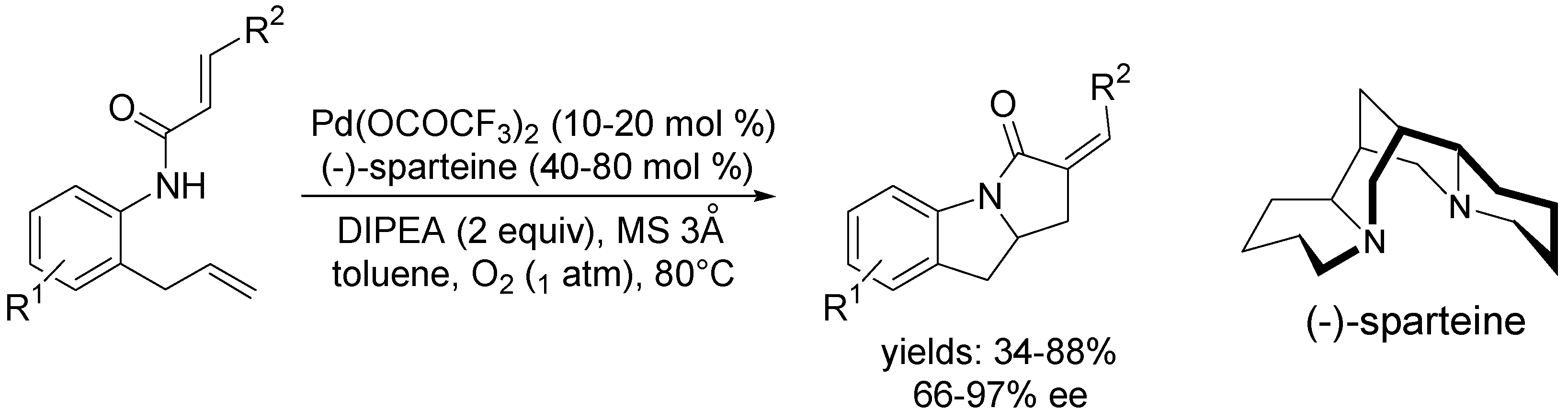
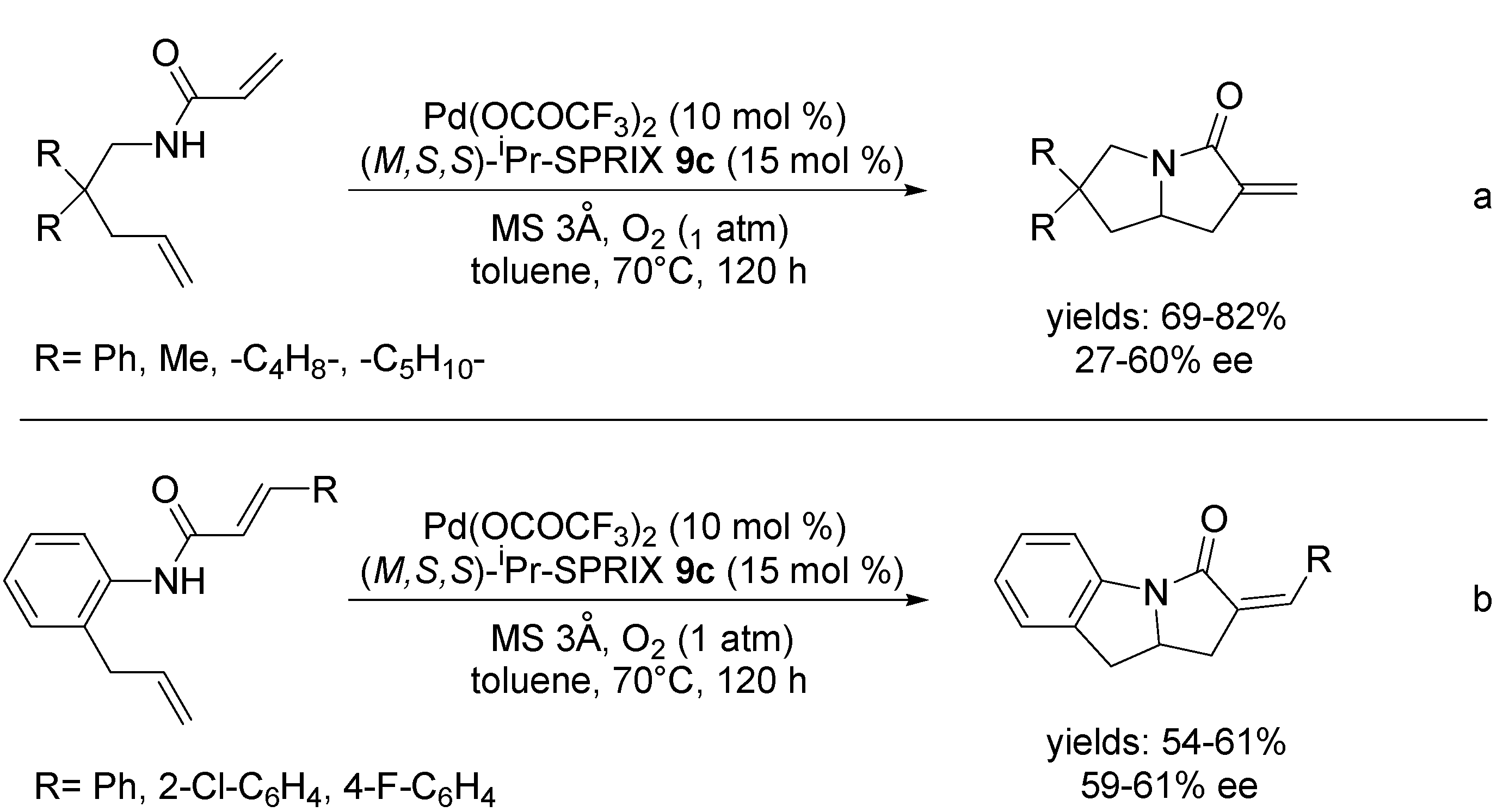
3.2. Coupling with Aryl Halides

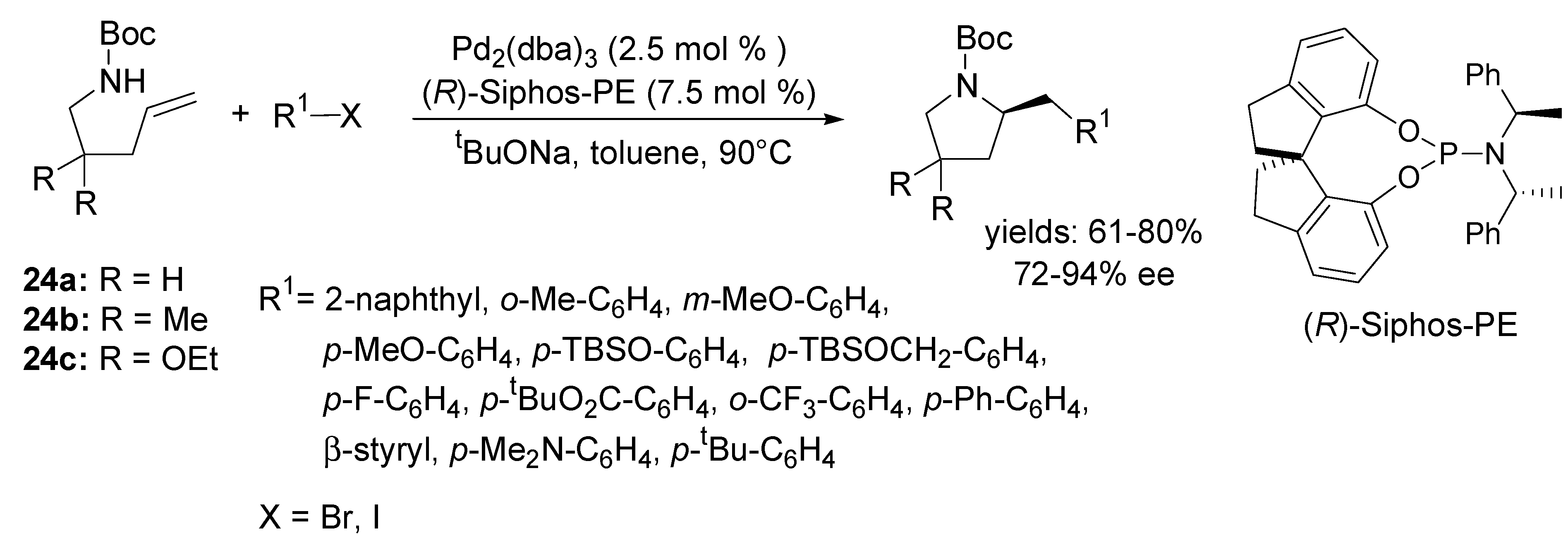
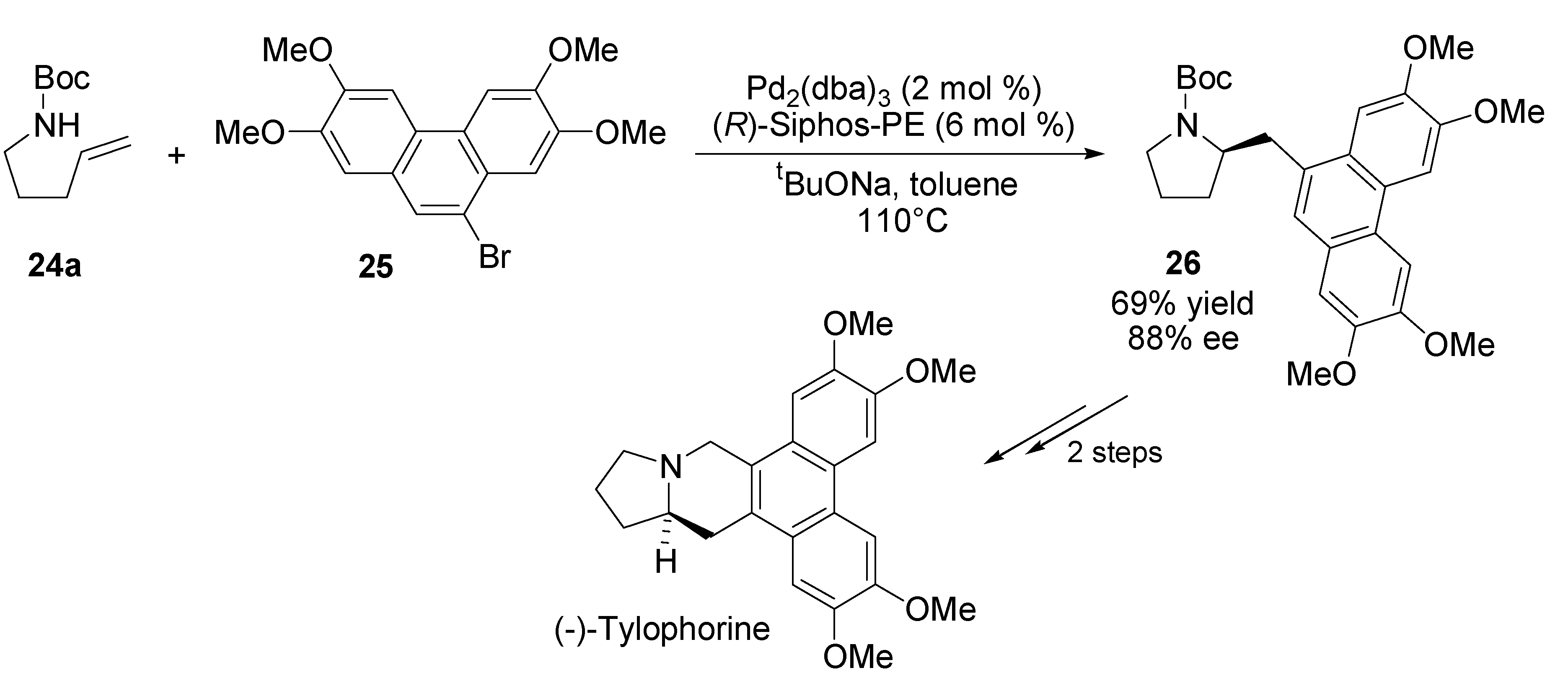
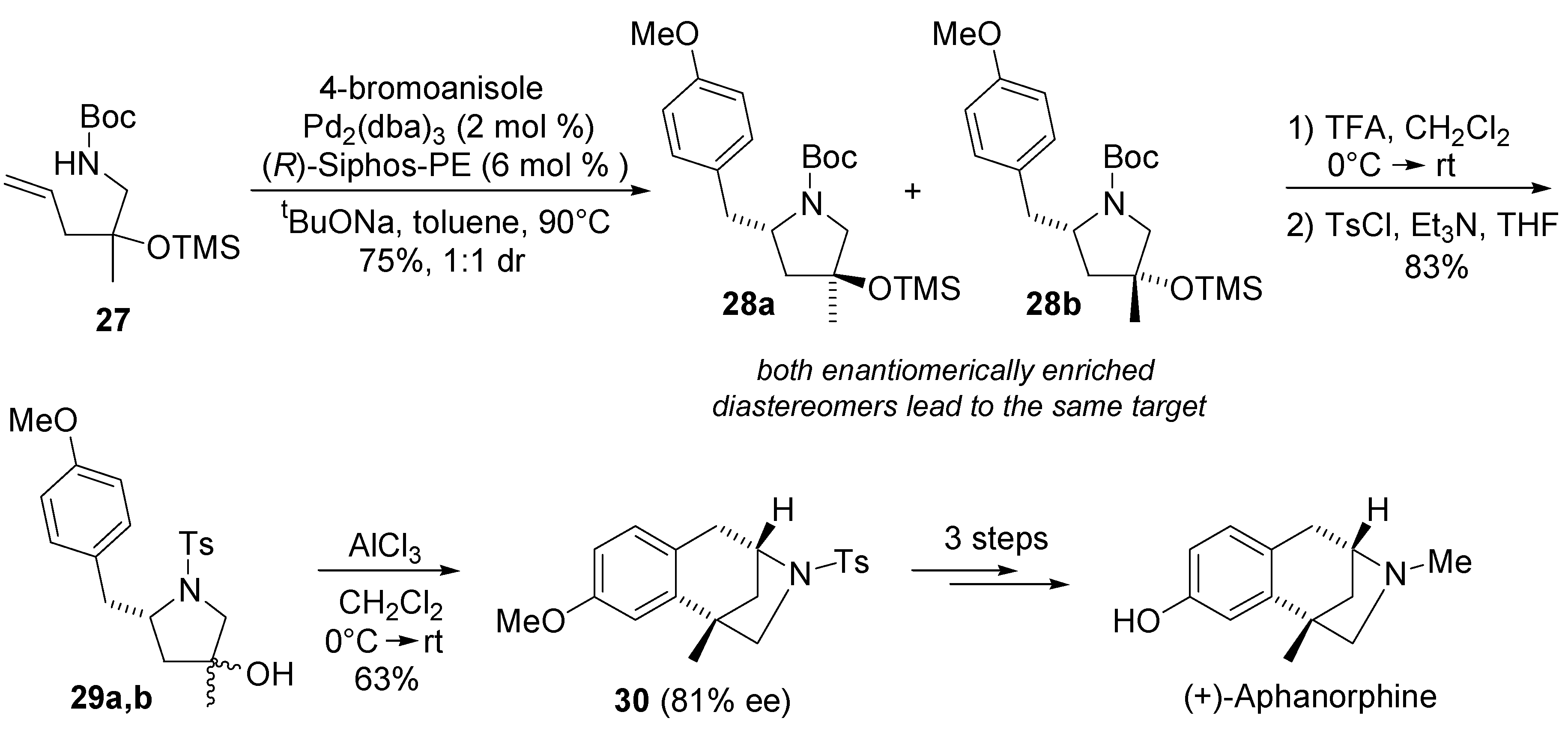

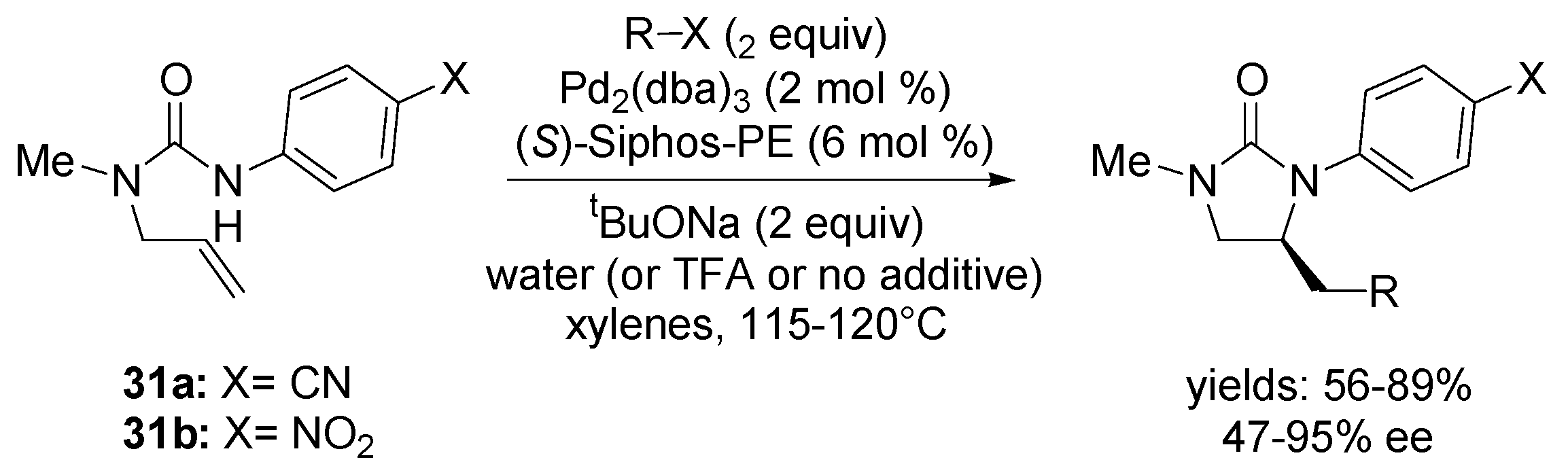
4. Asymmetric Domino Wacker-Type Cyclisation/Carbonylation Reactions
4.1. Intramolecular Alkoxylation/Methoxycarbonylation
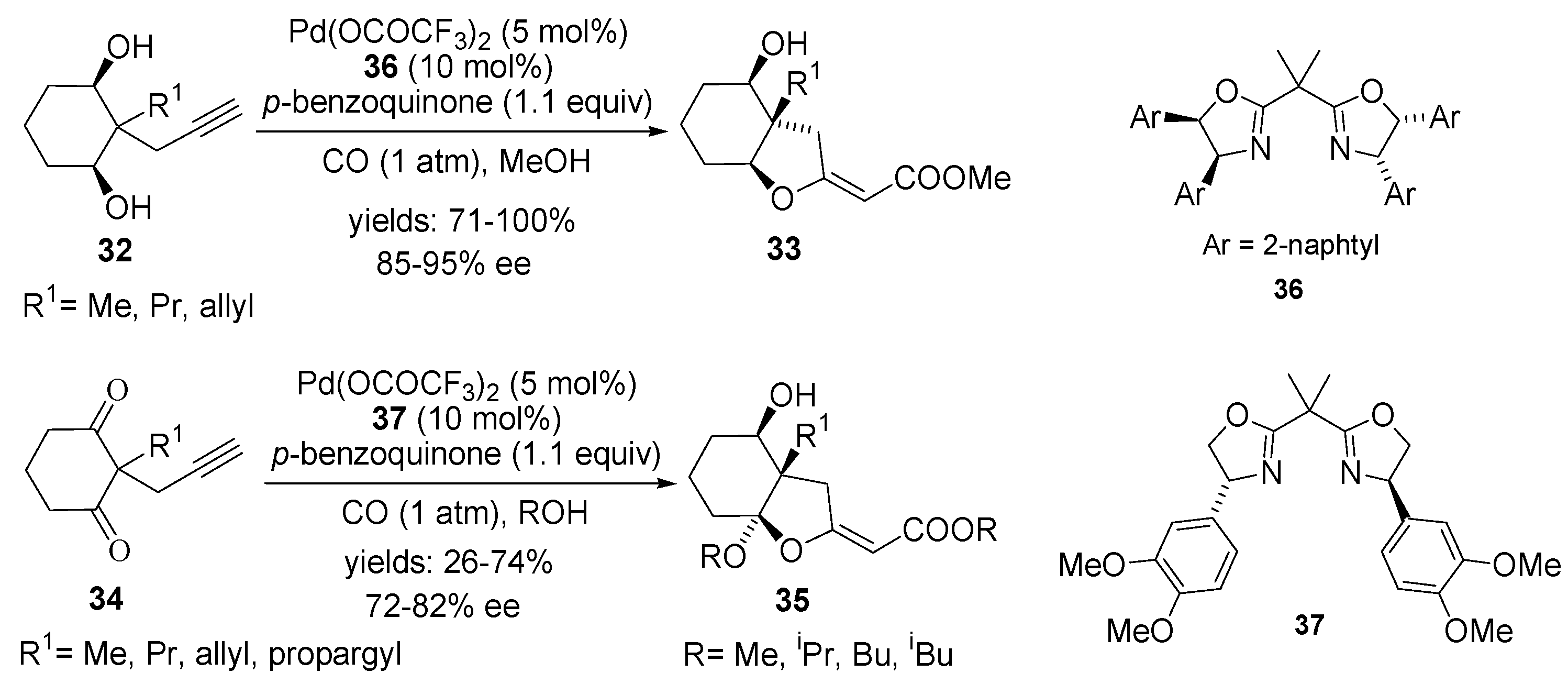
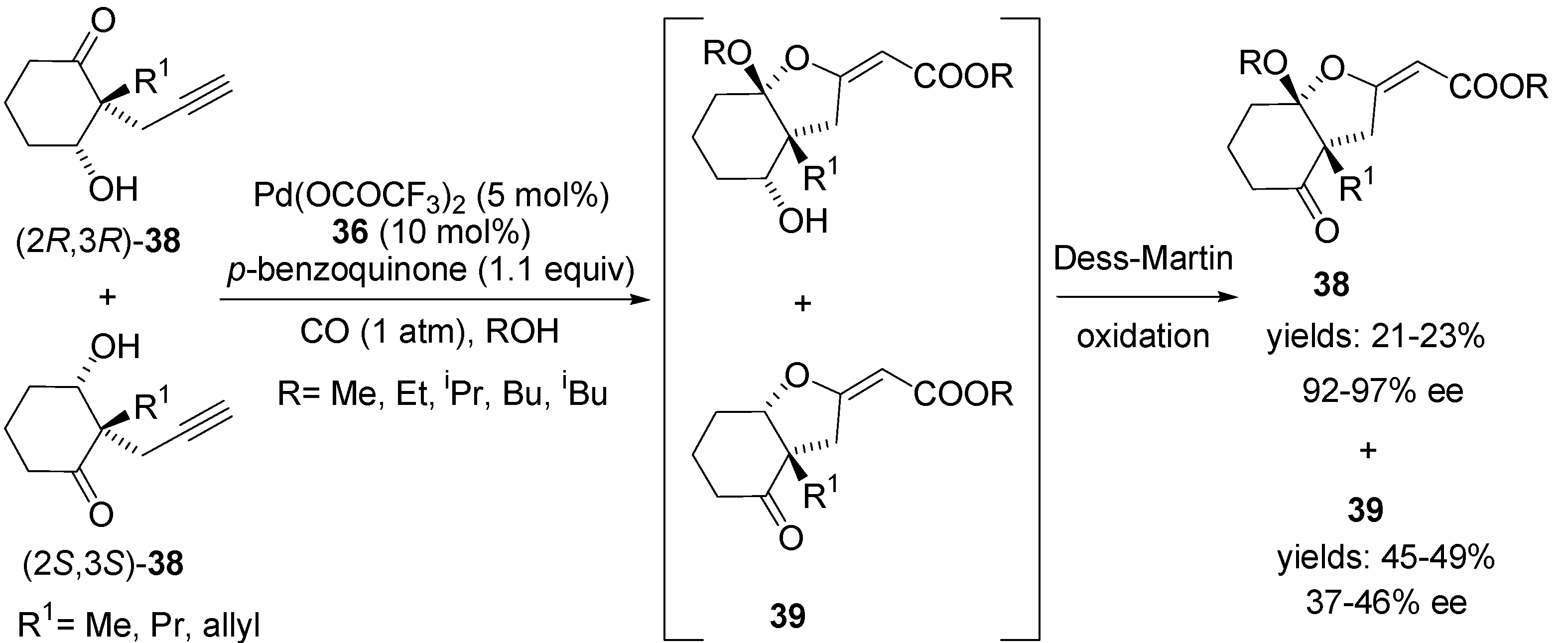
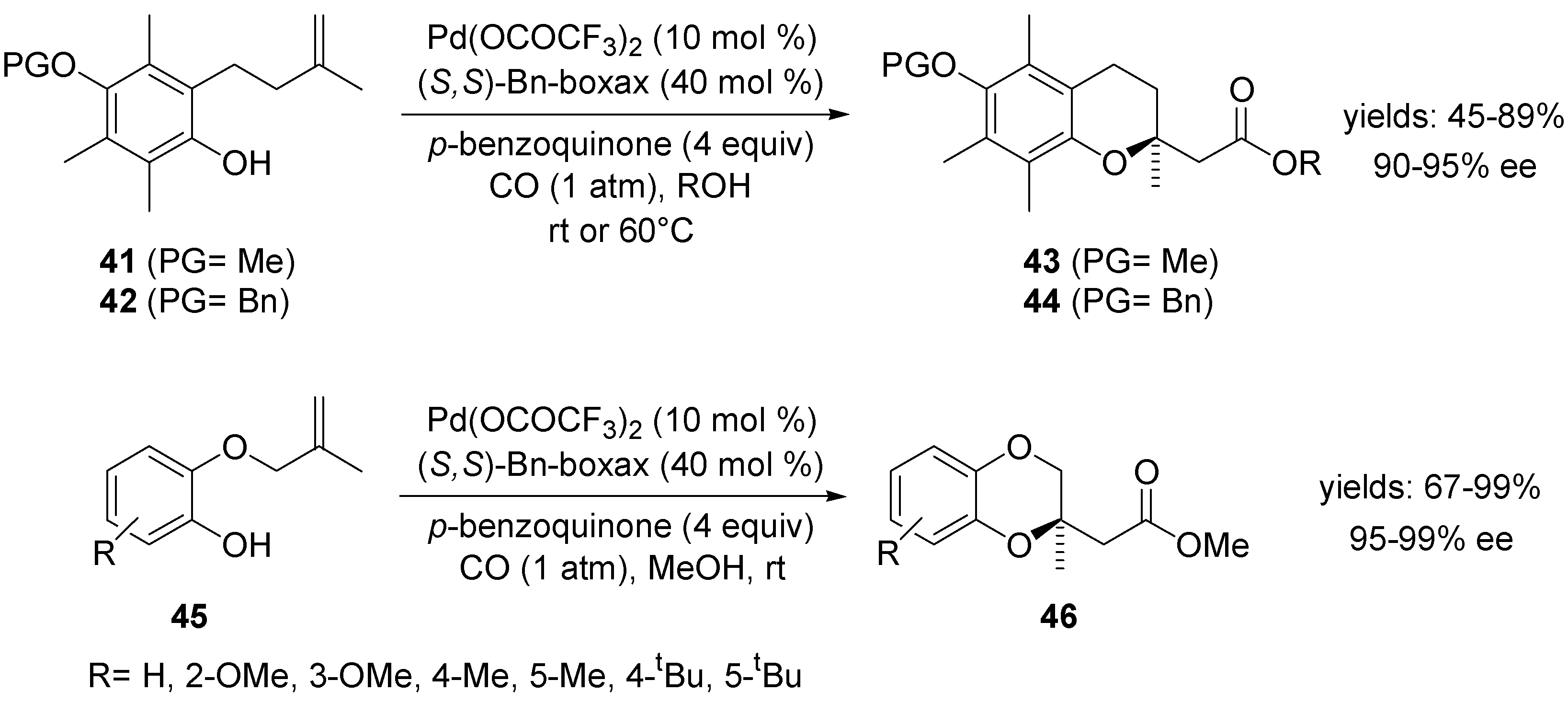
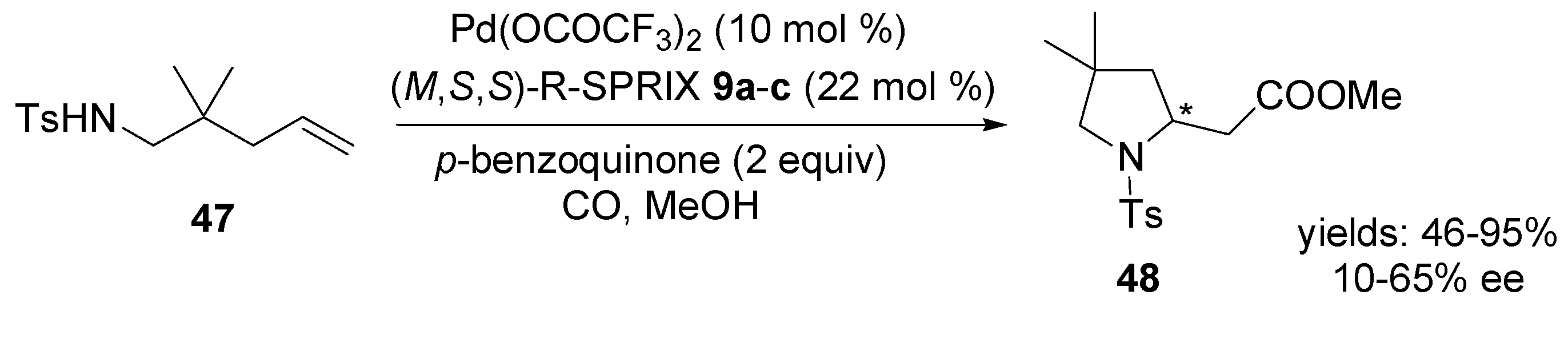
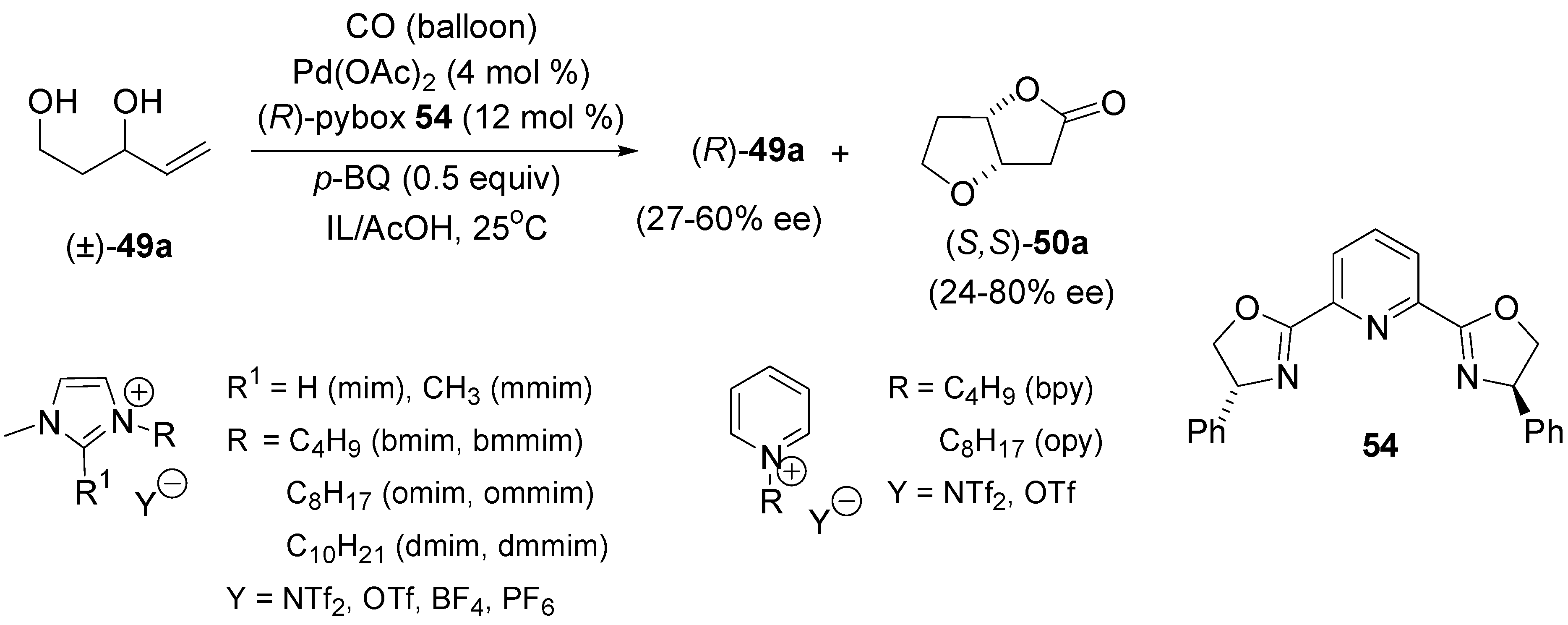
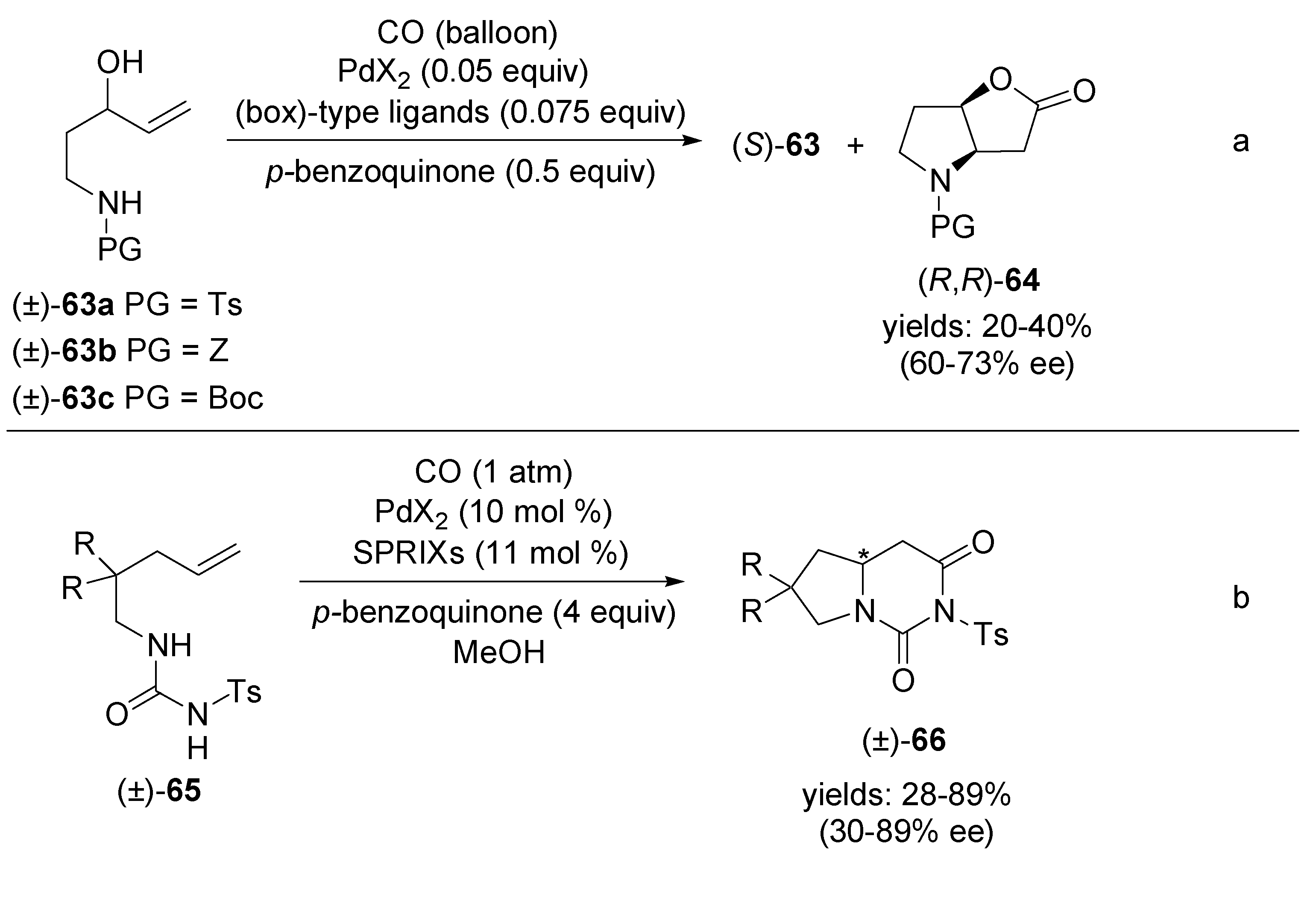
4.2. Intramolecular Alkoxylation/Lactonisation
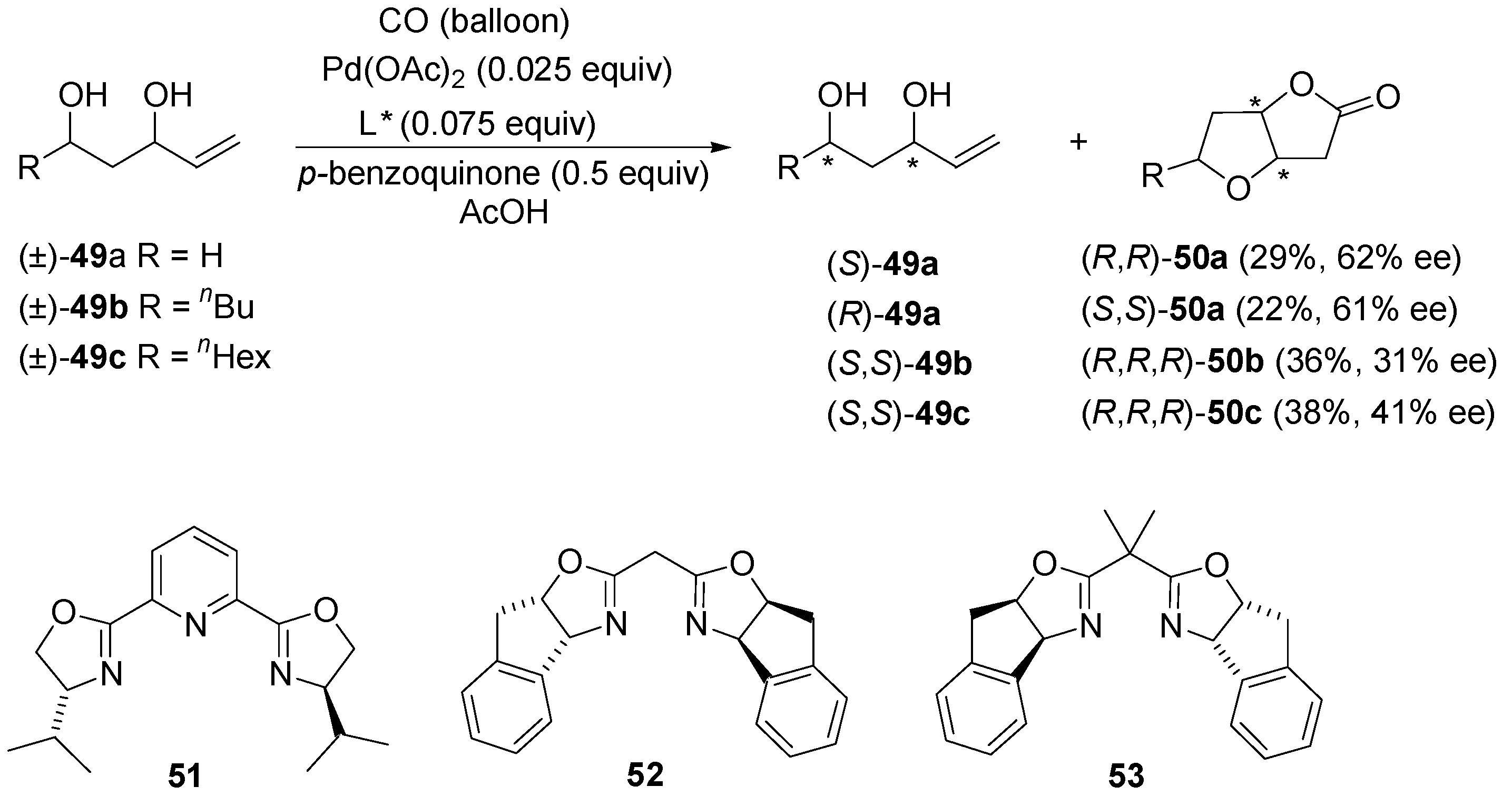

5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tsuji, J. Palladium Reagents and Catalysis; John Wiley & Sons Ltd.: Chichester, UK/Hoboken, NJ, USA, 2004. [Google Scholar]
- Malleron, J.-L.; Fiaud, J.-C.; Legros, J.-Y. Handbook of Palladium-Catalyzed Organic Reactions; Academic Press: London, UK, 1997. [Google Scholar]
- Tietze, L.F.; Ila, H.; Bell, H.P. Enantioselective palladium-catalyzed transformations. Chem. Rev. 2004, 104, 3453–3516. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.I.; Liu, G.; Stahl, S.S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 2011, 111, 2981–3019. [Google Scholar] [CrossRef] [PubMed]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry. Chem. Rev. 2004, 104, 2285–2310. [Google Scholar] [CrossRef] [PubMed]
- Beccalli, E.M.; Broggini, G.; Martinelli; Sottocornola, S. C–C, C–O, C–N Bond formation on sp2 carbon by Pd(II)-catalyzed reactions involving oxidant agents. Chem. Rev. 2007, 107, 5318–5365. [Google Scholar] [CrossRef] [PubMed]
- Jäger, V.; Gracza, T.; Dubois, E.; Hasenohrl, T.; Hümmer, W.; Kautz, U.; Kirschbaum, B.; Lieberknecht, A.; Remen, L.; Shaw, D.; et al. Pd(II)-Catalyzed Carbonylation of Unsaturated Polyols and Aminopolyols. In Organic Synthesis via Organometallics OSM 5; Helmchen, G., Dibo, J., Flubacher, D., Wiese, B., Eds.; Vieweg: Braunschweig, Germany, 1997; pp. 331–360. [Google Scholar]
- Semmelhack, M.F.; Bodurow, C. Intramolecular alkoxypalladation/carbonylation of alkenes. J. Am. Chem. Soc. 1984, 106, 1469–1498. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Kim, C.; Zhang, N.; Bodurow, C.; Sanner, M.; Dobler, W.; Meier, M. Intramolecular alkoxy-carbonylation of hydroxy alkenes promoted by Pd(II). Pure Appl. Chem. 1990, 62, 2035–2040. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Zhang, N. Stereoselective formation of tetrahydrofuran rings via intramolecular alkoxycarbonylation of hydroxyalkenes. J. Org. Chem. 1989, 54, 4483–4485. [Google Scholar] [CrossRef]
- Tamaru, Y.; Kobayashi, T.; Kawamura, S.-I.; Ochiai, H.; Hojo, M.; Yoshida, Z.-I. Palladium catalyzed oxycarbonylation of 4-penten-1,3-diols: Efficient stereoselective synthesis of cis 3-hydroxytetrahydrofuran 2-acetic acid lactones. Tetrahedron Lett. 1985, 26, 3207–3210. [Google Scholar] [CrossRef]
- Gracza, T.; Hasenöhrl, T.; Stahl, U.; Jäger, V. Synthesis of 3,6-Anhydro-2-deoxy-1,4-glyconolactones by Pd(II)-catalyzed, regioselective oxycarbonylation of C5- and C6-enitols, ω-homologation of aldoses to produce intermediates for C-glycoside/C-nucleoside synthesis. Synthesis 1991, 1108–1118. [Google Scholar] [CrossRef]
- Babjak, M.; Zálupský, P.; Gracza, T. Regiocontrol in the palladium(II)-catalysed oxycarbonylation of unsaturated polyols. ARKIVOC 2005, v, 45–57. [Google Scholar]
- Tamaru, Y.; Yoshida, Z. Heterocyclic synthesis by the use of the oxidizing potential of palladium(II). J. Organomet. Chem. 1987, 334, 213–223. [Google Scholar] [CrossRef]
- Tamaru, Y.; Hojo, M.; Yoshida, Z. Palladium(2+)-catalyzed intramolecular aminocarbonylation of 3-hydroxy-4-pentenylamines and 4-hydroxy-5-hexenylamines. J. Org. Chem. 1988, 53, 5731–5741. [Google Scholar] [CrossRef]
- Babjak, M.; Remeň, Ľ.; Szolcsányi, P.; Zálupský, P.; Miklóš, M.; Gracza, T. Novel bicyclisation of unsaturated polyols in PdCl2-CuCl2-AcOH catalytic system. J. Organomet. Chem. 2006, 691, 928–940. [Google Scholar] [CrossRef]
- Szolcsányi, P.; Gracza, T. Novel Pd(II)-catalysed N,O-bicyclisation as an efficient route to the 6-oxa-2-azabicyclo[3.2.1]octane skeleton. Chem. Commun. 2005, 2005, 3948–3950. [Google Scholar] [CrossRef] [PubMed]
- Palík, M.; Karlubíková, O.; Lásiková, A.; Kožíšek, J.; Gracza, T. Total synthesis of (+)-varitriol. Eur. J. Org. Chem. 2009, 2009, 709–715. [Google Scholar] [CrossRef]
- Tietze, L.F.; Sommer, K.M.; Zinngrebe, J.; Stecker, F. Palladium-catalyzed enantioselective domino reaction for the efficient synthesis of vitamin E. Angew. Chem. Int. Ed. 2005, 44, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Stecker, F.; Zinngrebe, J.; Sommer, K. Enantioselective palladium-catalyzed total synthesis of vitamin E by employing a domino Wacker-Heck reaction. Chem. Eur. J. 2006, 12, 8770–8776. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Spiegl, D.A.; Stecker, F.; Major, J.; Raith, C.; Große, C. Stereoselective synthesis of 4-dehydroxydiversonol employing enantioselective palladium-catalysed domino reactions. Chem. Eur. J. 2008, 14, 8956–8963. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.P. Palladium-catalyzed carboetherification and carboamination reactions of γ-hydroxy- and γ-aminoalkenes for the synthesis of tetrahydrofurans and pyrrolidines. Eur. J. Org. Chem. 2007, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.P. Stereoselective synthesis of saturated heterocycles via palladium-catalyzed alkene carboetherification and carboamination reactions. Synlett 2008, 2913–2937. [Google Scholar] [CrossRef]
- Gracza, T. Intramolecular Oxycarbonylation in Stereoselective Synthesis. In Stereoselective Synthesis of Drugs &Natural Products; Andrushko, V., Andrushko, N., Eds.; Wiley: Hoboken, NJ, USA, 2013; Chapter 15. [Google Scholar]
- Szolcsányi, P.; Gracza, T.; Špánik, I. Short racemic syntheses of calvine and epicalvine. Tetrahedron Lett. 2008, 49, 1357–1360. [Google Scholar] [CrossRef]
- Heumann, A.; Réglier, M. The stereochemistry of palladium catalysed cyclisation reactions. Part C: Cascade reactions. Tetrahedron 1996, 52, 9289–9346. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed reactions of alcohols. Part B: Formation of C–C and C–N bonds from unsaturated alcohols. Tetrahedron 2005, 61, 4179–4212. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed reactions of alcohols. Part C: Formation of ether linkages. Tetrahedron 2005, 61, 5955–6008. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed reactions of alcohols. Part D: Rearrangements, carbonylations, carboxylations and miscellaneous reactions. Tetrahedron 2005, 61, 9423–9463. [Google Scholar] [CrossRef]
- Hyland, C. Cyclisations of allylic substrates via palladium catalysis. Tetrahedron 2005, 61, 3457–3471. [Google Scholar] [CrossRef]
- Muzart, J. Pd0- and PdII-catalyzed oxaheterocyclization of substrates having both an allylic leaving group and a hydroxylated tether. J. Mol. Cat. A 2010, 319, 1–29. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Fasana, A.; Rigamonti, M. Palladium-catalyzed C–N bond formation via direct C–H bond functionalization. Recent developments in heterocyclic synthesis. J. Organomet. Chem. 2011, 696, 277–295. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem 2013, 6, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Uno, T.; Inui, S.; Murahashi, S.-I. Palladium(II)-catalyzed asymmetric oxidative cyclization of 2-allylphenols in the presence of copper(II) acetate and molecular oxygen. Study of the catalysis of the Wacker-type oxidation. J. Am. Chem. Soc. 1981, 103, 2318–2323. [Google Scholar] [CrossRef]
- Hosokawa, T.; Okuda, C.; Murahashi, S.-I. Substituent effects on palladium(II)-catalyzed enantioselective cyclization of a series of 2-(2-butenyl)phenols. J. Org. Chem. 1985, 50, 1282–1287. [Google Scholar] [CrossRef]
- Uozumi, Y.; Kato, K.; Hayashi, T. Catalytic asymmetric Wacker-type cyclization. J. Am. Chem. Soc. 1997, 119, 5063–5064. [Google Scholar] [CrossRef]
- Uozumi, Y.; Kato, K.; Hayashi, T. Cationic palladium/boxax complexes for catalytic asymmetric Wacker-type cyclization. J. Org. Chem. 1998, 63, 5071–5075. [Google Scholar] [CrossRef]
- Uozumi, Y.; Kyota, H.; Kato, K.; Ogasawara, M.; Hayashi, T. Design and preparation of 3,3′-disubstituted 2,2′-bis(oxazolyl)-1,1′-binaphtyls (boxax): New chiral bis(oxazoline) ligands for catalytic asymmetric Wacker-type cyclization. J. Org. Chem. 1999, 64, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Trend, R.M.; Ramtohul, Y.K.; Ferreira, E.M.; Stoltz, B.M. Palladium-catalyzed oxidative Wacker cyclization in nonpolar organic solvents with molecular oxygen: A stepping stone to asymmetric aerobic cyclizations. Angew. Chem. Int. Ed. 2003, 42, 2892–2895. [Google Scholar] [CrossRef] [PubMed]
- Trend, R.M.; Ramtohul, Y.K.; Stoltz, B.M. Oxidative cyclizations in a nonpolar solvent using molecular oxygen and studies on the stereochemistry of oxypalladation. J. Am. Chem. Soc. 2005, 127, 17778–17788. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Tanigaki, Y.; Patil, M. L.; Rao, C.V.L.; Takizawa, S.; Suzuki, T.; Sasai, H. Enantioselective 6-endo-trig Wacker-type cyclization of 2-geranylphenols: Application to a facile synthesis of (−)-cordiachromene. Tetrahedron: Asymmetry 2010, 21, 767–770. [Google Scholar] [CrossRef]
- Takenaka, K.; Mohanata, S.C.; Patil, M.L.; Rao, C.V.L.; Takizawa, S.; Suzuki, T.; Sasai, H. Enantioselective Wacker-type cyclization of 2-alkenyl-1,3-diketones promoted by Pd-SPRIX catalyst. Org. Lett. 2010, 12, 3480–2010. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.J.; Yang, G.; Zhang, W. Highly enantioselective Pd(II)-catalyzed Wacker-type cyclization of 2-allylphenols by use of bisoxazoline ligands with axis-unifixed biphenyl backbone. Tetrahedron Lett. 2007, 48, 4179–4182. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, F.; Zhang, W. Chelation-induced axially chiral palladium complex system with tetraoxazoline ligands for highly enantioselective Wacker-type cyclization. J. Org. Chem. 2007, 72, 9208–9213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, Z.; Zhang, W. Pd-catalyzed asymmetric aza-Wacker-type cyclization reaction of olefinic tosylamides. Tetrahedron Lett. 2010, 51, 5124–5126. [Google Scholar] [CrossRef]
- Bajracharya, G.B.; Arai, M.A.; Koranne, P.S.; Suzuki, T.; Takizawa, S.; Sasai, H. Development of chiral spiro ligands for metal-catalyzed asymmetric reactions. Bull. Chem. Soc. Jpn. 2009, 82, 285–302. [Google Scholar] [CrossRef]
- Arai, M.A.; Kuraishi, M.; Arai, T.; Sasai, H. A new asymmetric Wacker-type cyclization and tandem cyclization promoted by Pd(II)-spiro bis(isoxazoline) catalyst. J. Am. Chem. Soc. 2001, 123, 2907–2908. [Google Scholar] [CrossRef] [PubMed]
- Koranne, P.S; Tsujihara, T.; Arai, M.A.; Bajracharya, G.B.; Suzuki, S.; Onitsuka, K.; Sasai, H. Design and synthesis of chiral hybrid spiro (isoxazole–isoxazoline) ligands. Tetrahedron: Asymmetry 2007, 18, 919–923. [Google Scholar] [CrossRef]
- Takenaka, K.; Nagano, T.; Takizawa, S.; Sasai, H. Asymmetric synthesis of chiral spiro bis(isoxazoline) and spiro (isoxazole–isoxazoline) ligands. Tetrahedron: Asymmetry 2010, 21, 379–381. [Google Scholar] [CrossRef]
- Takenaka, K.; Akita, M.; Tanigaki, Y.; Takizawa, S.; Sasai, H. Enantioselective cyclization of 4-alkenoic acids via an oxidative allylic C–H esterification. Org. Lett. 2011, 13, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Ramalingan, C.; Takenaka, K.; Sasai, H. Pd(II)-SPRIX catalyzed enantioselective construction of pyrrolizines/pyrroloindoles employing molecular oxygen as the sole oxidant. Tetrahedron 2011, 67, 2889–2894. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Epa, W.R. Catalytic tandem oxy-palladation and vinylation. Tetrahedron Lett. 1993, 34, 7205–7208. [Google Scholar] [CrossRef]
- Tietze, L.F.; Wilckens, K.F.; Yilmaz, S.; Stecker, F.; Zinngrebe, J. Synthesis of 2,3-dihydrobenzo[1,4]dioxins and -oxazins via a domino Wacker-Heck reaction. Heterocycles 2006, 70, 309–319. [Google Scholar] [CrossRef]
- Tietze, L.F.; Heins, A.; Soleiman-Beigi, M.; Raith, C. Synthesis of anulated 1,4-dioxanes and perhydro-1,4-oxazines by domino-Wacker-carbonylation and domino-Wacker-Mizoroki-Heck reactions. Heterocycles 2009, 77, 1123–1146. [Google Scholar] [CrossRef]
- Yip, K.-T.; Yang, M.; Law, K.-L.; Zhu, N.-Y.; Yang, D. Pd(II)-Catalyzed enantioselective oxidative tandem cyclization reactions. Synthesis of indolines through C–N and C–C bond formation. J. Am. Chem. Soc. 2006, 128, 3130–3131. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Wolfe, J.P. Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis 2012, 351–361. [Google Scholar] [CrossRef]
- Hosokawa, T.; Murahashi, S.-I. New aspects of oxypalladation of alkenes. Acc. Chem. Res. 1990, 23, 49–54. [Google Scholar] [CrossRef]
- Hegedus, L.S. Transition metals in the synthesis and functionalization of indoles. Angew. Chem. Int. Ed. 1988, 27, 1113–1126. [Google Scholar] [CrossRef]
- Mai, D.N.; Wolfe, J.P. Asymmetric palladium-catalyzed carboamination reactions for the synthesis of enantiomerically enriched 2-(arylmethyl)- and 2-(alkenylmethyl)pyrrolidines. J. Am. Chem. Soc. 2010, 132, 12157–12159. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.N.; Rosen, B.R.; Wolfe, J.P. Enantioconvergent synthesis of (+)-aphanorphine via asymmetric Pd-catalyzed alkene carboamination. Org. Lett. 2011, 13, 2932–2935. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.A.; Wolfe, J.P. Synthesis of enantiomerically enriched imidazollidin-2-ones through asymmetric palladium-catalyzed alkene carboamination. Angew. Chem. Int. Ed. 2012, 51, 9886–9890. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Tanaka, M.; Yamamoto, Y.; Akita, H. Asymmetric cyclization-carbonylation of cyclic-2-methyl-2-propargyl-1,3-diols. Tetrahedron Lett. 2002, 43, 1511–1513. [Google Scholar] [CrossRef]
- Kato, K.; Tanaka, M.; Yamamura, S.; Yamamoto, Y.; Akita, H. Asymmetric cyclization-carbonylation of 2-propargyl-1,3-dione. Tetrahedron Lett. 2003, 44, 3089–3092. [Google Scholar] [CrossRef]
- Kato, K.; Matsuba, Ch.; Kusakabe, T.; Takayama, H.; Yamamura, S.; Mochida, T.; Akita, H.; Peganova, T.A.; Vologdin, N.V.; Gusev, O.V. 2,2´-Isopropylidene-bis[(4S,5R)-4,5-di(2-naphtyl)-2-oxazoline] ligand for asymmetric cyclization-carbonylation of meso-2-alkyl-2-propargylcyclohexane-1,3-diols. Tetrahedron 2006, 62, 9988–9999. [Google Scholar] [CrossRef]
- Kusakabe, T.; Kato, K.; Takaishi, S.; Yamamura, S.; Mochida, T.; Akita, H.; Peganova, T.A.; Vologdin, N.V.; Gusev, O.V. Asymmetric cyclization-carbonylation of 2-alkyl-2-propargylcyclohexane-1,3-diones: facile access to optically active hydradanes. Tetrahedron 2008, 64, 319–327. [Google Scholar] [CrossRef]
- Kato, K.; Motodate, S.; Takaishi, S.; Kusakabe, T.; Akita, H. Parallel kinetic resolution of propargyl ketols: formal synthesis of (+)-bakkenolide A. Tetrahedron 2008, 64, 4627–4636. [Google Scholar] [CrossRef]
- Tietze, L.F.; Zinngrebe, J.; Speigl, D.A.; Stecker, F. Palladium-catalyzed domino-Wacker-carbonylation reaction for the enantioselective synthesis of chromans and benzodioxins. Heterocycles 2007, 74, 473–789. [Google Scholar] [CrossRef]
- Shinohara, T.; Arai, M.A.; Wakita, K.; Arai, T.; Sasai, H. The first enantioselective intramolecular aminocarbonylation of alkenes promoted by Pd(II)-spiro bis(isoxazoline) catalyst. Tetrahedron Lett. 2003, 44, 711–714. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Asymmetric intramolecular Pd(II)-catalysed oxycarbonylation of alkene-1,3-diols. ARKIVOC 2008, viii, 8–17. [Google Scholar]
- Doháňošová, J.; Lásiková, A.; Toffano, M.; Gracza, T.; Vo-Than, G. Kinetic resolution of pent-4-ene-1,3-diol by Pd(II)-catalysed oxycarbonylation in ionic liquids. New J. Chem. 2012, 36, 1744–1750. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Stereocontrolled oxycarbonylation of 4-benzaloxyhepta-1,6-diene-3,5-diols promoted by chiral palladium(II) complexes. Tetrahedron: Asymmetry 2008, 19, 38–44. [Google Scholar] [CrossRef]
- Koóš, P.; Špánik, I.; Gracza, T. Asymmetric intramolecular Pd(II)-catalysed amidocarbonylation of unsaturated amino alcohols. Tetrahedron: Asymmetry 2009, 20, 2720–2723. [Google Scholar] [CrossRef]
- Tsujihara, T.; Shinohara, T.; Takenaka, K.; Takizawa, S.; Onitsuka, K.; Hatanaka, M.; Sasai, H. Enantioselective intramolecular oxidative aminocarbonylation of alkenylureas catalyzed by palladium-spiro bis(isoxazoline) complexes. J. Org. Chem. 2009, 74, 9274–9279. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doháňošová, J.; Gracza, T. Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols. Molecules 2013, 18, 6173-6192. https://doi.org/10.3390/molecules18066173
Doháňošová J, Gracza T. Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols. Molecules. 2013; 18(6):6173-6192. https://doi.org/10.3390/molecules18066173
Chicago/Turabian StyleDoháňošová, Jana, and Tibor Gracza. 2013. "Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols" Molecules 18, no. 6: 6173-6192. https://doi.org/10.3390/molecules18066173



